Formation mechanisms of ethyl acetate and organic acids in Kluyveromyces marxianus L1-1 in Chinese acid rice soup
N Liu, Likng Qin*, Lili Hu Song Mio*
a School of Liquor and Food Engineering, Guizhou University, Guiyang 550025, China
b Key Laboratory of Plant Resource Conservation and Germplasm Innovation in Mountainous Region (Ministry of Education), Collaborative Innovation Center for Mountain Ecology & Agro-bioengineering (CICMEAB), College of Life Sciences/Institute of Agro-bioengineering, Guizhou University, Guiyang 550025, China
c Teagasc Food Research Centre, Moorepark, Fermoy, Co. Cork P61 C996, Ireland
Keywords:Kluyveromyces marxianus Fermented rice soup Ethyl acetate Organic acid Formation mechanism
A B S T R A C T This study aims to explore the formation mechanism of ethyl acetate and organic acids in acid rice soup(rice-acid soup) inoculated with Kluyveromyces marxianus L1-1 through the complementary analysis of transcriptome and proteome. The quantity of K. marxianus L1-1 varied significantly in the fermentation process of rice-acid soup and the first and third days were the two key turning points in the growth phase of K. marxianus L1-1. Importantly, the concentrations of ethyl acetate, ethanol, acetic acid, and L-lactic acid increased from day 1 to day 3. At least 4 231 genes and 2 937 proteins were identified and 610 differentially expressed proteins were annotated to 30 Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways based on the analysis results of transcriptome and proteome. The key genes and proteins including up-regulated alcohol dehydrogenase family, alcohol O-acetyltransferase, acetyl-CoA C-acetyltransferase, acyl-coenzyme A thioester hydrolase, and down-regulated aldehyde dehydrogenase family were involved in glycolysis/gluconeogenesis pathways, starch and sucrose metabolism pathways, amino sugar and nucleotide sugar metabolism pathways, tricarboxylic acid (TCA) cycle, and pyruvate metabolism pathways, thus promoting the formation of ethyl acetate, organic acids, alcohols, and other esters. Our results revealed the formation mechanisms of ethyl acetate and organic acids in rice-acid soup inoculated with K. marxianus L1-1.
1. Introduction
Acid rice soup (rice-acid soup) is a traditional fermentation food in Guizhou, China and widely consumed as seasonings. The rice-acid soup fermentation process has two steps. First, the mixture of rice and water is fermented at room temperature for 30 days to obtain the‘primary rice-acid soup’. Second, the obtained primary rice-acid soup is subjected to additional fermentation at 28–35 °C for 4–7 days and then the final product, rice-acid soup, is obtained. The fermentation process is approximately 40 days and its flavor is unstable due to uncontrolled changes in the microbial community. In our previous studies [1,2], the rice-acid soup fermentation method withKluyveromyces marxianusL1-1 (K. marxianusL1-1) could reduce fermentation time from 40 days to 4 days. Importantly, rice-acid soup inoculated withK. marxianusL1-1 had a unique flavor. However,the formation mechanism of flavor in rice-acid soup inoculated withK. marxianusL1-1 is not clear.
Recently, more chemical and biotechnological applications of non-saccharomyces have been applied in the food field because these yeasts show advantageous metabolism features in the production of flavor components of interest [3].K. marxianusas a non-saccharomyces yeast, has various advantages over mesophilic yeasts, such as fast growth rate, wide spectrum of substrates, and reduced cooling cost [4]. It is a haploid, homothallic, thermotolerant,and hemiascomycetous yeast and closely related toKluyveromyces lactis[5].Notably,K. marxianusgrows in a wide temperature ranging from 4 to 52 °C and the high temperature can prevent the growth of microorganisms sensitive to heat [6,7]. UnlikeS. cerevisiae,K. marxianuscan assimilate lactose, glucose and xylose at high temperatures [6]. Furthermore, the probiotic properties ofK. marxianushave been extensively explored [8].
Interestingly,K. marxianusshows a great potential in the production of esters, which are key aromatic compounds in the food industry [9]. Ethyl acetate and other short-chain volatile esters are used as industrial solvents and perfume ingredients. It was reported that the global market demand of ethyl acetate was more than 1.7 million tons per year [10]. Therefore, the production of ethyl acetate is significant in the food production industry. Three synthesis ways of ethyl acetate have been reported [11]: hemiacetal oxidation(spontaneous formation of hemiacetal from acetaldehyde and ethanol under the action of enzymatic oxidization), condensation of ethanol and acetyl-CoA, and esterification of acetate and ethanol. However,it is difficult to produce ethyl acetate through esterification of ethanol and acetate because the ester-hydrolyzing activity of esterase is much higher than its ester-synthesizing activity [12]. The previous report demonstrated that the synthesis of ethyl acetate was characterized by the direct utilization of ethanol as a substrate or the hemiacetal reaction between sugar and acetaldehyde [12]. Ethyl acetate as an aroma component plays an increasingly important role in foods and other fields. However, the synthesis mechanism of ethyl acetate inK. marxianusin rice-acid soup is unknown. Most studies onK. marxianusfocused on its role in alcoholic fermentation in the fuel industry and food field [13], but the production of ethyl acetate or organic acids withK. marxianuswas seldom reported. The flavor accumulation metabolism ofK. marxianusis less understood than that ofS. cerevisiae. The formation mechanisms of ethyl acetate,other esters or flavor compounds in fermented foods inoculated withK. marxianusare not completely understood.
More and more multi-omics analysis technologies have been used to explore the synthesis, metabolism, and accumulation of nutrients and flavor components in foods. RNA sequencing is a novel highthroughput sequencing technology with many advantages, such as much information, low data redundancy and accurate analysis. It does not need a lot of the background of genomics, but it can analyze the transcriptional expression of new materials [14]. Proteomics can also enhance the understanding of the biochemical processes of flavor development in fermented foods [15]. However, due to non-coding RNA regulation, protein degradation, and protein secretion, the amount of differently expressed proteins (DEPs) are often less than that of differently expressed genes (DEGs). In addition, the detectable protein content, protease hydrolysis and operational errors in proteomics also limit the application of proteomic analysis [16]. Many factors lead to the difference in the analysis results of transcriptomics and proteomics. Notably, sequence features contributed to 15.2%–26.2% of total variations of mRNA and proteins [17]. Therefore, the combination of transcriptomics and proteomics may reveal the flavor formation mechanism inK. marxianusL1-1 in rice-acid soup.
In order to clarify the formation mechanism of ethyl acetate and organic acids in rice-acid soup inoculated withK. marxianusL1-1,volatile compounds and organic acids in rice-acid soup were analyzed in this study. In addition, we analyzed the differentially expressed genes and proteins ofK. marxianusL1-1 in rice-acid soup in the key fermentation period (from day 1 to day 3) through the complementary analysis of mRNA sequencing and proteomics. Based on gene ontology (GO) enrichment analysis and KEGG pathway enrichment analysis, the formation mechanisms of ethyl acetate and organic acids inK. marxianusL1-1 in rice-acid soup were explored.
2. Materials and methods
2.1 Strain culture and growth determination
K. marxianusL1-1 (CCTCC 2021073) isolated in traditional fermented rice-acid soup could produce lots of aroma compounds [2]and was used in rice-acid soup fermentation in this study. The strainK.marxianusL1-1 grew at 30 °C for 72 h in yeast peptone dextrose (YPD)agar. The culture was stored at 4 °C and subcultured bi-weekly in order to maintain its viability. For a long-term storage, stock cultures were maintained in 30% glycerol at −80 °C.
2.2 Preparation methods of fermented rice-acid soup
Rice-acid soup was fermented according to our previous study [18].Briefly, 8.0% selenium rice flour and 92.0% water was boiled under stirring conditions to obtain rice soup. After saccharification and liquefaction, the prepared raw materials were sterilized at 90 °C for 20 min. After rice soup was cooled to 30 °C,K. marxianusL1-1 was inoculated according to the concentration of 4.95 × 105CFU/mL. Then,the inoculated raw materials were poured into a sterilized rice-acid soup fermentation tank (Earthenware type, Sichuan, China) and sealed immediately. We analyzed and compared two rice-acid soup samples(Y 1 day and Y 3 day) inoculated withK. marxianusL1-1 respectively obtained in the early stage and late stage of the fermentation process(day 1 and day 3).
2.3 Determination of the counts of K. marxianus L1-1
Each sample (5 g) was mixed separately with 45 mL of 0.85%(m/V) NaCl solution and shaken at 150 r/min for 30 min at room temperature. Then, the supernatant was serially diluted by 105times with 0.85% (m/V) NaCl solution and spread on YPD agar.K. marxianusL1-1 were cultured at 30 °C for 72 h, and the number ofK. marxianusL1-1 was counted on day 0, day 1, day 2, day 3, and day 4, respectively.
2.4 Determination of the activities of key enzymes
Glucokinase 1 (GK-1),L-lactate dehydrogenase (L-LDH),acetyl-coenzyme A (ACA), and glucan 1,3-β-glucosidase (SCW4)were determined according to the manuals of the enzyme-linked immunoassay (ELISA) kit. Alcohol dehydrogenase (ADH) was determined according to the instructions of ADH (Solarbio) Kit.
2.5 Determination of volatile compounds
Through SPME-GC-MS analysis, volatile compounds were determined according to the previous methods [19,20]. Retention time and mass spectral data were used to identify each compound. The retention time of volatile compounds was determined with a C6–C26alkane standard based on our previous study [18]. The concentrations of volatile components were calculated with the peak areas of the internal standard (10 μL of 10 mg/L 2-methyl-3-heptanone). The mass spectra and retention indices were determined on at least two different GC columns with stationary phases of different polarities and then compared. The odor activity value (OAV) is defined as the quotient between the concentration of a volatile and its perception threshold [21].
2.6 Determination of organic acids
After the settlement, rice-acid soup samples inoculated withK. marxianusL1-1 were filtered with double-layer filter paper. Organic acids were determined according to our previous study [18].
2.7 Transcriptome sequencing and data analysis
Three independent biological replicates of transcriptome sequencing ofK. marxianusL1-1 were used in the samples of Y 1 day and Y 3 day. RNA extraction ofK. marxianusL1-1 cells, libraries construction, RNA-seq, and primary data analysis were carried out in PTM BIO Co. Ltd. (Hangzhou, China). Transcriptome sequencing and data analysis were performed according to our previous study [18].
2.8 Proteomic sequencing and data analysis
Similarly, three independent biological replicates of proteomic sequencing ofK. marxianusL1-1 were used in the samples of Y 1 day and Y 3 day. Proteomic sequencing and data analysis were performed according to our previous study [18].
2.9 Correlation analysis between transcriptomic and proteomic results
The DEGs and DEPs ofK. marxianusL1-1 were separately counted and the Venn diagrams were plotted according to the counted results. Correlation analysis (Pearson correlation coefficient) was performed according to our previous study [18].
2.10 Parallel reaction monitoring (PRM) analysis
Target analysis was performed through PRM. PRM analysis was performed according to previous methods [22,23]. The peptides were dissolved in the mobile phase A of liquid chromatography and then separated with the EASY-nLC 1000 ultra-high performance liquid system. Mobile phase A was an aqueous solution containing 0.1% formic acid and 2% acetonitrile and mobile phase B was an aqueous solution containing 0.1% formic acid and 90% acetonitrile.Gradient elution was programmed as follows: 0–40 min, 6%–25% B;40–52 min, 25%–35% B; 52–56 min, 35%–80% B; 56–60 min, 80%B. The flow rate was 500 NL/min. The peptides were separated by the ultra-high performance liquid system and injected into the NSI ion source for ionization and then analyzed by Q ExactiveTM Plus mass spectrometer.
2.11 Statistical analysis
All experiments were conducted in triplicate. Data were expressed as means ± standard deviation. Duncan’s multiple range test andt-test were carried out to analyze significant differences in SPSS version 20.0 (SPSS Inc., Chicago, IL, USA), whereP< 0.05 was considered to be statistically significant.
3. Results
3.1 Variations of the quantity and enzyme activity of K. marxianus L1-1 in the fermentation process of rice-acid soup
K. marxianus, which is able to utilize various sugars, may be a suitable microbe for lignocellulose hydrolysis and grain fermentation at 30 °C [24]. Similarly, in this study, the fermentation temperature was determined as 30 °C based on our previous study [25]. The quantity ofK. marxianusL1-1 varied significantly in the fermentation process. The quantity ofK. marxianusL1-1 gained the most significant increase rate from day 0 to day 1 (Fig. 1A) and decreased from day 1 to day 2. Interestingly, it increased from day 2 to day 3.The above variations might be related to the oxygen content in the fermentation tank. In addition, carbon dioxide gas was produced during the fermentation process and could be discharged in the form of bubbles to maintain good anaerobic conditions in the fermentation tank. After adding water to the fermentation tank and sealing the tank,the external microbial invasion was prevented. The limited supply of oxygen (the terminal electron acceptor) also initiated the synthesis of some esters, but it primarily forced ethanol production during the growth ofK. marxianusDSM 5422 [12]. We explored the formation of ethyl acetate, other esters and organic acids based on the growth ofK. marxianusL1-1 in this study. We investigated the key fermentation process of rice-acid soup in day 1 and day 3 and analyzed the formation mechanism of flavors inK. marxianusL1-1. The enzyme activities of GK-1, L-LDH and SCW4 were determined from day 0 to day 1 and from day 2 to day 3, displaying the increasing trend (Fig. 2).The enzyme activity of ADH decreased from day 0 to day 1 and the enzyme activity of acetyl-coenzyme A (ACA) decreased from day 2 to day 3. However, the enzyme activities of ADH and ACA on day 3 were higher than those in the initial fermentation stage.
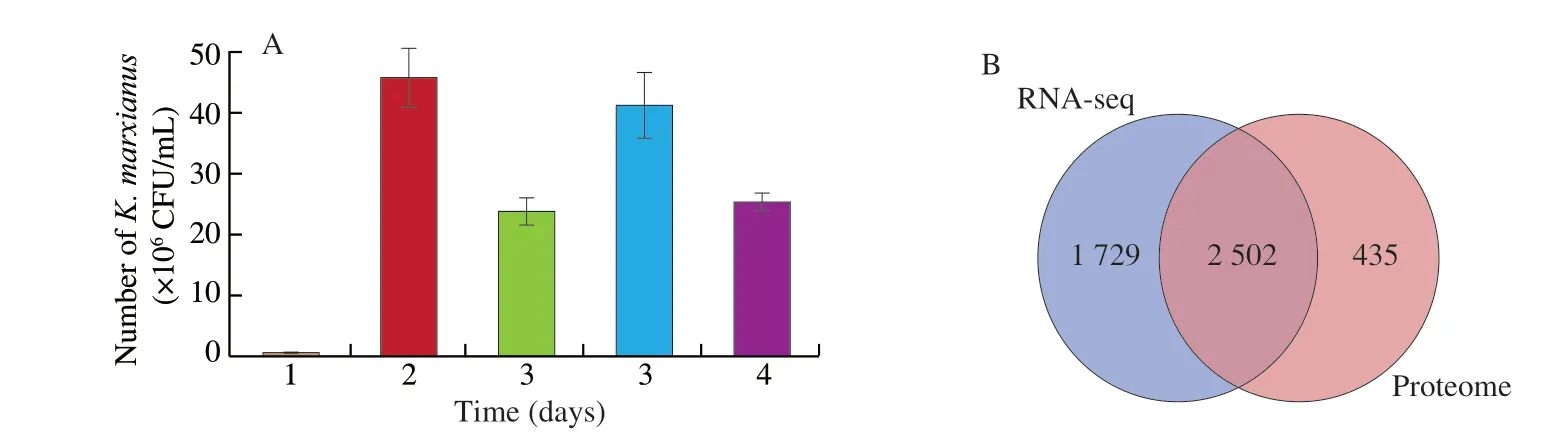
Fig. 1 (A) The number of K. marxianus L1-1during the rice-acid soup fermentation process. (B) Venn diagram of gene and protein in RNA-seq and proteome.(C) Venn diagram of up-RNA, down-RNA, up-protein and down-protein. (D) Scatter diagram of the correlation between the proteins and transcripts of K.marxianus L1-1 in 3 day and 1 day when rice-acid soup fermentation (Pearson correlation = 0.320 16, P <0.01).
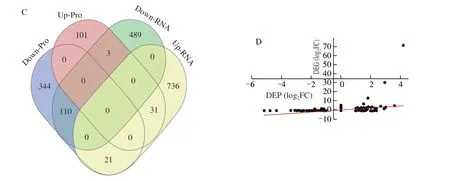
Fig. 1 (Continued)
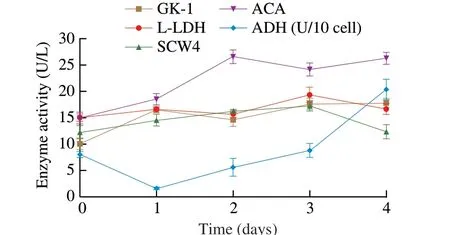
Fig. 2 The enzyme activity of (GK-1, L-LDH, ACA, SCW4 and ADH in K. marxianus L1-1.
3.2 Variations of key volatile compounds in the fermentation process of rice-acid soup
The variations of flavor compounds on day 1 and day 3 were explored. The formation of ethyl acetate required acetic acid,ethanol and some key enzymes, as discussed above. Based on the concentrations and thresholds of flavor compounds, 5 key acids, 13 key alcohols, and 12 key esters (Table 1) were determined as the key volatile compounds in the flavor accumulation. From day 1 to day 3,ethyl acetate concentration increased from (162.98 ± 5.02) mg/L to(241.37 ± 6.20) mg/L, which was higher than that in our previous study [18]. Ethanol concentration increased from (36.11 ± 4.54) mg/L to (52.68 ± 14.45) mg/L and acetic acid concentration increased from(0.21 ± 0.06) mg/L to (32.67 ± 1.57) mg/L. Acetic acid, 2-phenylethyl ester, 2-methyl-propanoic acid, ethyl ester, and 9 other esters were also found. Notably, ethyl acetate was the most important volatile compounds because of its high content and low odor threshold in the rice-acid soup fermentation, as confirmed in our previous study [26]. Ethyl acetate made a significant contribution to the formation of the fruity flavor and promoted the overall flavor balance in rice-acid soup. Moreover, ethyl acetate significantly increased the activities of glutathione reductase, glutathione peroxidase, glutathioneS-transferase, and glucose-6-phosphate dehydrogenase linked to the antioxidant function in fruits [27]. The formation of esters in the alcoholization stage was closely related to the enzyme activity of yeasts. Therefore, it is necessary to explore the formation mechanism of ethyl acetate and other esters.
3.3 Variations of organic acids in the fermentation process of rice-acid soup
Seven organic acids were found in rice-acid soup, includingL-lactic acid, acetic acid, malic acid, succinic acid, citric acid,oxalic acid, and tartaric acid (Table 1). Among 7 organic acids,L-lactic acid had the highest concentration. The concentration ofL-lactic acid increased from (3.01 ± 0.61) g/kg in day 1 to (6.02 ± 1.67) g/kg in day 3, and the concentration of malic acid increased from (0.77 ± 0.06) g/kg in day 1 to (1.14 ± 0.08) g/kg in day 3. The concentrations of the other 5 organic acids did not show the significant increase during the fermentation process. However, although the concentrations of the other 5 organic acids were low, they interacted with each other to promote the formation of the sourness and taste of riceacid soup [1]. In our study, both volatile components and organic acids affected the formation of the flavor of rice-acid soup. Lactic acid exists in two isomeric forms:L-(+) andD-(−)-lactic acid. It is produced by microbial fermentation and chemical synthesis and used in food, cosmetic, pharmaceutical, and chemical industries [28].In the study, we mainly focused on the increase inL-(+)-lactic acid caused by microbial fermentation.L-(+)-Lactic acid with a high enantiomeric purity is required in many industries, especially in medical, pharmaceutical and food industries, sinceD-(−)-lactic acid is harmful to humans and can cause decalcification or acidosis [29].L-(+)-Lactic acid not only promoted the formation of the flavor in rice-acid soup, but also had an important effect on health. We further explored the genes, proteins and enzymes associated with the formation of organic acids below.
3.4 Transcriptomic analysis of K. marxianus L1-1 during the key fermentation period of rice-acid soup
In the fermentation process of rice-acid soup, the expressions of a series of genes changed between Y 1 day and Y 3 day.The two independent cDNA libraries (Y 1 day and Y 3 day)constructed for high-throughput sequencing respectively yielded 19 617 191-27 627 279 pair-end reads and 5 885 157 300-8 288 183 700 clean reads after stringent quality check and data filtering (Q20 bases > 97.28%, Q30 bases > 92.01%, G + C 42.63%-43.03%) (Table S1). All of high-quality clean reads were used for gene comparison and more than 88% of total reads of clean reads were mapped to the database by software Bowtie 2.By using |log2FC| > 1.5 and FDR < 0.05, we compared the samples of Y 3 day with Y 1 day (Fig. S1A) and identified 1 390 DEGs(788 up-regulated and 602 down-regulated) inK. marxianusL1-1.
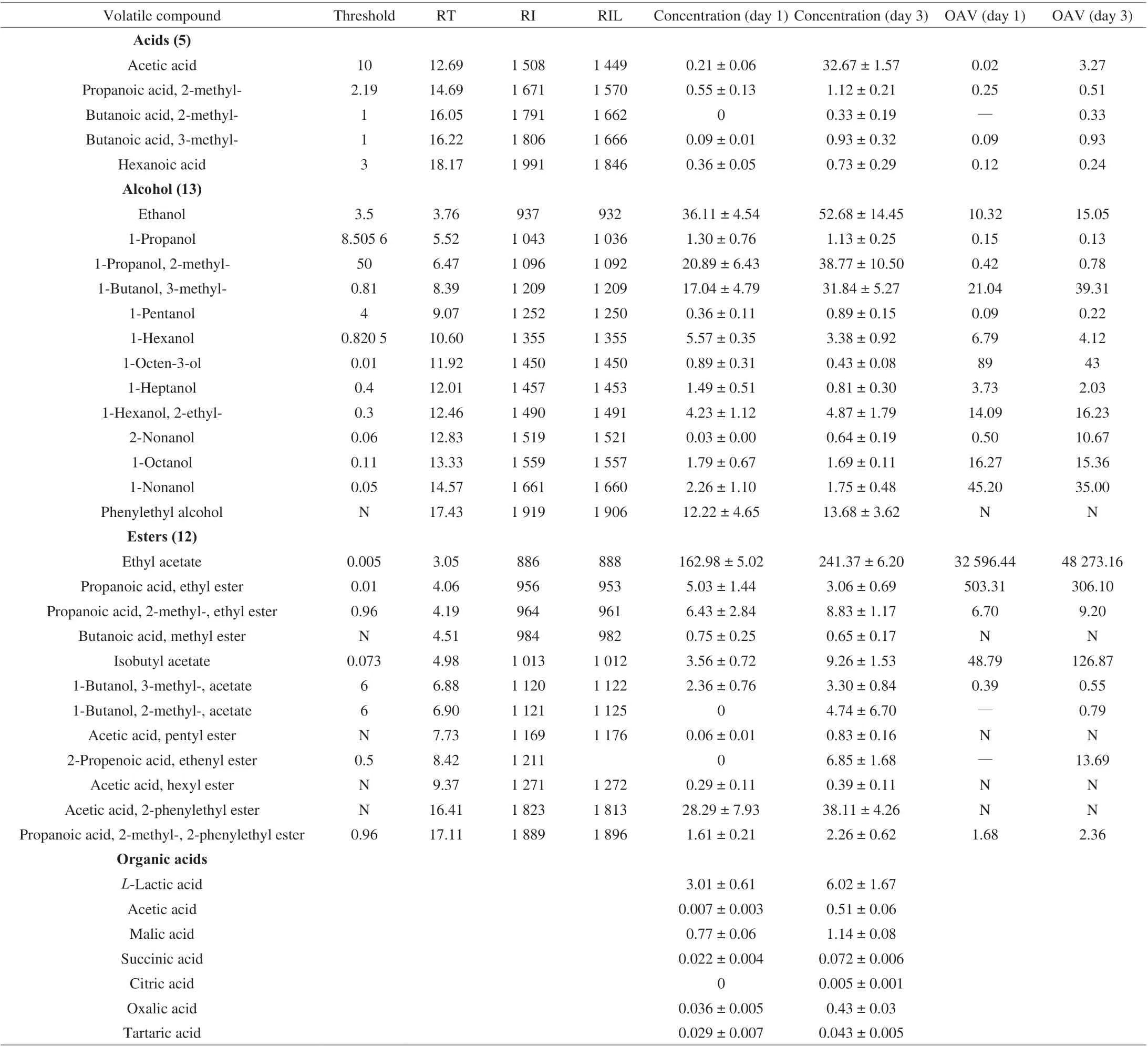
Table 1 The concentration (mg/L) and OAV of key volatile compounds and the concentration (g/kg) of organic acids in fermented rice-acid soup inoculated with K. marxianus L1-1 on day 1 and day 3.
GO analysis of the DEGs showed the enrichment of three major cellular components, biological processes, and molecular functions(Fig. S2). In terms of cellular components, most of the DEGs were enriched in nucleolus, small-subunit processome and preribosome,and large-subunit precursor. In terms of biological processes, most of the DEGs were enriched in endonucleolytic cleavage in ITS1 to separate SSU-rRNA from 5.8 S rRNA and LSU-rRNA from tricistronic rRNA transcript. In terms of molecular functions, these DEGs were enriched in structural constituent of ribosome, snoRNA binding and rRNA binding. All DEGs were subjected to KEGG pathway enrichment analysis. KEGG analysis assigned the DEGs of Y 1 day and Y 3 day to metabolic pathways. At least 4 231 genes were identified, including 1 390 DEGs annotated to 279 KEGG pathways (Table S2). The significantly enriched pathways (P< 0.05 andQ< 0.05) were ribosome, cytosolic DNA-sensing pathway, RNA polymerase, DNA replication, ribosome biogenesis in eukaryotes,pyrimidine metabolism, and purine metabolism. Importantly, the crucial KEGG pathways related to ethyl acetate and organic acids included amino sugar and nucleotide sugar metabolism, starch and sucrose metabolism, glycolysis/gluconeogenesis, pyruvate metabolism and tricarboxylic acid (TCA) cycle. These pathways showed the different roles ofK. marxianusL1-1 in the formation of flavor and taste of rice-acid soup.
3.5 Proteomics characterization
The total proteins ofK. marxianusL1-1 were extracted in the samples of Y 1 day and Y 3 day and subjected to 4D labelfree quantification (4D LFQ) proteomic analysis to complement the transcriptome analysis. According to the abundance levels of proteins, 610 proteins were identified as DEPs atP< 0.05, including 135 proteins with increased abundance levels and 475 proteins with decreased abundance levels (Fig. S1B). The number of up-regulated proteins was smaller than that of down-regulated proteins since the growth ofK. marxianusL1-1 was inhibited in the acidic environment in the later fermentation stage of rice-acid soup.
To obtain a global diagram of proteomic changes, at least 2 937 proteins were identified and 187 DEPs were annotated with GO analysis and KEGG analysis (Table S3). In GO functional analysis, 187 proteins were annotated to 59 GO terms. The results of GO analysis showed that the distributions of DEPs in functional classification were consistent with the distributions of transcription levels of DEGs (Fig. S3). In terms of cellular components, most of the up-regulated DEPs were enriched in mitochondrion, tricarboxylic acid cycle enzyme complex and mitochondrial matrix. In terms of the biological process, most of the up-regulated DEPs were enriched in citrate metabolic process, tricarboxylic acid metabolic process and galactose catabolic process. In terms of molecular functions,these up-regulated DEPs were enriched in oxidoreductase,L-malate dehydrogenase, malate dehydrogenase, and alcohol dehydrogenase.The 6 GO terms involved in alcohol dehydrogenase activity had the smallest (P< 0.01) and were related to the formation of ethyl acetate.The 187 DEPs were annotated to 30 KEGG pathways. Most of the up-regulated KEGG pathways obtained in the proteomic analysis were related to the formation of ethyl acetate and organic acids and explained reasonably the formation of flavor and taste in rice-acid soup inoculated withK. marxianusL1-1. In addition, the downregulated KEGG pathways obtained by the proteomic analysis were most related to the growth ofK. marxianusL1-1 (Fig. 3B), verifying that it was reasonable to select the third day as the ending of rice-acid soup fermentation. According to the above pathway analysis results(Fig. 3A), we could conclude that many proteins took part in various metabolism pathways, including metabolism pathways of amino sugar and nucleotide sugar, metabolism pathways of starch and sucrose,glycolysis/gluconeogenesis, metabolism pathways of pyruvate, and the citrate cycle. These metabolism pathways might largely affect the flavor formation metabolism ofK. marxianusL1-1 during the key fermentation period of rice-acid soup. However, the different KEGG pathways found in the proteomic analysis played different roles in the formation of flavor and taste in the rice-acid soup.
3.6 Correlation analysis of transcriptome and proteome data
Transcriptomic and proteomic analysis results are shown in Figs. 1B–D. At least 4 231 genes were identified and 1 390 DEGs were annotated to 279 KEGG pathways by using transcriptomic analysis. In addition, 2 937 proteins were identified and 610 DEPs were annotated to 30 KEGG pathways by using proteomic analysis.Pearson correlation coefficient was 0.376 1 and the results of the two analysis methods were significantly different. Therefore, the combination of transcriptome and proteome data could be an effective way to reveal the formation mechanism of the flavor in rice-acid soup inoculated withK. marxianusL1-1. The 5 KEGG pathways related to the synthesis of ethyl acetate and organic acids are provided in Table 2.

Fig. 3 The top 16 up-regulated KEGG pathways (A) and 14 down-regulated KEGG pathways (B) enriched scatter plot of DEPs in K. marxianus L1-1 which inoculated in rice-acid soup (Y 3 day vs Y 1 day).
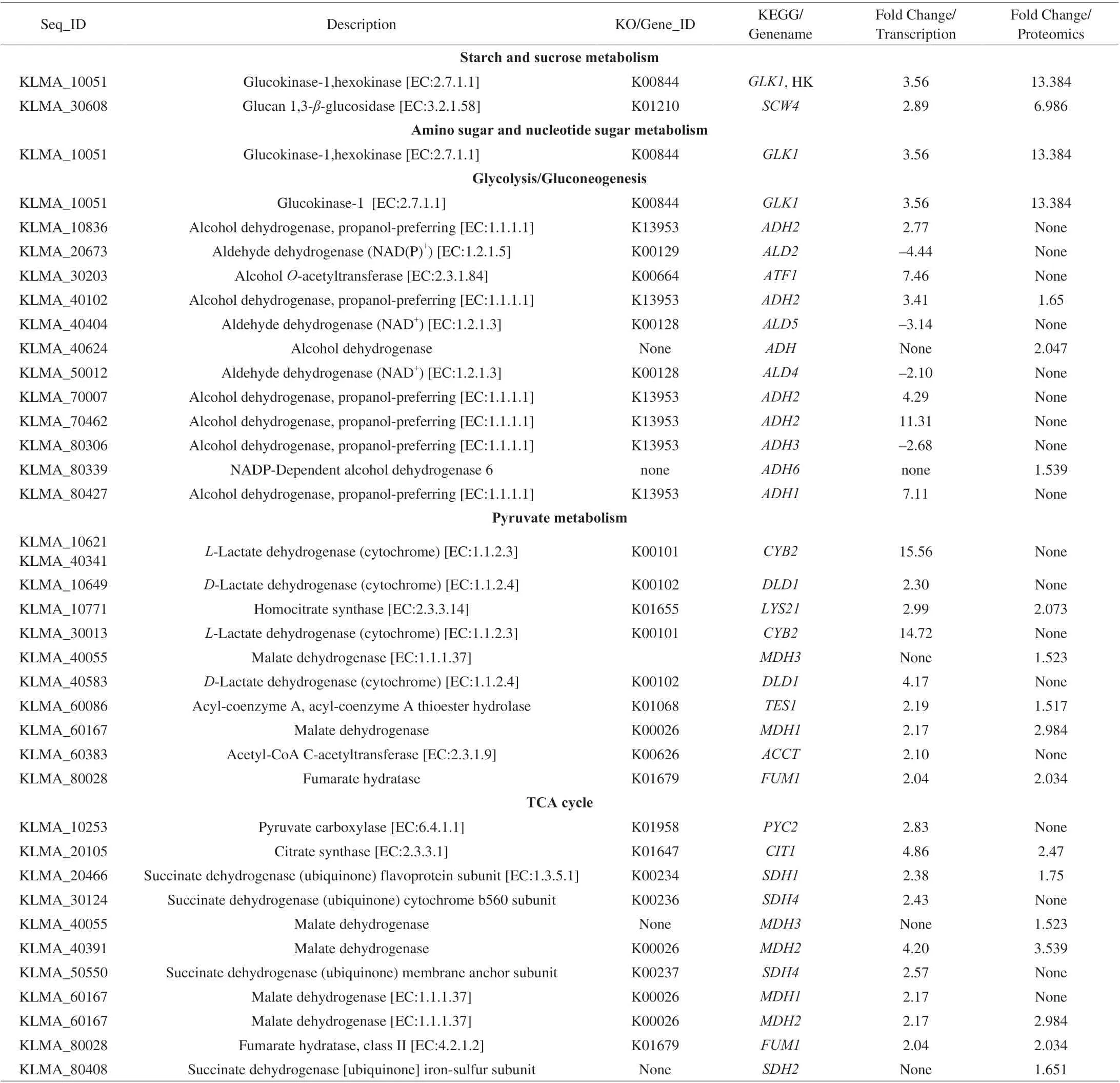
Table 2 The differentially expression genes and proteins (DEGs and DEPs) in key KEGG pathways of K. marxianus L1-1 when inoculated rice-acid soup (Y 3 day vs Y 1 day).
3.7 Comprehensive analysis of volatile flavor components,organic acids, and KEGG metabolic pathways
Starch and sucrose metabolism provides an important transient pool in the sugar accumulation pathways. The genes and proteins involved in the formation of the flavor and taste in rice-acid soup includedGLK1encoding glucokinase-1 and KLMA_10051 encoding hexokinase in the KEGG pathways (Table 2). Protein GLK1 is a glycolysis-initiating enzyme [30] and showed the 13.384-fold upregulation. The gene of KLMA_10051 encodes a hexokinase and showed the 3.56-fold upregulation. In addition,SCW4(KLMA_30608) encoding glucan 1,3-β-glucosidase showed the 6.986-fold upregulation in proteomic analysis results and the 2.89-fold upregulation in transcriptomic analysis results.
Glycolysis is the cytosolic pathway that converts glucose to pyruvate.ADH1,ADH2,ADH3(alcohol dehydrogenase) andADH6(NADP-dependent alcohol dehydrogenase 6) involved in glycolysis/gluconeogenesis respectively encode the proteins of BAO40648,BAO40126, and BAO42650 (Table 2 and Fig. 4A). In addition,we could infer that the formation of ethyl acetate in rice-acid soup fermentation inoculated withK. marxianusL1-1 was catalyzed by two enzymes named ATF1 (alcoholO-acetyltransferase) and TES1 (acyl-coenzyme A) (Table 2 and Fig. 4B), which possessed an acyl-coenzyme A: ethanolO-acyltransferase (AEATase) activity and the esterase activity. We found that ATF1 could use acetyl-CoA C-acetyltransferase (ACCT) to synthesize acetate esters,including ethyl acetate, acetic acid, 2-phenylethyl ester, and isobutyl acetate (Fig. 5). Furthermore, some genes and proteins related to the formation of ethyl acetate and other esters of rice-acid soup included GLK1 (glucokinase-1), KLMA_10051 (hexokinase), GPD2(glyceraldehyde 3-phosphate dehydrogenase) and ALD family(aldehyde dehydrogenase) (Table 2 and Fig. 5). However, we did not find a reverse esterase in the formation of ethyl acetate or other esters in our study (Fig. 4C).

Fig. 4 Ethyl acetate biosynthetic pathways and synthesis activities in K. marxianus L1-1 when inoculated rice-acid soup. (A) ADH oxidation of hemiacetal; (B) Ethyl acetate biosynthesis via ATF; (C) Reverse esterase activity.
Both TCA cycle and pyruvate metabolism played important roles in the formation of organic acids in rice-acid soup (Table 2 and Fig. 5). The up-regulated genePYC2(KLMA_10253)encoding pyruvate carboxylase played the crucial role in TCA cycle and pyruvate metabolism. The up-regulated geneLYS21(KLMA_10771) encoding homocitrate synthase was identified by the combined analysis of transcriptomics and proteomics in this study.The genesCYB2(KLMA_10621 and KLMA_40341) encodingL-lactate dehydrogenase were up-regulated, whereas anotherCYB2(KLMA_30013) was down-regulated.DLD1(KLMA_40583 and KLMA_10649) genes encodingD-lactate dehydrogenase were down-regulated in pyruvate metabolism and the down-regulation ofDLD1partially interpreted the increase inL-lactic acid during the fermentation process. The up-regulated genes and proteins includingMDH1(KLMA_60167),MDH2(KLMA_60167),MDH2(KLMA_40391), andMDH3(KLMA_40055) encoding malate dehydrogenase in pyruvate metabolism and TCA cycle were related to the increase in malic acid.FUM1(KLMA_80028) encoding fumarate hydratase in TCA cycle and pyruvate metabolism was upregulated and this upregulation reasonably explained the conversion from fumarate to malate.SDH1(KLMA_20466) encoding succinate dehydrogenase in TCA cycle was related to the increase in succinate.The up-regulated proteins SDH1 (KLMA_80408) was only detected in proteomics and the up-regulated genesSDH1(KLMA_30124 and KLMA_50550) were only detected in transcriptomics.
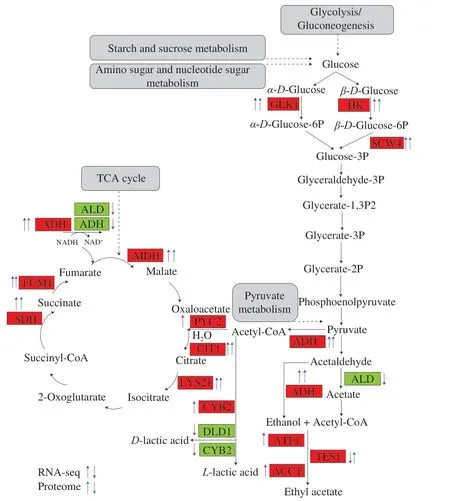
Fig. 5 The metabolic pathway of synthesis of ethyl acetate and organic acids in K. marxianus L1-1 when inoculated in rice-acid soup (Y 3 day vs Y 1 day).Red square means the up-regulate gene and protein, green square means the down-regulate gene and protein, purple up arrow means the up-regulate gene,purple down arrow means the down-regulate gene, blue up arrow means the up-regulate protein, blue down arrow means the down-regulate protein.
In our study, the down-regulated genes and proteins were involved in the KEGG pathways (Fig. 3B), including DNA replication,meiosis-yeast, homologous recombination, mismatch repair,autophagy-yeast, MAPK signaling pathway-yeast, phenylalanine metabolism, base excision repair, nucleotide excision repair, other types ofO-glycan biosynthesis, tryptophan metabolism, cell cycleyeast, penicillin and cephalosporin biosynthesis, andD-arginine andD-ornithine metabolism. Most of the down-regulated pathways were related to the growth ofK. marxianusL1-1.
3.8 PRM quantitative analysis to verify the key enzymes related to the formation of volatile flavor components and organic acids
Based on the joint analysis of DEGs and DEPs data corresponding toK. marxianusL1-1 transcriptome and proteome at day 3 and day 1 of rice-acid soup fermentation, 13 DEPs related to the formation of volatile flavor components and organic acids were selected. In the verification results (Fig. 6), GLK1 was the most significant upregulated protein and other key proteins included ADH family,MDH3, CYB2, SCW4 and CIT1. The PRM quantitative analysis verification results were basically consistent with the 4D LFQ quantitative results in the overall trend. Moreover, it verified the rationality of the analysis results of DEGs and DEPs on the KEGG pathway in this study.
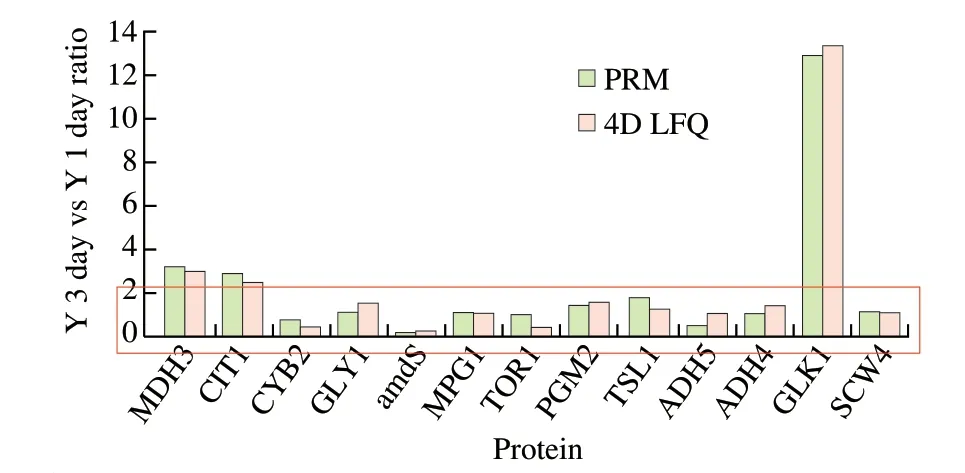
Fig. 6 PRM verification results of differentially expressed proteins.
4. Discussion
Rice-acid soup, as a cereal-based fermented food used for seasoning, is famous in China. However, the traditional rice-acid soup fermentation process requires two times of fermentation and the longterm fermentation time may lead to the unstable and non-persistent flavor. In this study, we adopted an inoculation strain (K. marxianusL1-1) isolated from original rice-acid soup. The inoculation ofK. marxianusL1-1 endowed the fermented rice-acid soup with a unique flavor and shortened the fermentation period of rice-acid soup from 40 days to 4 days [1,2]. Our previous study proved that this fermentation method could produce the high-quality flavor [2].However, the formation mechanism of the flavor in rice-acid soup inoculated withK. marxianusL1-1 is not clear. In our previous research, we did some measurements in the fermentation process of rice-acid soup inoculated withK. marxianusbased on the flavor, taste and physical and chemical and nutritional function indicators. We believed that day 1 and day 3 were respectively the key days of the fermentation early stage and the later stage. In this study, the RNAseq and 4D label-free technologies were used to explore the genes and proteins in the formation pathway of ethyl acetate and organic acids in rice-acid soup inoculated withK. marxianusL1-1. DEGs and DEPs inK. marxianusL1-1 were identified and annotated to key KEGG metabolism pathways, including metabolism pathways of starch and sucrose metabolism, metabolism pathways of amino sugar and nucleotide sugar, glycolysis/gluconeogenesis, metabolism pathways of pyruvate, and TCA cycle. The key metabolism pathways in this study were different from our previous study [18]. When riceacid soup inoculated with two strains (L. paracaseiH4-11 andK.marxianusL1-1), the KEGG pathways involving the up-regulated genes and proteins inK. marxianusL1-1 mainly included glycolysis/gluconeogenesis, TCA cycle, pyruvate metabolism. In a word, the differentially expressed genes and proteins were different in the two studies. Furthermore, the results of transcriptome and proteome analyses were combined to reveal the formation mechanism of ethyl acetate and organic acids. We provided a comprehensive interpretation and exact measurements of genes and proteins involved in the formation of the flavor of rice-acid soup inoculated withK. marxianusL1-1 for the first time.
4.1 Up-regulated proteins and genes involved in starch and sucrose metabolism and amino sugar and nucleotide sugar metabolism provide the energy for the formation of acids,alcohol and esters in rice-acid soup
Up-regulated GLK1 indicated an increase in NADPH amount and more energy generated inK. marxianusL1-1. Lane et al. [31] also reported that the catabolite repression could be reduced by modulating the expression of glucose-phosphorylating enzymes, such as GLK1 and hexokinase (HK).SCW4(KLMA_30608) encoding glucan 1,3-β-glucosidase might have the hydrolytic activity and provide the energy for the formation of the flavor and taste in rice-acid soup. It was also indicated that a 1,3-β-glucosidase, BGL1, purified from pilei, showed the hydrolytic activity toward laminarin and laminarioligosaccharides including laminaribiose andp-nitrophenylβ-D-glucopyranoside (pNPG) [32]. In addition, another study also reported that 1,3-β-glucosidase had a certain hydrolytic activity towards gentiobiose, cellobiose, and related polysaccharides [33].Therefore, the hydrolysis of sugar compounds might provide substrates and energy for orderly metabolism. Eventually, with hydrolytic enzymes, carbohydrates were easily converted into glucose inK. marxianusL1-1. Our fermentation method belonged to a static fermentation method.K. marxianusseemed to enhance glucose metabolism and shift to fermentation, implying the correlation between oxygen and glucose-sensing pathways [24]. In the future study, we will explore the content of oxygen in the fermenter and its correlation with the flavor in rice-acid soup. However, genes and proteins were differentially expressed in the early fermentation stage and late fermentation stage (the first and third days) of rice-acid soup with the inoculation ofK. marxianusL1-1. The correlation between transcriptome and proteome data was not perfect and the results of the two methods showed some differences [14]. Protein expressions may be affected by various factors in the translational stage [17].The efficiency of protein biosynthesis and accumulation depends on various factors in the biological regulation process. Therefore, we adopted the complementary analysis method of transcriptomics and proteomics to reveal the formation mechanisms of ethyl acetate and organic acids in Chinese rice-acid soup inoculated withK. marxianusL1-1.
4.2 Up-regulated proteins and genes involved in glycolysis/gluconeogenesis and pyruvate metabolism played an important role in the formation of ethyl acetate and other esters of riceacid soup
Volatile esters are secondary metabolites produced by yeasts and fungi during fermentation of fermented foods [34]. Interestingly,compared to alcohol, more esters existed in rice-acid soup inoculated withK. marxianusL1-1. This difference contributed to the flavor formation in rice-acid soup. Ethyl acetate was one of most important volatile esters in rice-acid soup. Both DEGsand DEPs in glycolysis/gluconeogenesis and pyruvate metabolism played an important role in the formation of ethyl acetate and other esters in rice-acid soup(Fig. 5). This type of esterification was carried out with primary and secondary alcohols, aldehydes or ketones [35]. ADH enzymes catalyze the synthesis of ethyl acetate through the oxidation of hemiacetal. ADH3 was only found in the transcriptomic analysis in this study. ADH2 was constitutively expressed in aerobic growth with glucose as a carbon source, whereas ADH3 expression increased as cells entered the stationary phase. These results were in agreement with the previous analysis ofK. marxianus[36]. Our study proved that the ADH family was important in the complementary analysis of transcriptome and proteome. ADH family had a significant effect on the formation of ethyl acetate and ethanol and the up-regulated genes and proteins suggested that ADH1, ADH2, and ADH6 were the dominant enzymes in ethanol production when glucose was used as a carbon source. In the identified up-regulated genes and proteins,ADH2 and ADH6 were critical in the reduction of acetaldehyde to ethanol (a precursor to ethyl acetate). This result was consistent with the previous study [36]. Another report proved that the alcohol acetyltransferase was associated with intracellular lipid particles in cytosol [37]. We analyzed the up-regulated genes and proteins of ADH family and found that they promoted the formation of ethanol and ethyl acetate. The PRM quantitative analysis results verified that the ADH family was the key protein in the formation of ethanol and ethyl acetate. Herein, one possible formation pathway was the oxidation of hemiacetal (the spontaneous product of ethanol and acetaldehyde) under the catalysis action of ADH. Although the two methods of transcriptomics and proteomics showed some differences,all the up-regulated ADH genes and proteins indicated that the alternative biosynthetic routes of ethyl acetate existed inK. marxianusL1-1 and promoted the formation of ethyl acetate in rice-acid soup.
The previous studies on theATF1p[38,39] also found that acetyl-CoA was used to synthesize acetate esters. It was demonstrated that acetyl-CoA played a crucial role in the generation of more NADH and more ATP in glucose metablism [24]. The previous study postulated that ester biosynthesis inK. marxianusmight also occur through homologs to the medium-chain acyltransferases fromS. cerevisiae:the isoamyl acetate-hydrolyzing esterase, theN-acetyltransferase Sli1 and/or the alcohol-O-acetyltransferase [40]. Interestingly, we also found that the another important ester family including propanoic acid, 2-methyl-propanoic acid, ethyl ester, 2-propenoic acid, ethenyl ester and propanoic acid, 2-methyl-2-phenylethyl ester, and propanoic acid, ethyl ester was closely related to propanoate metabolism. The propanoate is expected to be converted to acetyl-CoA or pyruvate, as suggested by the analysis results of propanoate metabolism. It was also demonstrated that propanoate was converted to acetyl-CoA in three classes of mycolate [41]. However, the differentially expressed geneATF1was only found in the transcriptomics analysis. The combination method of transcriptome and proteome could provide the more reasonable explanation for the formation of ethyl acetate,propanoic acid, and ethyl ester.
Protein GLK1 is a glycolysis-initiating enzyme, which promotes the formation of ethyl acetate and other esters and also plays an important role in glycolysis/gluconeogenesis and pyruvate metabolism. Glyceraldehydes-3-P and pyruvate are the intermediate products in the glycolysis process and provide the carbon skeleton for volatile compound biosynthesis in rice-acid soup. A recent transcriptomic study suggested that aβ-glucosidase homolog inK. marxianusmight be responsible for cellobiose degradation [24].Interestingly, the genes ofALDfamily (aldehyde dehydrogenase)were down regulated, indicating that more energy was used inADHfamily encoding alcohol dehydrogenase. In the further study,we will explore the change of aldehyde dehydrogenase. Some different genes were involved in the formation of ethyl acetate inK. marxianusandS. cerevisiae, although the metabolism of ethyl acetate inK. marxianuswas seldom reported. The previous study suggested that the biosynthesis of acetate ester could be interpreted with the antagonistic activity of esterase IAH1 [42].Similarly, a previous study also reported that there was no esterase involved in the biosynthesis ethyl acetate or other esters inK. marxianusCBS 6556 [36].
4.3 Up-regulated genes and proteins involved in TCA cycle and pyruvate metabolism played important roles in the formation of organic acids of rice-acid soup
TCA cycle is crucial in mitochondrial membranes for respiration and can supply electrons during oxidative phosphorylation within the inner mitochondrial membrane. It was also reported that organic acids were closely related to TCA cycle in rice [43]. It was demonstrated that pyruvate carboxylase as an anaplerotic enzyme had a special effect and played an essential role in various cellular metabolic pathways including gluconeogenesis, glucose-induced insulin secretion, de novo fatty acid synthesis, and amino acid synthesis [44].Different from our previous results [18], the DEGs and DEPs related to TCA cycle and pyruvate metabolism in this study might interpret the formation mechanism of organic acids during the key fermentation period of rice-acid soup inoculated withK. marxianusL1-1. CIT1 was a key proteinin the PRM quantitative analysis and verified our result, Other related proteins including LYS21, CIT1, FUM1, and SDH, might also promote the formation of organic acids.
The previous study proved that homocitrate synthase was responsible for the first important step of the pathway and played the crucial role in pyruvate metabolism [45]. Notably, homocitrate synthase LYS21 was linked to the key process of DNA damage repair in a nucleus and TCA cycle in the cytoplasm [24,46]. The up-regulated geneCIT1(KLMA_20105) encoding citrate synthase reasonably interpreted the increase in citric acid in the fermentation process of rice-acid soup. CIT1 acts as a quantitative marker for healthy mitochondrion and is encoded by the nuclear DNA [47].Interestingly, lactic acid has optical isomers:L-lactic acid andD-lactic acid, which can be produced by chemical synthesis (DLlactic acid) or microbial fermentation (L-lactic acid,D-lactic acid, orDL-lactic acid). Compared to chemical synthesis processes, microbial fermentation processes present more advantages since they make use of renewable substrates from lactic acid bacteria [48]. Consistently,our study demonstrated that fermented rice-acid soup inoculated withK. marxianusL1-1 could produce moreL-lactic acid on day 3 than day 1. Furthermore, it was reported that fumarate hydratase (FUM1)and succinate dehydrogenase (SDH) were tumour suppressors [49],indicating thatK. marxianusL1-1 could have potential probiotic characteristics. Therefore, the enhanced activities of proteins and enzymes in TCA cycle and pyruvate metabolism might have an important effect on the formation of organic acids in rice-acid soup inoculated withK. marxianusL1-1, which will be further explored in the future.
4.4 Down-regulated proteins and genes indicated the stable formation of the flavor in rice-acid soup
Interestingly, most of the genes and proteins which were related to the formation of flavor and involved in the 5 KEGG pathways,including starch and sucrose metabolism, amino sugar and nucleotide sugar metabolism, glycolysis/gluconeogenesis, pyruvate metabolism,and TCA cycle, were up-regulated except the genesCTS1,ALDfamily andDLD1. The up-regulated genes and proteins played an active role in the formation of flavor during rice-acid soup fermentation,whereas the down-regulated genes and proteins played an important role in maintaining the stable key flavor. Many reports focused on the up-regulated genes or proteins in the role of promoting the maturity of the flavor in fermented foods, but the down-regulated genes or proteins were seldom reported. In addition, most of the downregulated pathways were related to the growth ofK. marxianusL1-1.This result provided a reasonable explanation of the decrease in the quantity ofK. marxianusL1-1 in rice-acid soup during the later stage of fermentation period (day 3). The down-regulated proteins in DNA replication had the small log2FC value (data were not displayed),indicating that related DEPs had small effects on the growth ofK. marxianusL1-1. Therefore, the third day was the proper end of rice-acid soup fermentation. The down-regulated KEGG pathways were related to the decomposition and utilization of substrates byK. marxianusL1-1. Consistently, a previous report demonstrated that down-regulated proteins related to advanced glycation end products were implicated in the aging process [50]. In the future, we will focus on the influences of the contents of substrates ofK. marxianusL1-1 on the formation of the flavor in rice-acid soup.
5. Conclusion
The transcriptome and proteome ofK. marxianusL1-1 in rice-acid soup were determined in the study. The differentially expressed genes and proteins related to the formation of ethyl acetate and organic acids were determined. DEGs and DEPs were identified and found to be enriched in the key KEGG metabolism pathways, including metabolism pathways of starch and sucrose, metabolism pathways of amino sugar and nucleotide sugar, glycolysis/gluconeogenesis,metabolism pathways of pyruvate, and TCA cycle. Through the complementary analysis of the transcriptome and proteome, we revealed the possible formation mechanisms of ethyl acetate and organic acids in Chinese rice-acid soup inoculated withK. marxianusL1-1. This study provides the basis for improving aroma and taste of fermented foods and revealing the flavor formation mechanisms.
Declaration of competing interest
The authors declare that they have no competing financial interests.
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (32060530), Guizhou University, Gui Da TeGang He Zi (2022) 39, Technology platform and talent team plan of Guizhou. China ((2018)5251), Graduate Research Fund Project of Guizhou (YJSCXJH(2019]028). Industry-University-Research Cooperation Project of Guizhou (701/700465172217) and China Scholarship Council (201906670006).
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://doi.org/10.1016/j.fshw.2022.07.017.
- 食品科学与人类健康(英文)的其它文章
- Wine, beer and Chinese Baijiu in relation to cardiovascular health:the impact of moderate drinking
- Comparative analysis of physicochemical properties, ginsenosides content and α-amylase inhibitory effects in white ginseng and red ginsen
- Monitoring and identif ication of spoilage-related microorganisms in braised chicken with modif ied atmosphere packaging during refrigerated storage
- Effect of cooking processes on tilapia aroma and potential umami perception
- Volatile prof ile and multivariant analysis of Sanhuang chicken breast in combination with Chinese 5-spice blend and garam masala
- Physicochemical, rheological and antioxidant prof iling of yogurt prepared from non-enzymatically and enzymatically hydrolyzed potato powder under refrigeration

