Correlation between dominant bacterial community and non-volatile organic compounds during the fermentation of shrimp sauces
Ruichng Go*, Huijie Liu Ying Li Hongying Liu, Yue Zhou Li Yun*
a School of Food and Biological Engineering, Jiangsu University, Zhenjiang 212013, China
b Ocean College of Hebei Agriculture University, Qinghuangdao 066000, China
Keywords:Shrimp sauces Non-volatile compounds Bacterial community Electronic tongue Correlation analysis
A B S T R A C T Shrimp sauce, one of the traditional salt-fermented food in China, has a unique f alvor that is inf ulenced by the resident microf olra. The quality of salt-fermented shrimp sauce was evaluated in this work by determining the total volatile basic nitrogen (TVB-N), the amino acid nitrogen (AAN), organic acid, 5′-nucleotide and free amino acids (FAA). Moreover, the dynamics of microbial diversity during processing was investigated by using high-throughput sequencing technology.The results showed that the AAN, TVB-N, organic acid, 5′-nucleotide and FAA content were in range of 0.93-1.42 g/100 mL, 49.91-236.27 mg/100 mL, 6.65-20.68 mg/mL, 3.51-6.56 mg/mL and 81.27-102.90 mg/mL.Among the microbial diversity found in the shrimp sauce, Tetragenococcus, Flavobacterium, Polaribacter,Haematospirillum and Staphylococcus were the predominant genera. Correlation analysis indicated that the bacteria Tetragenococcus and Staphylococcus were important in the formation of non-volatile compounds.Tetragenococcus positively correlated with a variety of FAAs; Staphylococcus positively correlated with 5′-nucleotides. The analysis indicated that Tetragenococcus and Staphylococcus were the core genera affecting non-volatile components. These f indings indicate the dynamics of the bacterial community and non-volatile components inter-relationships during shrimp sauce fermentation and provide a theoretical basis for improving the fermentation process of shrimp sauce.
1. Introduction
Shrimp sauce is one of the traditional fermented food ingredients used in Chinese and Southeast Asian cuisines. And shrimp sauce has a long history in China because of its unique f lavor and high nutritional value [1].Acetes chinensisis the main processing raw material of shrimp sauces. They grow rapidly, have a short life cycle, and are found only in the Bohai Sea, the Yellow Sea of China and North Korea.A. chinensishas high nutritional value and a unique flavor,and it is rich in protein, minerals and chitin [2]. Shrimp fermentation involves the conversion of organic substances into simpler compounds such as peptides, amino acids and other nitrogenous compounds either by the action of microorganisms or endogenous enzymes [3]. And non-volatiles water-soluble compounds of low molecular weight in shrimp sauce may be taste contributions which can be classif ied into nitrogenous (FAA, nucleotides, organic bases and related compounds,etc.) and non-nitrogenous compounds (sugar, organic acids, and inorganic ions) [4].
The raw materials and the native microorganisms are the most essential factors affecting the quality of shrimp paste. Spontaneous fermentation is one of the most ancient processes used to preserve food products, improve taste and f lavor, and enhance the nutritional profile. Shrimp sauce is generally fermented naturally since sterilization is seldom performed during production [5]. Spontaneous fermentation with unsterilized raw materials is intractable [6].Microbial ingredient types can be inf luenced by the raw materials [7].Meanwhile, the microbial structure and diversity of shrimp sauces are easily affected by the production methods adopted by different manufacturers, such as the fermentation process parameters,fermentation period, raw material sources, and technical skills and expertise. Differences in the natural climatic conditions and microbial activities combined with ingredient types may be responsible for their diversity of nutritional, organoleptic and quality in the final fermentation product [2]. Thus, the correlation between foods and their microbiota profiles is important for improving the repeatability of product quality. Commercial development of fermented products requires more effective information on the core microorganisms and their contributions in flavor formation to provide desirable products. A variety of microorganisms present in fermentation processes and contribute to complex biochemical changes, resulting in a great diversity of product properties. The production of aroma compounds and some chemical characteristics (such as organic acids and amino acids) were highly affected by the metabolic activity of microbes. At present, the high-throughput sequencing (HTS)technology has been extensively used to investigate the composition of the microbial community of fermented, such as shrimp paste [5],dry-cured ham [8], and kimchi [7]. The technology combines genome sequencing and bioinformatics analysis to provide bacterial information in samples without the need for the culture process.The various indigenous microbiota may establish competitive and/or cooperative microbial relationships during initial fermentation with the outgrowth of several dominant microbes during late fermentation [9]. Furthermore, it has been found that halophilic bacteria are considered to be the dominant microorganisms during shrimp sauce fermentation, such asStaphylococcus,Tetragenococcus,BacillusandAerococcus[10].
The main objectives of this study were to investigate non-volatile taste active compounds and bacterial community of shrimp sauce,and to establish the correlation between bacterial diversity and nonvolatile taste active compounds. High-throughput sequencing could be used to manifest dynamic changes in the core microorganisms and the structure of the bacterial community of the shrimp sauce. The nonvolatile taste active compounds including total volatile basic nitrogen(TVB-N), amino acid nitrogen (AAN), organic acids, free amino acids(FAAs) and 5′-nucleotides. This study will be helpful to elucidate the underlying mechanism during the fermentation process of shrimp sauces and provide new insights for further studies on non-volatile taste active compounds formation in this product. Furthermore, it will provide a foundation for the establishment of new technology for product quality control and be beneficial to improve the industrial production of safe and high-quality shrimp sauces products.
2. Materials and methods
2.1 Preparation of shrimp sauces
Shrimp sauces were collected from a traditional factory in Cangzhou, Hebei Province, China. The final salinity of the shrimp sauces collected from the factory in November 2019 was 6%. The shrimp sauces were placed into the pots which were sealed by triplelayer gauze and stored for the exact time for fermentation. The samples were randomly withdrawn from a fermentation vat at specific fermentation periods. Then, the shrimp sauces were transferred to the laboratory. All the shrimp sauce samples were stored at -80 °C until further analysis.
2.2 Illumina MiSeq sequencing analysis
Genomic DNA from the shrimp sauces was extracted using the HiPure Soil DNA Kits (Mage, Guangzhou, China) according to the manufacturer’s protocols. Polymerase chain reaction (PCR) was carried out with primers 341F (5′-CCTACGGNGGCWGCAG-3′)and 805R (5′-GGACTACHVGGGTATCTAAT-3′), targeting the V3 to V4 regions of the bacterial 16S rRNA genes. Amplification was conducted under the following conditions: 94 °C for 2 min, followed by 30 cycles at 98 °C for 10 s, 62 °C for 30 s (except for 16S V4:55 °C for 30 s), and 68 °C for 30 s and a final extension at 68 °C for 5 min. Following PCR, the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, U.S.) was used for the purification of the products. ABI Step One Plus Real-Time PCR System (Life Technologies, Foster City, USA) was used for quantifying the products. Purified amplicons were pooled in equimolar and pairedend sequenced (PE250) on an Illumina platform according to the standard protocols. The raw reads were deposited into the NCBI Sequence Read Archive (SRA) database.
2.3 Determination of TVB-N and AAN
TVB-N is one of the potential indicators to evaluate the fermented food quality and safety. TVB-N was measured by the micro diffusion method [11]. Twenty grams of shrimp sauces were homogenized in 100 mL of distilled water at 10 000 r/min for 30 s. The homogenate equilibrated for 30 min then filtered.One microliter boric acid solution (20 g/L) and one drop of the mixed indicator were added to the central chamber of a dish.Then, a diluted sample (1 mL) and saturated potassium carbonate solution (1 mL, 1 g/mL) were added into the dish. The mixed indicator was titrated with a 0.01 mol/L hydrochloric acid standard titration solution. The TVB-N was determined by the amount of hydrochloric acid consumed and expressed in g/100 g.
AAN content was determined by the titration method [2]. A diluted sample (10 mL) was mixed with 60 mL distilled water and titrated to pH 8.2 with 0.05 mol/L NaOH. Next, adding 10 mL formaldehyde, the mixture was titrated to pH 9.2 with 0.05 mol/L NaOH.
2.4 Determination of organic acids
Five grams of shrimp sauces was homogenized with 25 mL distilled water for 2 min and centrifuged at 10 000 r/min for 15 min. The water extract of shrimp sauces (5 mL) was mixed with 2 mL of zinc sulphate (300 g/L) and 2 mL of potassium ferrocyanide(106 g/L) diluted to 100 mL with distilled water and then centrifuged at 10 000 r/min for 10 min [12]. The supernatant was filtered through 0.22 μm filters before HPLC analysis. Ten microliters of the filtrate were injected into the high-performance liquid chromatograph(HPLC; LC-20A; Shimazu, Japan). The detection parameters were as follows: chromatographic column, Agilent Zorbax SB-Aq (4.6 mm ×250 mm); column temperature, 35 °C; detector wavelength, 210 nm;and mobile phase, methanol (A) and 0.1% phosphoric acid (B), flow rate: 1 mL/min.
2.5 Determination of FAAs
The analysis of FAAs was detected with an amino acid analyzer(Sykam, Germany). One gram of sample was homogenized at 10 000 r/min for 2 min in 9 mL of 1% sulfosalicylic acid (m/V) and centrifuged at 10 000 r/min for 15 min at 4 °C. Then, the supernatant neutralized (pH 2.2) with 2 mol/L HCl. Finally, the sample was determined by the amino acid analyzer.
2.6 Determination of nucleotide
Based on the method of previous research with a slight modification [13], the samples (5 g) were homogenized with 20 mL 10% (V/V) cold perchloric acid (4 °C) at 10 000 r/min for 2 min and centrifuged at 10 000 r/min for 15 min for 4 °C, and the strep repeated. The two supernatants were combined and neutralized(pH 5.8) with 1 mol/L KOH. Then, the mixtures were filtered using 0.22 μm filters before HPLC analysis. The HPLC and the column were the same for organic acids. The detection parameters were as follows:column temperature, 35 °C; detector wavelength, 254 nm; and mobile phase, methanol (A) and 0.1% phosphoric acid (B), flow rate: 1 mL/min.
2.7 Electronic tongue analysis
E-tongue analysis was performed according to a previous method with some modifications. This system was composed of five taste sensors: CA0 (to detect sour substances), C00 (to detect bitter substances), AE1 (to detect astringent substances), AAE (to detect umami substances), and CT0 (to detect salty substances). According to the material-liquid ratio of 1:4 (m/V), shrimp sauces (30.00 g) were mixed with 120 mL deionized water in a blender for 1 min. After centrifugation at 10 000 r/min for 10 min at 4 °C, the supernatant was filtered through filter paper and subsequently analyzed. Before analysis, sensors were calibrated and diagnosed. The sensors were washed in a cleaning solution (30 mmol/L KCl + 0.3 mmol/L tartaric acid, 120 s + 120 s). The sensors were dipped in the sample solution for 30 s. Each sample was repeated 4 times, and 3 stable sets of data were retained, and the average value was taken as the result of the test.
2.8 Statistics analysis
All measurements were done in triplicate. Statistics analysis of all results was implemented using SPSS Statistics 21 (SPSS Inc.,Chicago, IL, USA). Values were presented as the mean and standard deviation (SD). The statistical difference was determined by Analysis of variance (ANOVA). The significance level was defined atP< 0.05.In addition, figures were painted with Origin 2018 software (OriginLab Co., Northampton, MA, USA).
3. Results and discussion
3.1 Bacterial diversity changes
The Miseq sequencing approach was applied to analyze bacterial diversity and communities in samples during the fermentation period(0, 1, 4, 7 months (m0, m1, m4, m7)). As shown in Table 1, 1 338 047 sequencing reads of bacterial 16S rRNA genes were retrieved from the samples. After removing the low-quality and chimera reads and trimming the PCR primers, high-quality reads with an average of approximately 1 281 365 clean tags were obtained. The clean tags were allocated to 11 077 OTUs.
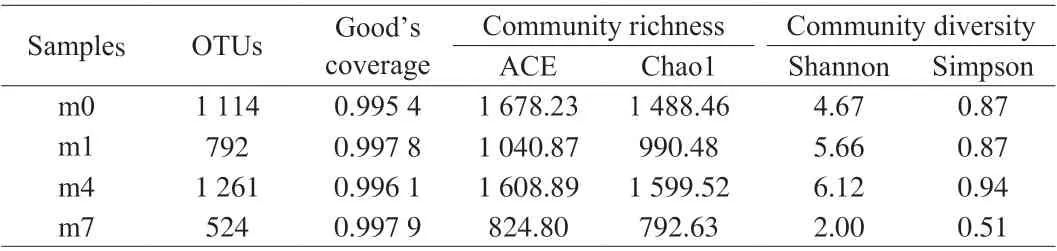
Table 1 OTUs, Good’s coverage, community richness indices (ACE and Chao1), and community diversity indices (Simpson and Shannon) in shrimp sauces from different fermentation stages.
The Good’s coverage value regarding the microbial community abundance was found between 99.54% and 99.79%, demonstrating that the sample had a high coverage rate. Chao1 and ACE were used to calculate the number of microorganism species and Shannon’s index and Simpson’s index are used to estimate the changes in richness and evenness in samples [13]. Shannon and Simpson, Chao1 and ACE indices at the initial stage of fermentation were higher than that at the later stage of fermentation, which indicated that alpha diversity in shrimp sauces at the initial stage was more diverse than other parts of the samples. As the fermentation progresses, the number of microbial species in the samples gradually increased before the 4thmonth and then decreased significantly during the following fermentation. That may be because the temperature is lower (below 0 °C) before the 4thmonth, while the temperature gradually rose at the end of the fermentation. Low-temperature preservation could facilitate maintaining the diversity of microbiota, and storage at room temperature might be more liable to the formation of dominant bacteria and lead to a reduction in the diversity.
3.2 Microbiology analysis
The bacterial diversity of the shrimp sauces was determined by high-throughput sequencing, which was classified at the phylum(Fig. 1A) and genus level (Fig. 1B). At the phylum level,Bacteroidetes, Actinobacteria and Proteobacteria were dominant during the early fermentation. As the fermentation progressed,the initially dominant phylum gradually diminished and the phylum Firmicutes increased after the 4thmonth of fermentation.In the 7thmonth, the relative abundance of Firmicutes increased to 98.65% and it gradually became the dominant phylum of the microbial flora. In previous studies, Firmicutes was also the main constituents of the microbial flora in the fermentation of shrimp sauces [14], sour meat [15] and fish sauce [16].This indicated that Firmicutes plays a vital role in the quality of shrimp sauces. At the genus,Flavobacteriumwas dominant from the m0 to m1 and then dropped rapidly.Flavobacteriumas pathogenic bacteria was associated with the ability of some species to cause disease,which was commonly isolated from freshwater environments [17].The proportions ofStaphylococcusandHaematospirillumincreased from 0.01% and 0.09% at the beginning of fermentation to 8.70% and 8.58% in the 4thmonth.Staphylococcuscan prevent the formation of off-odor and rancidity due to their antioxidant activities [18].And some strains ofStaphylococcuspossessed high lipase and protease activities [19]. There was a dramatic increase in the proportion ofTetragenococcusin the 7thmonth of fermentation and becoming the predominant genus (98.52%). This indicated that the diversity of the microbial flora decreased as fermentation progresses.Tetragenococcuscan tolerate the high concentration of salt in the fermented food, such as fish sauce, jeotgal and soybean products [20].In previous research,T.halophiluscould increase the total amino acid content [21] and repressed the formation of BAs during food fermentation [22]. Therefore,Tetragenococcusmay improve flavoring, taste characteristics, and safety of shrimp sauces.Furthermore, the relative abundance ofPseudomonas,PsychrobacterandPolaribacterdecreased during fermentation and ripening.These bacteria were associated with components degradation, slime formation, odors and appearance variation [23].
The microbial functions were revealed based on PICRUSt analysis of 16S rRNA gene sequence data. For the shrimp sauce samples, the 20 most abundant KEGG pathways of the four samples were shown in Fig. 1C. The largest proportion in samples was metabolism-related genes (level 1), indicating that metabolism was the primary function of the bacterial community. A total of 34 KEGG pathways (level 2)were observed. Among them, Carbohydrate metabolism, Amino acid metabolism, and Metabolism of cofactors and vitamins were the most abundant pathways in samples (Fig. 1C). Genes related to amino acid metabolism and lipid metabolism were abundant in the early stage of fermentation, while those related to carbohydrate metabolism were more abundant in the middle and late stages of fermentation. It has been demonstrated that carbohydrate metabolism is closely related to the presence of Firmicutes, while amino acid and lipid metabolisms are close to the presence of Proteobacteria [24]. It indicated that the microbial activities in naturally fermented shrimp sauces were related to amino acid, carbohydrate, and lipid metabolism. Moreover,microorganisms contribute to flavor compounds by undergoing amino acid metabolism [25] and genes related to amino acid metabolism may correlate to the nutritional value of the product [26].
3.3 TVB-N and AAN analysis
The TVB-N and AAN content of the shrimp sauces were determined and the results were showed in Fig. 2. The TVB-N level of seafood is an important parameter for quality and freshness that is associated with the metabolism of spoilage bacteria and the activity of endogenous enzyme, producing volatile nitrogen including ammonia (NH3), trimethylamine (TMA), and dimethylamine [27].The initial TVB-N level in samples was 0.93 mg/mL and the content in samples increased with the extension of the fermentation and storage time. After fermentation for the 7thmonth, the TVB-N content reached (236.27 ± 3.98) mg/100 mL. The higher bacterial producers of TVB-N in fish wereEnterobacteriaceae,Photobacteriumspp., andLactobacillusspp. [28]. Various microbial metabolites derived from protein hydrolysis associated with spoilage have been reported to cause undesirable sensory attributes in seafood products [27]. The reproduction ofTetragenococcusalso affected the content of TVB-N, which can produce organic acid to neutralize volatile basic nitrogenous compounds [29].Changes in TVB-N content also affect the AAN content [2].
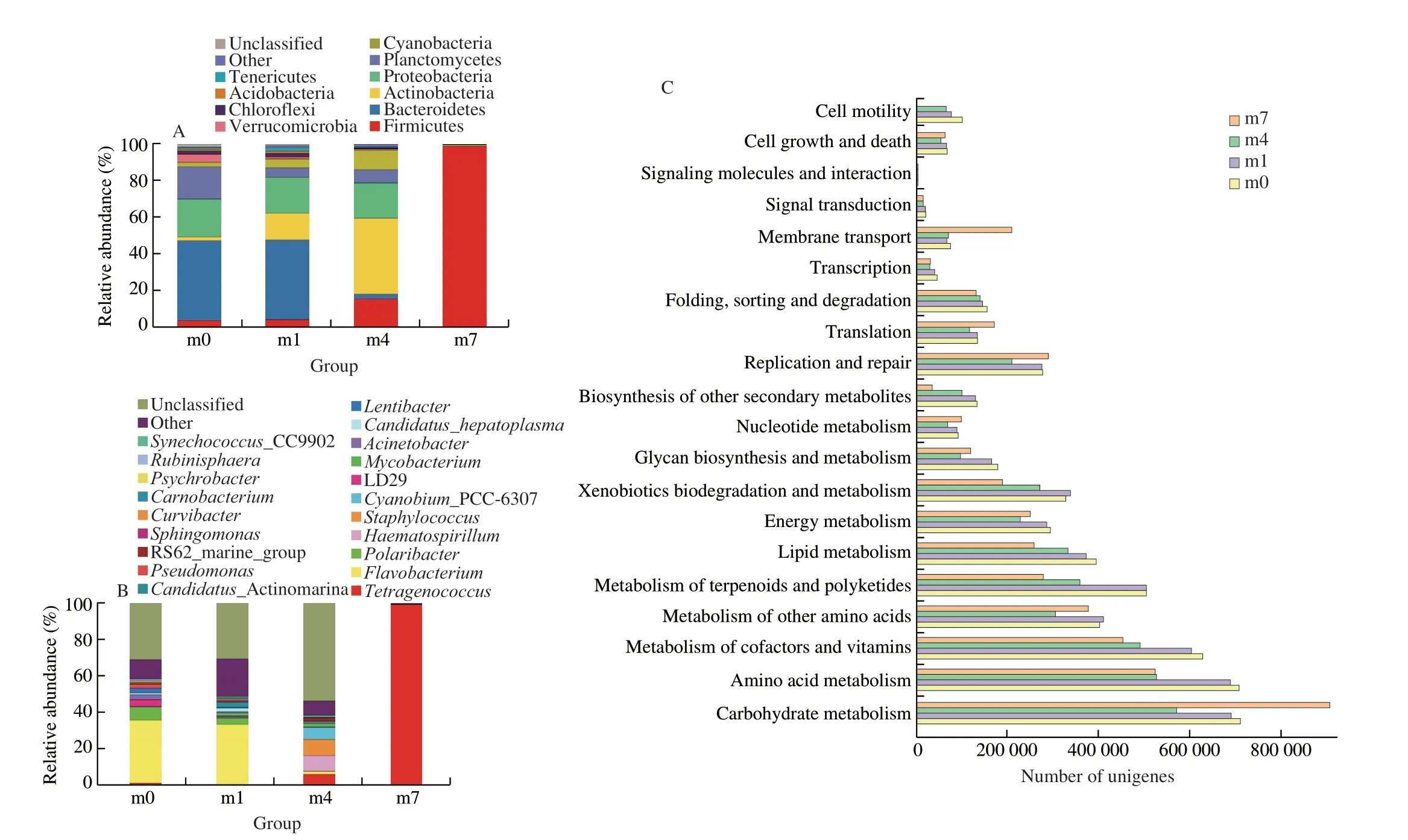
Fig. 1 Relative abundance of bacterial composition in shrimp sauces during the fermentation process at phylum level (A) and genus level (B). Picrust2 assignment of predicted genes within bacterial community of shrimp sauces (C).
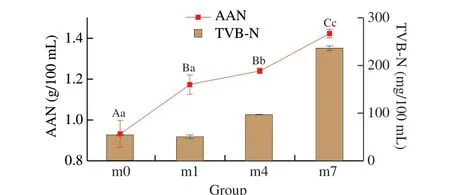
Fig. 2 Changes of amino nitrogen and total volatile base nitrogen in shrimp sauce samples during the fermentation process. Different uppercase letters among the contant of amino nitrogen at the different times indicate significant differences (P < 0.05), and lowercase letters among the content of total volatile base nitrogen at the different times indicate significant differences (P < 0.05).
AAN is one of the most crucial quality indexes of fermentation products which is highly related to nutritional value and sensory characteristics [30]. The AAN content of samples increased with increasing fermentation time (P< 0.05). At the beginning of fermentation, the content of AAN was 0.93 g/100 mL. An increase in AAN in the fermented shrimp paste was observed during the fermentation period. And the AAN content in the 7thmonth was 1.42 g/100 mL. The aforementioned results suggested that the nitrogenous compounds were hydrolyzed to small fragments,particularly amino acids. AAN content was used as a convenient index of the degree of protein hydrolysis [31], which was due to the extracellular proteases secreted by microorganism.
3.4 Organic acids
Organic acids were important flavor components in traditional fermented food, which contributed significantly to taste and impart unique umami and sour taste [32]. Organic acids were the crucial acidifiers and exhibited the proper concentration to endow the natural sour taste and could inhibit the growth of miscellaneous bacteria to extend shelf life. In this study, a total of 6 kinds of organic acids were detected during the fermentation process. Among various organic acids, succinic acid had the highest concentration (6.13 mg/mL),followed by lactic acid (4.38 mg/mL), tartaric acid (2.48 mg/mL),malic acid (3.98 mg/mL), and acetic acid (1.97 mg/mL). The concentration of citric acid was the lowest (1.73 mg/mL). In the early stage of shrimp sauces, concentrations of lactic acid, acetic acid, citric acid, succinic acid and malic acid have been reduced to varying degrees. As fermentation progressed, the concentrations of organic acids gradually increased in the 4thmonth until the end of fermentation. Several factors, such as fermentation environment, types of microorganisms and the state of raw materials can affect the ratio of the contents of organic acids [33]. The decline in tartaric acid might be related to the formation of potassium hydrogen tartrate [34]. The rapid decrease of malic acid during the 4thmonth may be attributed to malolactic fermentation, in which malic acid was converted to lactic acid [35]. The LAB strains have a high ability to form organic acids [36]. This might account for the increase in organic acid content after the 4thmonth.
Lactic acid can enhance the contribution ofL-amino acids to taste [37]. According to reports, succinic acid was an indispensable taste component in some seafood [4] and has been reported to contribute to a special sour and umami taste [38,39]. The production of organic acids in samples provided an acidic environment unfavorable for the growth of many pathogenic and spoilage microorganisms [36].
3.5 5′-Nucleotides
Flavor nucleotides are among the main substances contributing to the flavor of shrimp sauces. The level of five 5′-nucleotides (CMP,UMP, GMP, IMP and AMP) were analyzed in shrimp sauce samples from different fermentation stages. As shown in Fig. 3, GMP was the main component (1.82 mg/mL) in initial samples, followed by AMP,IMP and UMP, and the content of CMP was least (0.27 mg/mL).As the fermentation time increased, the content of 5′-nucleotides increased. At the end of fermentation, the contents of UMP, AMP,GMP and IMP reached the maximum, and their concentrations were 0.69, 2.49, 2.29 and 0.53 mg/mL. The content of CMP (0.56 mg/mL)reached the maximum in the 4thmonth. And AMP was the dominant 5′-nucleotide, which was the main umami 5′-nucleotide in seafood [4].It is generally believed that AMP, GMP and IMP have outstanding umami tastes, and can present taste even at low levels. GMP is a flavor-enhancer, which gave a stronger meaty flavor [40]. AMP at low concentrations contributed sweetness and elicited umami and complexity tastes [38]. IMP and GMP activated the umami receptors and were described as positively correlating with umami and kokumi [41].Moreover, Flavor nucleotides (IMP, GMP and AMP) and umami-taste amino acids (aspartic and glutamic acids) express greater umamitaste [42]. It has been reported that flavor nucleotide content is closely related to its degradation and the secondary metabolic activities of the microorganisms [43]. These microorganisms can produce nucleotides at different rates, thus differentially affecting the umami taste of shrimp sauces.
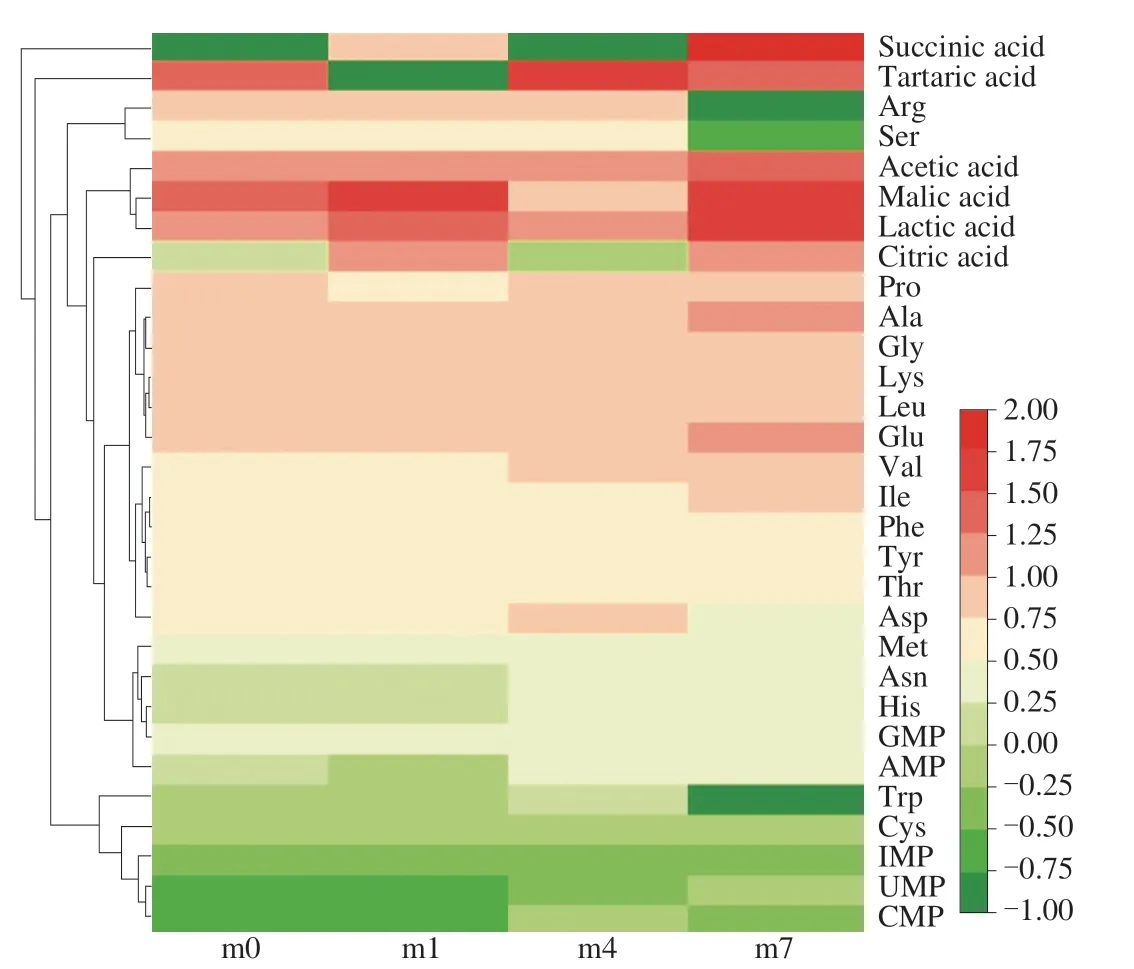
Fig. 3 Heatmap and dendrogram of the non-volatile organic compounds present in shrimp sauces from different fermentation stages. The heatmap plot indicates the relative abundance of non-volatile organic compounds in different samples (variables clustered on the vertical axis). The phylogenetic tree is mainly concerned with the correlation between non-volatile flavour compounds. The phylogenetic tree was calculated using the neighbour-joining method. The colour intensity is proportional to the relative abundance of nonvolatile organic compounds.
3.6 Amino acid analysis
FAAs not only have a certain flavor capacity of their own but also are precursors to many volatile flavor compounds. As shown in Fig. 3,a total of 19 FAAs were detected in all shrimp sauces. The abundance of FAAs changed throughout fermentation. The concentrations of total free amino acids (TFAA) increased from 81.27 mg/mL to 102.90 mg/mL. Shrimp sauces fermented for 4 months had the highest TFAA content. But the abundance of umami amino acids and sweet amino acids was the highest in the 7thmonth, contributing to the improvement of the sweet and umami flavor of shrimp sauces. During the fermentation, changes of FAAs were promoted by endogenous enzyme and microorganism [44]. Microorganisms continuously decomposed proteins in the early stages of fermentation, leading to an increase in the content of amino acids. In the later stages of fermentation, there was some microbial death, resulting in a reduced ability to decompose proteins [45]. In addition, the bacterial autolysis phenomenon itself also led to a certain increase in the free amino acid content.Staphylococcusexhibited protease activities and can hydrolyze protein into peptides and FAAs [46]. The abundance ofStaphylococcusin the 4thmonth is 8.44%. It was responsible for the growth of TFAA at this time point. FAAs contribute directly to taste.Alanine and glutamic were the main FAAs. The contents of alanine and glutamic acid were 11.97 and 11.78 mg/mL. Tryptophan (Trp)and arginine (Arg) were scarcely detected except for the first four months, while the content of threonine (Thr) was also less in the late fermentation stage. Glutamic acid is crucial to the umami taste, while alanine contributes to a sweet taste [47]. Alanine with IMP has been implicated to strongly increase the umami taste [48]. Amino acids are also precursors of various volatile flavor compounds. Amino acids can be converted toα-ketoglutarate acids, which can be metabolized directly or indirectly into the corresponding aldehydes [49]. Volatiles originated from amino acids are important for the flavor of seafood and meat products. For instance, sulfides components act as aroma components in shrimp sauce [50] and methionine was considered to be an essential precursor of Sulphur-containing volatile organic compounds [51].
3.7 E-tongue analysis
E-tongue can detect taste properties by electronic sensors which are based on the changes in the membrane potential of the lipid membrane of each artificial sensor to determine the basic taste sensory indexes. This is a technical method for analyzing lowvolatile or non-volatile compounds. Fig. 4A showed the radar map for taste determination of shrimp sauces by the E-tongue. There were significant differences in sourness, aftertaste-A, umami, saltiness,sweetness between the groups of shrimp sauces (P< 0.05). Among them, with the increase in fermentation time, the umami and saltiness increased. The differences in the concentration of FAAs of shrimp sauces may affect the flavor. They could contribute to the sweet and bitter taste. In addition, flavor nucleotides (IMP, GMP and AMP)express greater umami-taste, when they company with umami-taste amino acids (aspartic and glutamic acids) [42]. Umami substances appear to enhance the perceived sweetness or saltiness, or other flavor characteristics [52].
PCA of the taste response to shrimp sauces with different fermentation time was presented in Fig. 4B. PC1 and PC2 explained 39.6% and 34.0% of the total variance, respectively. As shown in Fig.4B, samples from different fermentation time were well distinguished.The m7 samples were discriminated from other samples by umami and saltiness with negative score values at PC1, which indicated higher umami and saltiness in these samples compared to other samples. The m0 and m4 samples were differentiated by sweetness with negative score values at PC2, which indicated higher sweetness in these samples compared to other samples. The differences in the concentration of FAAs of shrimp sauces with different stages may affect the flavor. The FAAs themselves could contribute to sweet,umami and bitter taste and influence saltiness and sourness together with acids and inorganic salts.
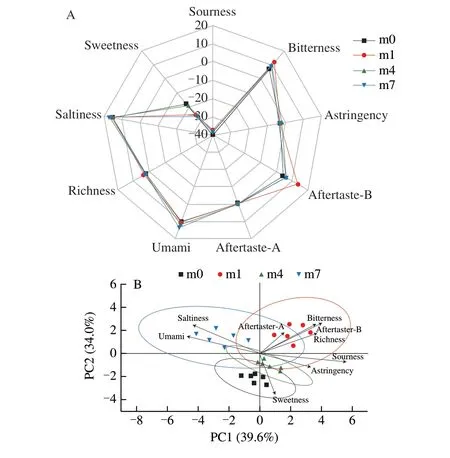
Fig. 4 Radar chart (A) and principal component analysis (B) of electronic tongue data for shrimp sauces from different fermentation stages.
3.8 Correlation between microbiota and non-volatile active compounds
Many non-volatile compounds contribute significantly to the unique flavor of fermented products. Microbial communities are critical to flavor formation due to reactions catalyzed by their different enzymes. The relationship between the abundance of the individual genus within the bacterial community and non-volatile flavor substances, including FAAs, organic acid and flavor nucleotides was evaluated by the spearman correlation heatmap analysis. The results were showed in Fig. 5. Most of the genera were found to exhibit a certain degree of correlation with non-volatile flavor substances. The result showed that the abundances of theTetragenococcusformed a significantly positive correlation with 10 FAAs, including some delicious amino acid such as glutamic and alanine,Flavobacteriumformed a significantly positive correlation with a variety of amino acids.Staphylococcuswas significantly positively correlated with CMP and GMP.Lactobacillushas formed a significantly positive correlation with AMP, Glu and His. And organic acids were positively correlated withTetragenococcusandHaematospirillum.
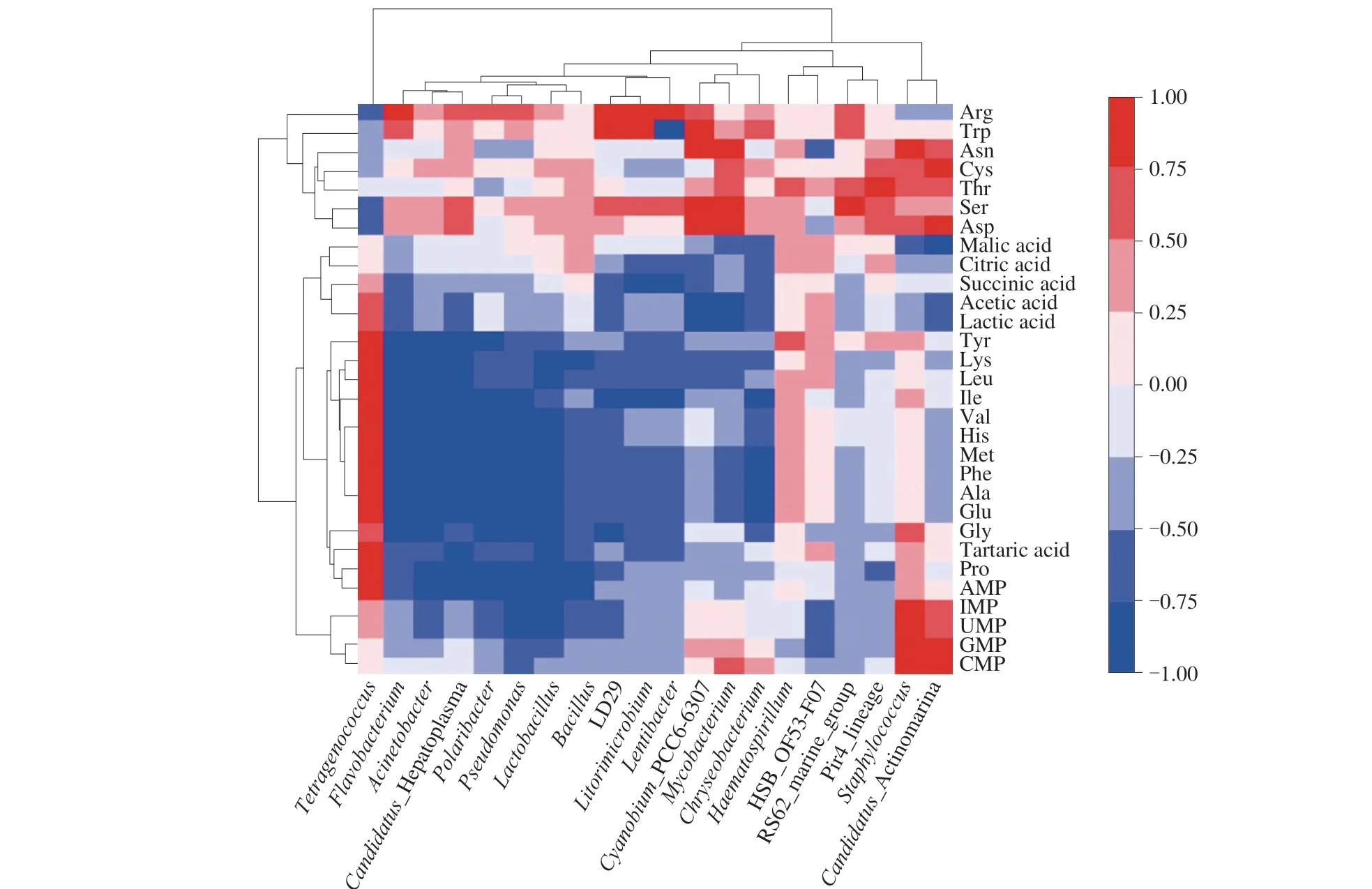
Fig. 5 Correlation analysis between the relative abundance of dominant bacterial community and non-volatile organic compounds in shrimp sauces from different fermentation stages, shown as a heatmap. Shades of red and blue indicate strong positive and negative correlations, respectively.
LAB can produce organic acids and bacteriocin, thereby inhibiting the growth of putrefying and pathogenic bacteria, ensuring the safety of the fermentation process, and promoting the formation of flavor, nutrients, and color.Tetragenococcusspp. is related to various salted fermented foods, such as soy sauce, soy bean paste, fermented fish and shrimp paste [53]. It has been reported thatT. halophilusmay play important roles in the production of organic acids, amino acids and flavoring compounds during the fermentation of salted foods [54].Staphylococcushas protease activity, which could degrade proteins into micromolecular peptides and FAAs, providing nutrition for the growth and reproduction of other microorganisms [44].And as reported [55], protease and lipase activities of coagulasenegative staphylococci produce various flavor compounds, which can be responsible for flavor development in shrimp sauces during the beginning of fermentation. The generaStaphylococcusandTetragenococcususually dominate during the production of fermented seafood, which contributed to lowering the pH and reducing the risk of putrefaction.
4. Conclusions
In this study, physicochemical parameters, taste substance(6 organic acids, 5 5′-nucleotides and 19 FAAs), and microbial profiles of the naturally fermented shrimp sauces were detected, and their correlations were explored. The results of E-tongue detection can distinguish the taste characteristics of different samples. This study provides a better understanding of the dynamic changes in the bacterial community. The phylum Firmicutes and the genusTetragenococcuswere dominant at the late stage of fermentation,while the diversity of the bacteria in shrimp sauces at the initial stage was more diverse than other parts of the samples. Correlation analysis indicated thatTetragenococcuspositively correlated with a variety of FAAs;Staphylococcuspositively correlated with 5′-nucleotides. The analysis indicated thatTetragenococcusandStaphylococcuswere the core genera affecting non-volatile components. These findings indicated the dynamics of the bacterial community and non-volatile components inter-relationships during shrimp sauce fermentation and provide a theoretical basis for improving the fermentation process of shrimp sauce. Moreover, there were limitations that should be addressed in our study. Addressing the correlation between representative bacteria and a few non-volatile components with a variation on each stage is not enough to reveal the complex interweaving relationships of bacteria.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This project had support from the National Key R&D Program of China (2019YFD0901903) and the Innovation Team Project of Hebei (Province) Modern Agricultural Industry Technology System(HBCT2018170207), and the Postgraduate Research & Practice Innovation Program of Jiangsu Province (SJCX20_1426).
- 食品科学与人类健康(英文)的其它文章
- Wine, beer and Chinese Baijiu in relation to cardiovascular health:the impact of moderate drinking
- Comparative analysis of physicochemical properties, ginsenosides content and α-amylase inhibitory effects in white ginseng and red ginsen
- Monitoring and identif ication of spoilage-related microorganisms in braised chicken with modif ied atmosphere packaging during refrigerated storage
- Effect of cooking processes on tilapia aroma and potential umami perception
- Formation mechanisms of ethyl acetate and organic acids in Kluyveromyces marxianus L1-1 in Chinese acid rice soup
- Volatile prof ile and multivariant analysis of Sanhuang chicken breast in combination with Chinese 5-spice blend and garam masala

