Effect of Tremella fuciformis polysaccharide on the stalling and f lavor of tteok during storage
Hongxiu Fan, Hongcheng Liu, Wenyi Li, Wenjing Su, Dawei Wang,Shanshan Zhang, Tingting Liu,*, Yanrong Zhang,*
a School of Food Science and Engineering, Jilin Agricultural University, Changchun 130118, China
b Scientif ic Research Base of Edible Mushroom Processing Technology Integration of Ministry of Agriculture and Rural Affairs, Changchun 130118, China
c Engineering Research Center of Grain Deep-processing and High-effeciency Utilization of Jilin Province, Changchun 130118, China
d Key Laboratory of Technological Innovations for Grain Deep-processing and High-effeciency Utilization of By-products of Jilin Province, Changchun 130118, China
Keywords:Anti-staling Tremella fuciformis polysaccharide Tteok Volatile f lavor compounds
A B S T R A C T The effect of Tremella fuciformis polysaccharide (TFP) on the retrogradation property and aroma profile changes of tteok during storage was investigated. Results indicated that addition of TFP significantly increased the stability to thermal and mechanical shearing of starch, decreased short-term retrogradation, the hardness and the retrogradation enthalpy (ΔH) of tteok during storage, but had no signif icant effect on the amylopectin chain length of tteok. The possible mechanism for the retarding staling effect of TFP is related to the interaction between TFP and starch chains that interferes with the alignment of starch chains. Electronic nose and g as chromatography-mass spectrum (GC-MS) analysis results showed that the difference in the concentrations of volatile compounds and fatty acids of tteoks at different storage time gradually increased with the advancement of storage period. The addition of TFP to tteok inhibited the development of unpleasant volatile compounds, probably by delaying the oxidation and decomposition of lipids and preserving the antioxidant phenolic compounds in tteok, thus slowing down the f lavor deterioration of tteok and contributing to f lavor maintainace. Overall, this study could help food manufacturers to choose a high-effective and natural polysaccharide to control the retrogradation rate and f lavor loss of tteok.
1. Introduction
Tteok is a type of glutinous rice cake and has been traditionally consumed as a snack or table food in Asian countries, particularly South and North Korea and China. Conventionally, tteok is made from glutinous rice. The glutinous rice was steamed and pounded by a wooden hammer right after cooking to lose the integrity of rice kernel and to form the unique viscoelastic property. The product has an enticing color and is soft, chewy and slightly sticky with some elasticity and therefore is widely popular [1,2]. H owever, when tteok is kept at room temperature, it tends to be tough and rigid and l ose its characteristic flavor within a few days due to starch retrogradation. Such staling process can cause reduced consumer acceptance and shorter shelf-life of tteok, leading to food waste and making it difficult to establish a global market. In order to control staling, several food additives such as amylase, lipids, surfactants,hydrocolloid and emulsifiers have been used. These additives have direct effect on starch retrogradation, mainly by partially degrading starch (use of enzymes) [3], formation of complexes with amylose or the outer branches of amylopectin (use of lipids, emulsifiers and surfactants) [4,5], reduction in the swell and rupture of starch granule (use of emulsif iers) [6], etc. At present, many researchers are exploring the use of polysaccharides in controlling the retrogradation rate and prolonging the shelf life of starch-based products due to their safety, easy accessibility, and high effectiveness. There are a number of studies concerning the application of polysaccharides to modify the retrogradation behavior of starch, including dextran, chitosan,galactomannan, soybean polysaccharide and tea polysaccharide [7,8],and researchers are dedicated to exploring the general rules for the anti-staling effect of polysaccharides on the starch-based foods. Sozer et al. [9] reported that polysaccharides enhance water retention and limit its redistribution within starchy food structures,providing an anti-staling effect. In addition, possible interactions among polysaccharides and starch molecules, i.e. polysaccharides interacted with leached starch molecules through hydrogen bonds and electrostatic forces are hypothesized to retard the aggregation of amylose that led to retrogradation [10]. The anti-staling ability of polysaccharides is greatly influenced by the type, source and molecular weight of polysaccharides as well as the specific starchy foods [11].Zhou et al. [7] reported that tea polysaccharide exhibited more pronounced inhibiting effect on the retrogradation of wheat starch compared with carboxymethyl cellulose. Pongsawatmanit et al. [12]found that addition of xanthan facilitated amylose retrogradation, but retarded amylopectin retrogradation of tapioca starch.
Tremella fuciformisis a colloidal edible mushroom and is often used as traditional medicine and food in Asia because of its excellent nutritional value and biological activities.T. fuciformispolysaccharide(TFP) is the main active ingredient inT. fuciformis, and many studies reported that it have various biological activities, including antioxidation, anti-inflammatory, antitumor, antihyperlipidemic,antidiabetic, and improving the immune system [13,14]. The TFP has gained great attention in natural food additives, pharmaceutical and health food industries due to its rich sources, safety and various bioactivities. Researchers found that tremella polysaccharide is mainly composed of homogeneous polysaccharide (70%-75% of the total polysaccharide), and it can increase the viscosity of solution and possess emulsion stabilizing property. Thereby TFP has been widely used as thickener or stabilizer in food products such as beverage, dairy products and ice-cream [15,16]. However, to the our best knowledge,TFP has been rarely studied on anti-staling analysis of starch-based products in the literature.
The objective of the current work was to investigate the potential usefulness of TFP on the anti-staling effect in tteok during storage.Since staling is a complex phenomenon affected by several factors, it is unlikely that any single method can provide a complete picture of the staling properties of tteok. Therefore, multiple methods, including rapid viscosity analysis (RVA), differential scanning calorimetry(DSC), texture profile analysis (TPA) were used to study the effect of TFP on the thermal transition properties, starch recrystallization and the length of amylopectin chain of tteok. Since the aroma profile is also regarded as one of the key criteria utilized to assess the sensory quality of rice products, and content changes in the volatile compounds could also indicate the staling degree [17], therefore,in this study, we use electronic nose and gas chromatography-mass spectrum (GC-MS) to study the changes in the volatile compounds and fatty acid composition of tteok during storage. The present study will enhance the understanding of anti-staling effect of tremella polysaccharide on tteok and provide theoretical foundation for the industrial processing of tteok with high quality.
2. Methods and materials
2.1 Materials
Glutinous rice was obtained from Harui Professional Rice Planting Cooperative (Wuchang, Jilin, China). Moisture content and amylose and amylopectin contents of glutinous rice were measured according to American Association of Cereal Chemists (AACC)method 44-15.02 [18] and the method of Ji et al. [19]. The contents of moisture, amylose and amylopectin in the glutinous rice were 13.47%, 1.71% and 75.22%, respectively. Dried fruit bodies ofT. fuciformiswas obtained from Bolin Biotechnology Co., Ltd. (Jilin,China).β-Amylase of barley (EC 3.2.1.2, activity 1 000 U/mL)and pullulanase (EC 3.2.1.41, activity 20 U/mg) were purchased from Megazyme (Wicklow, Ireland). The C7-C25n-alkane standards were purchased from Sigma-Aldrich (St. Louis, MO, USA). Other chemicals used in this study were of analytical grade were supplied by Beijing Chemical Reagents Co. (Beijing, China).
2.2 Preparation of TFP
The TFP was extracted and isolated according to Xu et al. [16]. In brief, the driedT. fuciformisfruit body was crushed, screened through a 120-mesh sieve and mixed with distilled water at a materialliquid ratio of 1:75 (g/mL). Then the sample was extracted in a high-pressure extraction kettle at 120 °C, 1.0 MPa for 40 min under nitrogen protection. The resultant extract was centrifuged to remove the precipitate, and the supernatant was collected and subjected to the Sevage method for deproteinization three times. The obtained polysaccharide solution was concentrated to 1/6 of the original volume by a rotary evaporator and mixed with four equivalent volumes of ethanol at 4 °C overnight. The precipitate was then collected by centrifugation, re-dissolved in distilled water and dialyzed(8 000–12 000 Da) for 48 h. The dialysis liquid was lyophilized to obtain TFP. The purity of TFP was 85.64%, which was determined using phenol-sulfuric acid colorimetric method.
2.3 Pasting properties of flour
The pasting properties of glutinous rice flour/TFP blends were analyzed using a Rapid Visco-Analyzer (Brookfield, USA) according to the AACC Method 61-02.01 [18]. For the preparation of glutinous rice flour, glutinous rice was ground using a crusher mill and the flour was sifted through a 100-mesh sieve. The glutinous rice flour was replaced by TFP at 0%, 2%, 4%, 6% and 8% levels (m/m, based on glutinous rice flour weight). Then the glutinous rice/TFP composite flour was suspended in distilled water (10%,m/m) and transferred to an RVA container. The flour slurries were heated from 50 °C to 95 °C at 6 °C/min, held at 95 °C for 5 min, cooled to 50 °C at 6 °C/min, and subsequently held at 50 °C for 2 min. The pasting parameters were determined from the RVA curves, including peak, trough and final viscosities, pasting temperature and breakdown and setback values.
2.4 Preparation of tteok
Control tteok was prepared following a traditional method for tteoks [20]. 200 g of glutinous rice was weighed and soaked in 600 g of distilled water at 25 °C for 36 h. Afterwards, the soaked grains were drained, spread on a wet gauze and put into in a steamer.The glutinous rice was steamed for 20 min, added 80 mL of distilled water, cooked 20 min, added 80 mL of distilled water again, stopped heating and then hold for 20 min. The cooked glutinous rice was put onto a board and pounded using a mallet for 20 min to lose the integrity of rice kernel and to form a glutinous rice dough with viscoelastic property. After pounding, the tteok sample was divided into small pieces and kept in a ziplock bag at 4 °C for further analysis.Four levels of TFP (2%, 4%, 6%, and 8% (m/m), based on glutinous rice weight) were used to partially replace the glutinous rice. TFP was weighed and dissolved in 80 mL of water at first. The glutinous rice was soaked in the same way as control tteok, then the soaked glutinous rice was steamed for 20 min, added with 80 mL of distilled water, continued to cook 20 min, mixed with TFP solution, stopped heating and then hold for 20 min. All the samples were then pounded in the same way as control tteok.
2.5 Textural analysis
Tteok samples were stored at 4 °C for 0, 1, 2, 3, 4, 5 and 6 days and their textural property was determined using a TA-XT Plus texture analyzer (Stable Micro System, Surrey, UK). The tteok samples were molded into a cylinder shape of 20 mm (diameter) ×20 mm (height) and were compressed twice to 30% of the original sample height using a P/36R probe. The interval time between compressions was set as 5 s and the pre-test, test, and post-test were 2.00 mm/s. The hardness (maximum force at the first compression)was calculated from the textural profile analysis curves.
2.6 DSC analysis
Thermal analysis of the retrogradation behavior of tteok samples was performed using a differential scanning calorimeter (Discovery DSC 25, TA Instruments, USA). Freeze-dried powdered tteok (6 mg) was weighed into a stainless-steel DSC pan, and the pan was sealed and allowed to stand for 1 h. An empty aluminum pan was used as a reference point. The sample pan was heated from 25 °C to 100 °C at a heating rate of 10 °C/min. The peak temperatures (Tp) and endothermic enthalpy (ΔH, J/g) of the endothermic transition were determined using the Trios software version 2.0.
2.7 Determination of amylopectin chain length
2.7.1 Isolation of amylopectin
Amylopectin was isolated according to a method described by Guo et al. [21]. Briefly, the dried tteok sample (0.6 g) was steeped in 2 mL ethanol, and then the softened sample was dissolved in 20 mL 0. 5mol/L NaOH by heating in a boiling water bath for 30 min with vigorous stirring. After cooling to room temperature,the sample solution was centrifuged (8 000 r/min, 10 min) to remove the precipitate, and the solution was neutralized with 2 mol/L HCl. A 13 mL mixture of isoamyl alcohol andn-butanol (1:1,V/V) was added to the sample solution and the mixture was heated in a boiling water bath for 10 min with vigorous stirring. After cooling to room temperature, the sample solution was placed in the refrigerator at 4 °C overnight and then centrifuged (8 000 r/min, 10 min). The precipitate was the crude amylose, and the supernatant was the crude amylopectin solution. The obtained amylopectin solution was transferred to a 10 mL mixture of isoamyl alcohol andn-butanol (1:1,V/V) and the solution was heated in a boiling water bath for 30 min with vigorous stirring. After cooling to room temperature, the solution was stored at 4 °C overnight, centrifuged (8 000 r/min, 10 min) and the obtained supernatant was added to two equivalent volumes of anhydrous ethanol. The mixture was held at 4 °C overnight, centrifuged(8 000 r/min, 10 min) and the precipitate was dried at 40 °C to obtain the purified amylopectin.
2.7.2 Determination of β-amylolysis limit
The obtained amylopectin sample (0.5 g) was suspended in 25 mL of sodium acetate buffer (pH 5.0, 0.02 mol/L) andβ–amylase was added at as dose of 100 U. The mixture was incubated at 37 °C for 48 h, followed by heating at 100 °C for 10 min to deactivateβ-amylase.The total carbohydrate and reducing residues of the hydrolysate were determined by the phenol-sulfuric acid method and the Park-Johnson method, respectively. Theβ-amylolysis limit of amylopectin was calculated according to the method of Wang et al. [22].
2.7.3 Interior chain length and average exterior chain length
The amylopectin sample (22 mg) was dispersed in 10 mL of sodiumacetate buffer solution (0.02 mol/L, pH 5.5). Pullulanase was then added at a dose of 4 U for debranching at 37 °C for 24 h. The debranched sample was heated at 100 °C for 20 min to deactivate the enzyme. The average chain length (CL) was calculated as the ratio of reducing residues equivalent to glucose to the total carbohydrate. The average interior chain length (ICL) and average exterior chain length(ECL) were calculated using the Eq.1 and Eq.2, respectively.
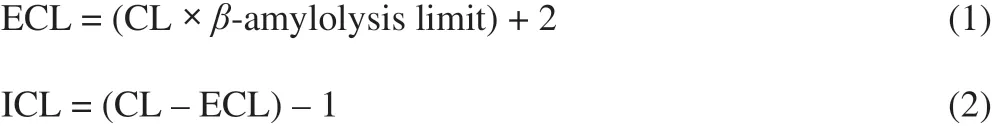
2.8 Fatty acids analysis
Total lipid was extracted from 200 g of tteok sample using the Folch method with chloroform/methnol (2:1,V/V) [23]. The extracted crude lipid (50 mg) was transferred into a 25 mL test tube and added with 2.5 mL hexnane and 0.5 mL sodium hydroxide methanol solution (0.5 mol/L). The tube was shaken with a vortex mixer for 5 min. After methylization, the upper hexane phase was collected for GC-MS analyisis.
Fatty acid methyl esters were analysed on a HP-88 capillary column (100 m × 0.25 mm × 25 μm). The temperature gradient of GC oven was as follows: initial temperature 125 °C maintained for 0.5 min, increased to 145 °C at 10 °C/min, then increased to 180 °C at 5 °C/min, holding for 15 min, and then raised to 230 °C at 5 °C/min,holding for 15 min. The helium gas flow rate was 1.0 mL/min and the split ratio was 1:50. The injector temperature, ion source temperature and transfer line temperature were 250, 230 and 250 °C,respectively. Mass range was set at 60–600m/z. Indification of fatty acids in the samples was carried out by comparing their mass spectra with the mass spectra from NIST MS Search 2.3 database, and the quantification analysis was carried out using undecanoic acid methyl ester as an internal standard [24].
2.9 Electronic nose analysis
The odour fingerprint map of tteoks after 0, 1, 2, 3, 4, 5, 6 and 7 days of storage at 4 °C was extracted using a portable electronic nose(PEN 3 Airsence, Schwerin, Germany) which included a set of 10 commercial metal oxide sensors. The specification of the electronic nose sensors was described in Table 1. For each measurement, a tteok sample (5 g) was placed in a headspace vessel and left at 25 °C for 20 min to equilibrate the volatiles in the headspace. During the measurement, the headspace gas of the sample was pumped into the sensor chamber at a flow rate of 400 mL/min. The detection time was 60 s and the flush time was 80 s. The electronic nose data of tteok samples were analyzed by principal component analysis (PCA)to reduce the dimensionality of datasets and to differentiate flavor features of tteoks during storage time. The analyses of the data were carried out using DPS version 3.11.
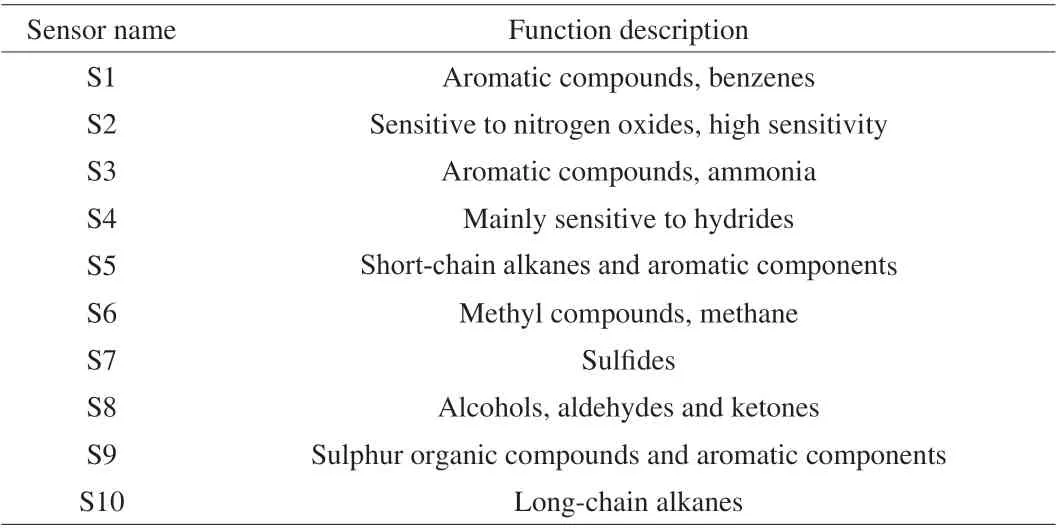
Table 1 Performance description of the electronic nose sensors.
2.10 Volatile compound analysis via solid phase microextraction combined with gas chromatography-mass spectrum(SPME/GC-MS)
The volatile compounds of tteok during storage were extracted by SPME, and the identification of volatile compounds was conducted by gas chromatography-mass spectrometry (TSQ 9000, Thermo Scientific, USA). 5 g of tteok samples with a solution (4 μL) of 2-methyl-3-heptanone in methanol (81.6 μg/mL) added as internal standard were placed in a 20 mL headspace vial and was sealed tightly. The samples were incubated in an agitator at 50 °C for 10 min, and the SPME fiber (DVB/CAR/PDMS, 50/30 μm) was inserted into the headspace of the vial to absorb volatile compound at 50 °C for 30 min [25]. After extraction, the SPME fiber was injected into the GC inlet (250 °C) to desorb for 5 min. The volatile compounds were separated using a TG-5MS capillary column (30 m × 0.25 mm ×0.25 μm). The temperature gradient of GC oven was set as: initial temperature 40 °C maintained for 4 min, increased at 1.5 °C/min to 65 °C, then increased to 230 °C at 7 °C/min, and the final temperature was held for 7 min. The helium flow rate was set as 1.0 mL/min.The injector temperature, ion source temperature and transfer line temperature were 250, 230 and 250 °C, respectively. The MS was operated in full scan range from 50 u to 350 u. Volatile compounds from the samples were identified by matching their mass spectra with the NIST MS Search 2.3 database and by comparing their retention indices (RIs) with the literature values. The RI value was calculated using the standard C7–C25n-alkanes under the same chromatographic conditions [25]. The content of volatile compounds was calculated with a semi-quantitative method, by comparing their peak areas to the peak area of the internal standard [26].
2.11 Sensory evaluation of tteok
The sensory evaluation of the flavor of tteoks during storage was performed by a panel of 10 well-trained judges (5 females and 5 males, aged between 20 to 55) at a standard sensory laboratory. The evaluation standards (scoring system) of the flavor of the samples was established according to the methods of Tiparat et al. [27]. The sensory evaluation was divided into 5 levels according to the flavor quality of the samples, and the sensory attributes were scored on a scale from 0 to 5 (4-5: with rich fragrance of glutinous rice; 3-4: with fragrance of rice, but weak; 2-3: no fragrance of rice, no unpleasant smell;1-2: slightly unpleasant smell; 0-1: serious unpleasant smell) [17].The samples were maintained at 25 °C for 30 min before testing to avoid the influence of temperature on the sensory evaluation. The judges had a 3 min forced break after finishing their evaluation of each sample test. The average scores of sensory attributes based on the scores given by the 10 judges (each repeated three times) were used as evaluation results for quantitative analyses.
2.12 Statistical analysis
All determinations were repeated at least three times the results were expressed as mean ± standard deviation (SD). Differences between samples were assessed using one-way analysis of variance with SPSS 16.0 software. Differences withP< 0.05 were regarded as statistically significant. PCA analysis was conducted using Origin 2019b.
3. Results and discussion
3.1 Effects of TFP on the pasting properties of glutinous rice flour
As seen in Table 2, the peak, trough or final viscosities of glutinous rice paste with 2%-8% TFP were significantly lower than the control sample. This result might be due to the adsorbing of TFP onto the starch granules to prevent abrasions between the starch granules or due to association of the TFP with leached out amylose and/or amylopectin. Furthermore, the glutinous rice/TFP composite system exhibited increased peak, trough and final viscosities as TFP concentration increased from 2% to 8%. Luo et al. [28] hypothesized that the starch/hydrocolloids composite system is biphasic with hydrocolloids existing in the continuous phase. This increased concentration of TFP in the composite system was probably the main reason for the increased viscosity. Pasting temperature indicates the temperature at which starch begins to thicken and provides evidence of the minimum temperature required to cook glutinous rice. The pasting temperature was lower in the composite system with 2%-8%TFP (56.94–71.93 °C) compared to the control (76.87 °C), whichmight be due to the intermolecular associations between TFP with the leached amylopectin and/or amylose [29].
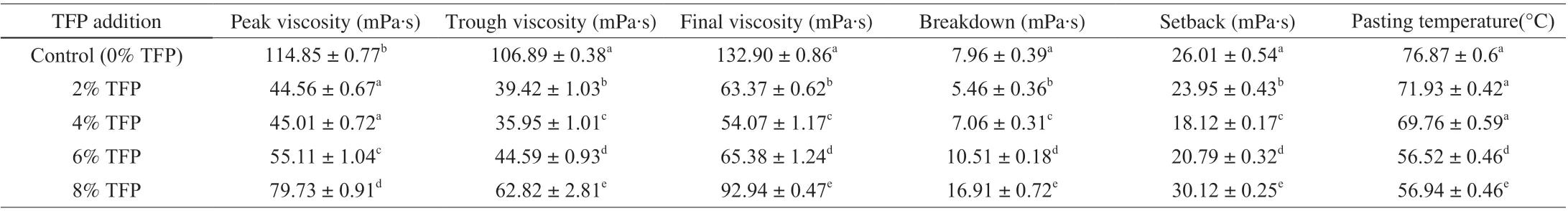
Table 2 The pasting parameters of glutinous rice pastes with different levels of TFP.
As shown in Table 2, the composite system with 2%–4% TFP had lower setback and breakdown values than the control, which indicated that addition of 2%–4% TFP to glutinous rice flour increased the stability to thermal and mechanical shearing, and decreased shortterm retrogradation of glutinous rice starch. Starch retrogradation is a process in which the disordered starch chains reassociate by strong hydrogen bonding to form more ordered structures [30]. Fu et al. [31]reported that the interactions between starch chains and hydrocolloids could interfere with the alignment of starch chains, resulting in the delaying of retrogradation in starch during cooling. Dangi et al. [29]reported that addition of hydrocolloids diminished water migration from the starch, which also hindered the formation of a highly ordered structure. In addition, the setback value and breakdown value increased as the TFP concentration increased from 4% to 8%,indicating that addition of TFP at higher levels caused instability of the composite system and increased short-term reaggregation. When the addition amount of TFP was higher, phase separation occurred due to thermodynamic incompatibility between glutinous rice starch and TFP molecules [32]. This phase separation could lead to mutual exclusion of each polymer in the composite system, which promoted amylose-amylose and amylopectin-amylopectin aggregation by concentrating same molecules in regions and increased starch retrogradation [31].
3.2 Effect of TFP on the texture characteristic of tteok during storage
The staling of starchy foods is typically related to increases in hardness due to starch retrogradation. Therefore, the hardness of the tteok was monitored to investigate anti-stalling effect of TFP during storage. As shown in Fig. 1, the hardness value of the control tteok increased significantly after 2 days of storage. When the addition amount of TFP was in the range of 2%–4%, the hardness of tteoks also showed an increase with the advancement of storage period,but the difference became significant after 3 or 5 days of storage.The results revealed that the increase of the hardness of tteok was delayed during storage. Thus the results indicated that TFP addition at lower concentration was highly effective in delaying the starch retrogradation of tteok during storage, which might be attributed to the fact that TPF at lower concentrations could interact with leached amylose or amylopectin in the continuous phase to interfere with the alignment of starch chains, thus contributing to hardness reduction.However, when the addition amount of TFP was higher than 4%,the tteoks displayed significantly higher hardness than the tteok with 4% TFP during storge. This might be due to the fact that when the addition amount of TFP was higher, phase separation occurred which promoted retrogradation of starch chains. In addition, excessive TFP molecules could cross-linked with each other, resulting in higher gel strength and therefore higher hardness of tteok.
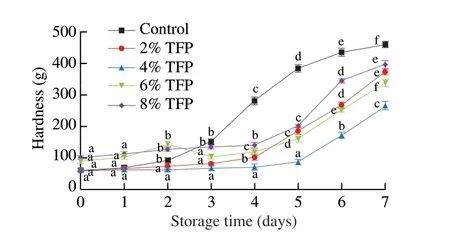
Fig. 1 Hardness of tteoks with different levels of TFP during storage. Letters a-f indicate significantly different (P < 0.05).
3.3 Effect of TFP on the thermal property of tteok during storage
The thermal transitions properties of tteok with different levels of TFP were analyzed after 7 days of storage to evaluate the retrogradation behaviors. An endothermic peak was observed in the tteok samples, which is related to melting of retrograded amylopectin and/or amylose-long amylopectin double helices, and the peak temperature (Tp) for endothermal transition and enthalpy values were presented in Table 3. It can be observed that theTpvalue of tteoks increased slightly during storage. It was reported that the increase ofTpusually reflected a higher degree of crystal perfection [33].However, addition of TFP to tteok at the level of 2%-8% did not led to significant difference in theTpvalues of tteok samples. The ΔHvalue could reflect a comprehensive measure of the thermal property of starch. It is related to the melting of amylopectin-based crystals and reflects the content of double helices in the tteok [34,35]. As shown in Table 3, the ΔHvalue increased significantly during storage for the control sample. However, the tteoks with 2-8% TFP had lower ΔHvalues than the control sample at the same storage time, indicating that addition of TFP to tteok was effective in reducing amylopectin retrogradation of tteok during storage, which might be due the fact that addition of TFP into the tteok could interfere with the alignment of starch chains during retrogradation (due to steric hindrance) [36].The ΔHvalue of tteok was lowest when TFP concentration was 4%.However, when the addition amount of TFP was higher than 4%,the ΔHvalue increased slightly. This might be due to the fact that addition of higher amount of TFP caused phase separation of the system. The results of thermal properties were consistent with the data of texture characteristics and pasting properties as described in this study.
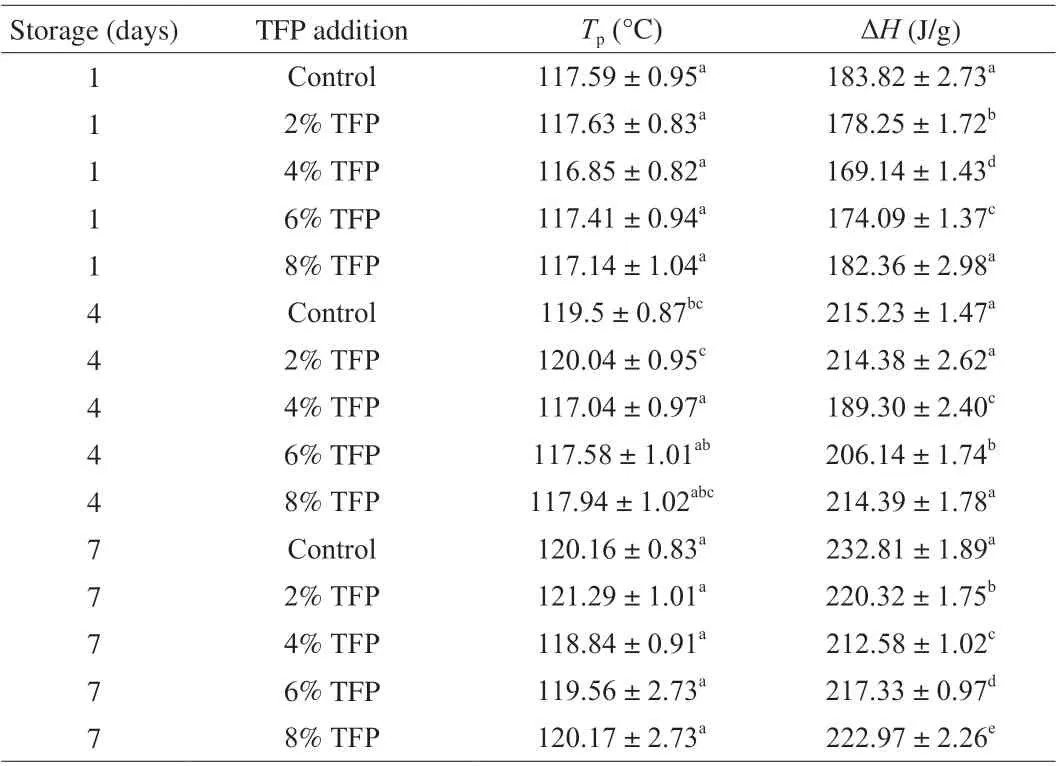
Table 3 DSC parameters of tteoks with different levels of TFP during storage.
3.4 Effect of TFP on the amylopectin chain length of tteok
Tteok contains a large amount of amylopectin (more than 98%of total starch) [37], therefore amylopectin plays an important role in the starch retrogradation properties of tteok. Previous studies showed that the rate of amylopectin retrogradation was positively correlated with the amylopectin fine molecular structure including molecular size distribution and average chain length. Würsch et al. reported that the starch retrogradation rate increased with the increase of amylopectin chain length, and short amylopectin chains with degree of polymerization (DP) less than 10-11 hardly retrograded [38].Jane et al. also reported that long amylopectin branched chains were rather easily reassociated after gelatinization [39], whereas short amylopectin chains hardly associate to form a crystalline structure because reassociation of amylopectin chains only occur when DP was higher than 15 [40]. As shown in Table 4, the CL of tteoks were in the ranges of 18-19, which were in line with the results of previous research investigating the amylopectins of maize, potato and rice [22,41]. The CL of tteok amylopectins decreased slightly with the addition of TFP, which might be due to the interactions between TFP and amylopectins in the tteok, which inhibited the reassociation of amylopectin chains into long chains. In addition, the ECL of amylopectins of tteok decreased slightly with the addition of TFP,while the ICL value remain unchanged with the increasing level of TFP. The results indicated that the addition of TFP to tteok could reduce the length of outer branches of amylopectin slightly, but did not affect the chain length between the two adjacent branches in the amylopectin.
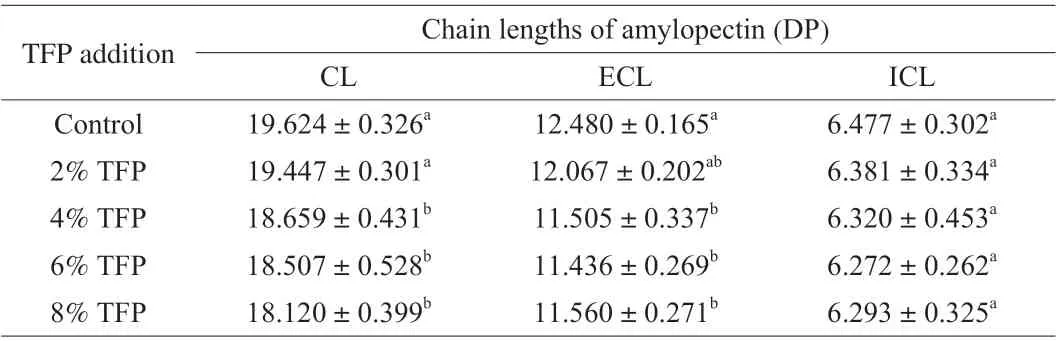
Table 4 The chain lengths of amylopectin of tteoks with different levels of TFP.
3.5 Fatty acids composition of tteok
Changes of the fatty acids compositions in the the control tteok or the tteok with 4% TFP during storage are shown in Table 5. Thirteen fatty acids were detected in the tteok samples during 7 days of storage, including six saturated fatty acids (SFAs), five monounsaturated fatty acids (MUFAs) and two polyunsaturated fatty acids (PUFAs).Linoleic acid andα-linolenic acid were the major PUFAs seen in all tteok samples during storage. It can be observed that the content of PUFA in the control tteok decreased with the advancement of storage period, but the difference became significant after 3 days of storage.This might be due to the PUFAs underwent lipolysis under the action of lipoxygenase or auto-oxidation during storage. Similarly, Lampi et al. [42]reported that the evolution of many flavor compounds, such as aldehydes, alcohols and ketones, largely depends on the enzymatic oxidation or auto-oxidation of linoleic acid and linolenic acid, which were easily oxidized. The content of PUFA in the tteok with 4% TFP also decreased during storage, but the difference became significant after 5 days of storage.
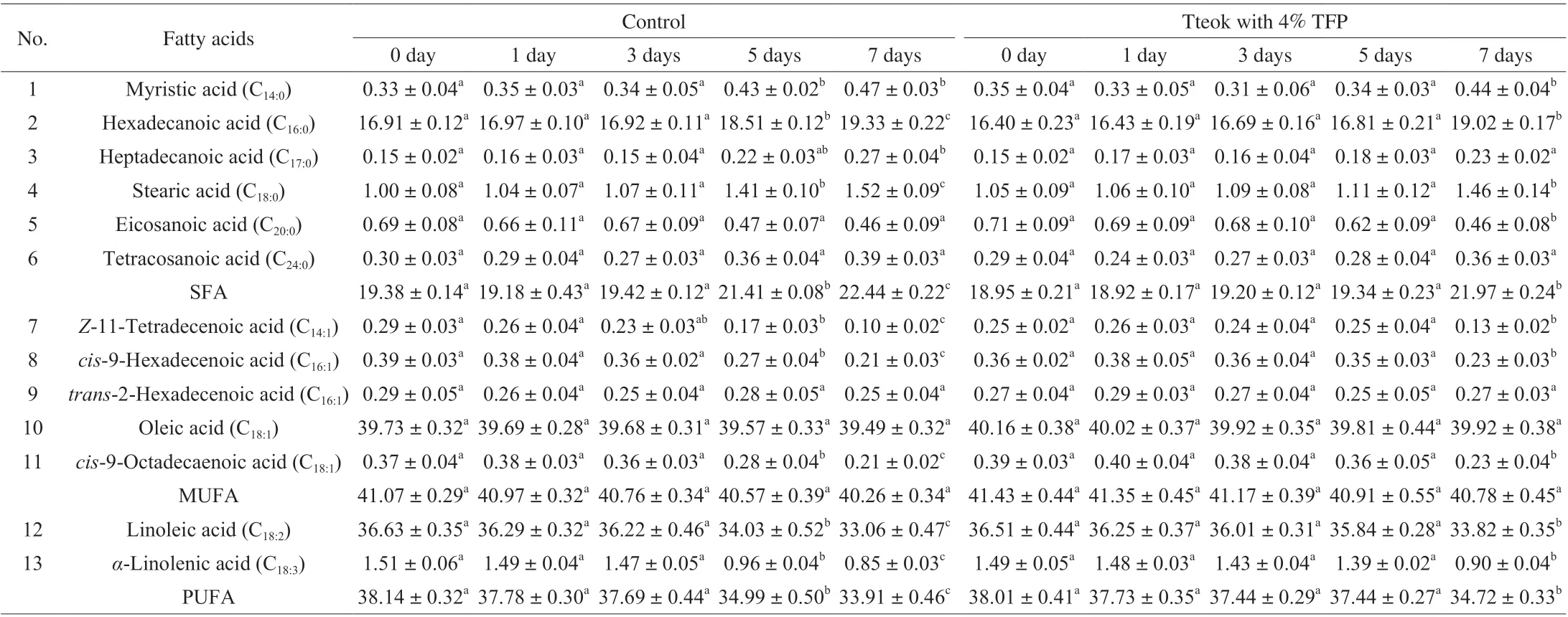
Table 5 Fatty acid composition (g/100 g oil) of the control tteok and the tteok with 4% TFP during storage.
In relation to MUFAs, oleic acid was the main constituent of MUFAs in all tteok samples during storage period. It can be seen that the content of MUFA in the control tteok and the tteok containing 4% TFP decreased with the advancement of storage period, but the difference was not significant.
In relation to SFAs, hexadecanoic acid and stearic acid were dominant SFAs in tteoks during storage period. It can be seen that the content of SFA in the control tteok showed non-significant variations up to the 3rdday, thereafter it showed significant increase up to the end of storage period. The increase in the content of SFA in the control tteok up to the end of storage period might be due to the oxidation of lipids. For the tteok with 4% TFP, the content of SFA also increased during storage, but the difference became significant on day 7. Overall, the results revealed that tteok with 4% TFP showed smaller changes in the fatty acid contents during storage compared with the control tteok. Thus it could be speculated that addition of 4% TFP might be effective in the retardation of MUFA oxidation and lipolysis in tteok.
3.6 Changes in the volatile profiles of tteok during storage
3.6.1 Electronic nose responses to the volatile compounds of tteok
Fig. 2 showed the response values of 10 electronic nose sensors for the tteok containing 4% TFP and the control tteok at different stages of storage. Considering the response values of 10 sensors during storage, S6, S7, S8 and S9 presented much higher and different responses to the volatile compounds of tteok samples than other sensors. Thus, these sensors were considered first. For the control sample (Fig. 2A), the response values of S6 and S8 showed nonsignificant variations during the first 3 days, and then they increased significantly up to the end of storage period. The numerous volatile compounds that lead to the unique flavor of tteok could be formed through Maillard reaction and via lipid oxidation and degradation during the rice cooking process or the rice dough pounding process.Until now, more than 300 volatile compounds have been identified from rice products, and the main constituents of these volatile compounds were normally aldehydes, acids, alcohols, ketones,esters and heterocyclic compounds [43]. Dias et al. [44] reported that aldehydes such as hexanal, nonanal and decanal; and ketones such as 6-methyl-5-hepten-2-one, (E)-5-methyl-4-hepten-3-one and 6,10,14-trimethyl-2-pentadecanone; and alcohols such as 1-octene-3-ol and 5-methyl-2- heptanol play key roles in the overall aroma profile of cooked rice. Hence, S6, which is sensitive to methyl compounds,exhibited a relatively higher response during the storage. It was reported that the contents of aldehydes and ketones in cooked rice increased during storage [45]. Aldehydes and ketones were thought to be mainly produced by the oxidation and decomposition of lipids [46].Thus the significant increases of S6 and S8 for the control tteok after 3 days of storage might be associated with the higher contents of aldehydes or ketones during tteok aging. As observed in Fig. 2,the response values of S7 and S9 for the control tteok increased significantly after storage for 5 days, indicating the contents of sulphur organic compounds and heterocyclic compounds increased with the aging of tteok sample. It was reported that sulphur organic compounds and heterocyclic compounds were the products of lipid decomposition and protein breakdown and would lead to undesirable flavor in tteok during storage [46,47]. For the tteok with 4% TFP(Fig. 2B), changes in the response values of S6, S8, S7 and S9 during storage were similar to those of the control sample. The response values of S6, S8, S7 and S9 showed non-significant variations during the first 5 days, thereafter they increased significantly up to the end of storage period. The results revealed that the time at which S6, S8, S7 and S9 presented significant increase was deferred compared to that of the control sample, indicating that changes in the volatile compounds over storage time were reduced, and that addition of 4% TFP into tteok was helpful for the retention of tteok flavor.
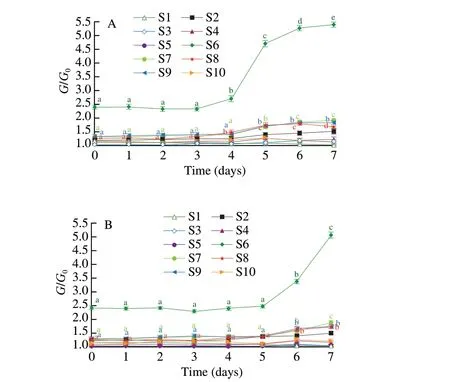
Fig. 2 PCA analyses of the electronic nose data for the tteok samples at different storage times (A represents the control tteok, B represents the tteok with 4% TFP). Letters a-f indicate significantly different (P < 0.05).
Fig. 3 presented the score values of the first and second principal components (PCs) of sensor responses of tteoks during storage by PCA. It can be seen that for the tteok with 4% TFP and the control tteok, the accumulative variance contribution rates of the first two PCs was higher than 90%, indicating that the first two PCs could reflect all the characteristics of volatile odor of the samples during storage [48].The tteok with 4% TFP and the control tteok at different storge stages are distributed along PC1 from the left to the right part of the plots,implying a trend related to the changes in the volatile compounds with an increase of storage time. In the PCA score plots of the control sample (Fig. 3A), sample points of day 0, day 1, day 2 and day 3 were partially overlapped along the abscissa (PC 1) and ordinate axes(PC 2), indicating that these samples had similar volatile characteristics and that they were hardly be distinguished by electronic nose. As storage proceeded, the day 5, day 6 and day 7 samples were far away from the day 0 sample along the PC 1 and PC 2 axes, which indicated significant differences in the volatile odor between day 0 and day 5 to day 7 sample. Day 5 was also the time when the hardness of the control tteok increased drastically. The PCA score plots for the tteok with 4% TFP are depicted in Fig. 3B. It can be seen that the samples from day 0 to day 5 were partially overlapped along the PC 1 and PC 2 axes, while samples from day 6 and day 7 were well separated from the day 0 sample, implying that the volatile compounds of the tteok with 4% TFP was more stable during storage compared with those of the control tteok.
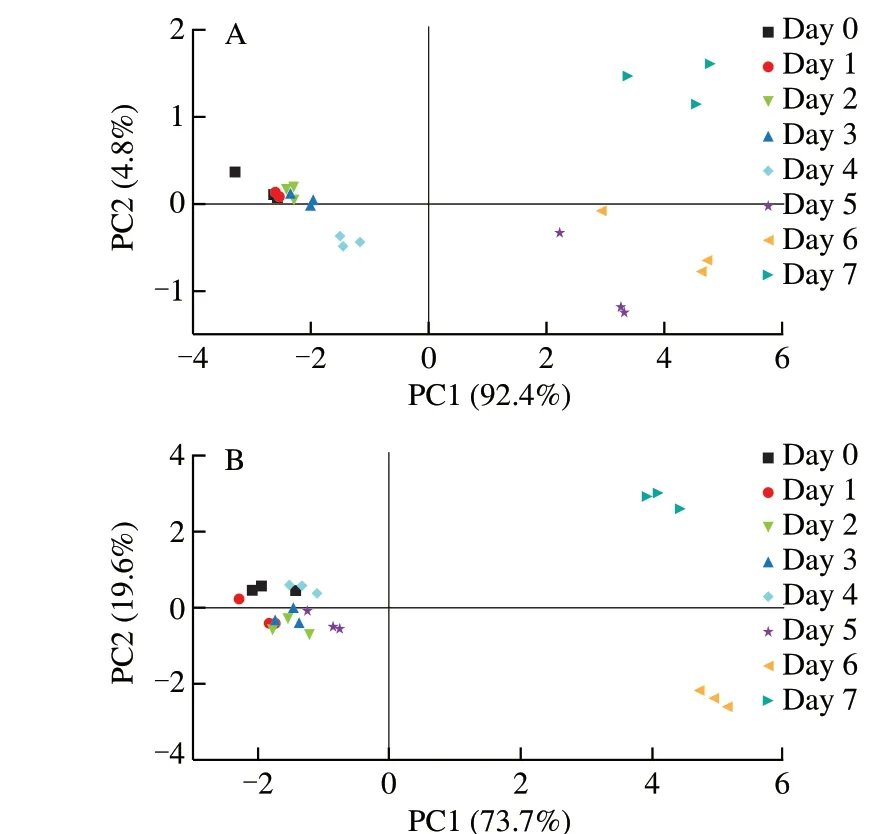
Fig. 3 The sensitivity of each electronic nose sensor for the tteok samples of different storage times (A represents the control tteok, B represents the tteok with 4% TFP).
3.6.2 SPME/GC-MS analysis of volatile compounds in tteok
GC-MS was utilized to analyze the volatile compounds of tteoks during storage to have a better understanding of the changes in the flavor of tteok during 7 days of storge. As shown in Table 6, a total of 53 volatile compounds were identified across all samples, which were classified into acids, ketones, aldehydes, alcohols, heterocyclic compounds, phenols, esters, sulphur-containing compounds and other compounds, respectively. Aldehydes are thought to be mainly produced via lipid decomposition, and often play a very important role in the overall flavor of rice because of relatively low odor threshold and high relative content. It can be observed that with the advancement of storage period the contents of nonanal and decanal in the control tteok decreased up to the 3rdday, and then increased significantly up to the end of storage period. It was reported that nonanal and decanal are secondary oxidation products of linoleic acid and contribute to flavor of fruity, floral and grass, with low odor threshold (0.8-3.1 ng/L) [46]. The significant decrease in the nonanal and decanal contents during the early stage of storage (0-3 days)might be due to their high volatility, unstable properties and relatively low contents [49,50]. However, as storage proceed, the degree of lipid oxidization increased, resulting in higher levels of lipid oxidation products (nonanal and decanal). The significant decrease in the contents of PUFAs in the control tteok after 3 days of storage (Table 5)could demonstrate this hypothesis. Other saturated aldehydes in the control tteok including pentadecanal, heptadecanal and methyltetradecanal showed non-significant variations during the first 3 days, thereafter they increased significantly up to the end of storage period due to the lipid oxidization. In addition, contents of unsaturated aldehydes ((E)-tetradec-2-enal,Z-12-tetradecenal) in the control tteok decreased significantly up to the 7thday, which might be related to the further autoxidation or decomposition of unsaturated aldehydes [50,51].In the tteok with 4% TFP, the contents of saturated aldehydes (nonanal,decanal, pentadecanal, heptadecanal, methyltetradecanal) decreased up to the 5thday, thereafter they showed significant increase up to the end of storage period. Combined with the fact that the content of PUFAs in the tteok with 4% TFP showed significant decrease after 5 days of storage (Table 5), it can be speculated that the significant increase in the the contents of saturated aldehydes after 5 days of storage might be due to the oxidative degradation of fatty acids. In addition, the rise in the levels of saturated aldehydes of the tteok with 4% TFP was significantly lower than that of the control sample,indicating that addition of TFP could inhibit the development of aldehydes in the stored tteok.
Ketones and heterocyclic compounds such as pyrazines, pyrroles,pyridines, etc. are thought to be formed by the oxidative degradation of fatty acids or by Maillard reactions, and their scents were normally described as caramel. However, they could also contribute to the unpleasant odor of rice products at high concentrations [51]. As shown in Table 6, the contents of ketones [5-(hydroxymethyl)2-pyrrolidinone, 2-(2-methyl-propenyl)- cyclohexanone,N-carbamoyl-1-pyrroid-2-one, 1,4,5,6-tetramethyl-2-pyrimidone, 3-methyl-1,4-diazabicyclo[4.3.0]nonan-2,5-dione, 5(4H)-oxazolone-2-methyl-4-phenylmethylene, 2-ethyl-1,3,4-trimethyl-3-pyrazolin-5-one, 3-butyl-6-methyl piperazine-2,5-dione, 7-propyl- pyrrolizin-1-one] and heterocyclic compounds [1-(1-oxo-2-butenyl)-piperidine, 2-(2-hydroxyhex-1-enyl)-3-methyl-5,6- dihydropyrazine,3-methoxy-2,5- dimethylpyrazine] in the control tteok showed nonsignificant difference during the early stage of storage (day 0 to day 3),and then increased significantly from day 5 to day 7. Pyrrole compounds such as 5-(hydroxymethyl)2-pyrrolidinone,N-carbamoyl-1-pyrroid-2-one and 7-propyl-pyrrolizin-1-one contribute to roasted,roasted nutty or cooked aroma in cocoa, coffee and roasted peanut.They are reported to be the products of browning reaction or Maillard reaction, and could increase the bitter taste and decrease the lightness of rice products at high concentrations [25,53]. In the tteok with 4% TFP, the contents of those ketones and heterocyclic compounds showed non-significant difference up to the 5thday, thereafter they increased significantly up to the end of storage period. In addition,the rise in the levels of ketones and heterocyclic compounds after 7 days of storage was also significantly lower than that of the control tteok. The results indicated that addition of TFP to tteok inhibited the development of ketones and heterocyclic compounds in tteok,suggesting the TFP could inhibit deterioration of the flavor of tteok.
Acids also accounted for a large portion of the volatile compounds in tteoks. It can be seen from Table 6 that in the control tteok and the tteok with 4% TFP, the contents of unsaturated fatty acids includingZ-11-tetradecenoic acid,cis-9-hexadecenoic acid andcis-9-octadecaenoic acid decreased with the advancement of storage period, which were in accordance with the results from fatty acids composition analysis (Table 5). These unsaturated fatty acids can be cleaved to different aldehydes and ketones depending on the oxidized lipids [51,54]. In addition, the contents of saturated fatty acids in the control tteok and the tteok with 4% TFP including hexadecanoic acid and heptadecanoic acid increased during storage. It was reported that the saturated fatty acids can be formed by the degradation of triglycerides [52]. Table 6 also showed that the changes in the unsaturated fatty acid contents of the tteok with 4% TPF were lower than those of the control tteok after 7 days of storage, suggesting that addition of TFP to tteok helped to reduce the degradation of unsaturated fatty acids to some extent.
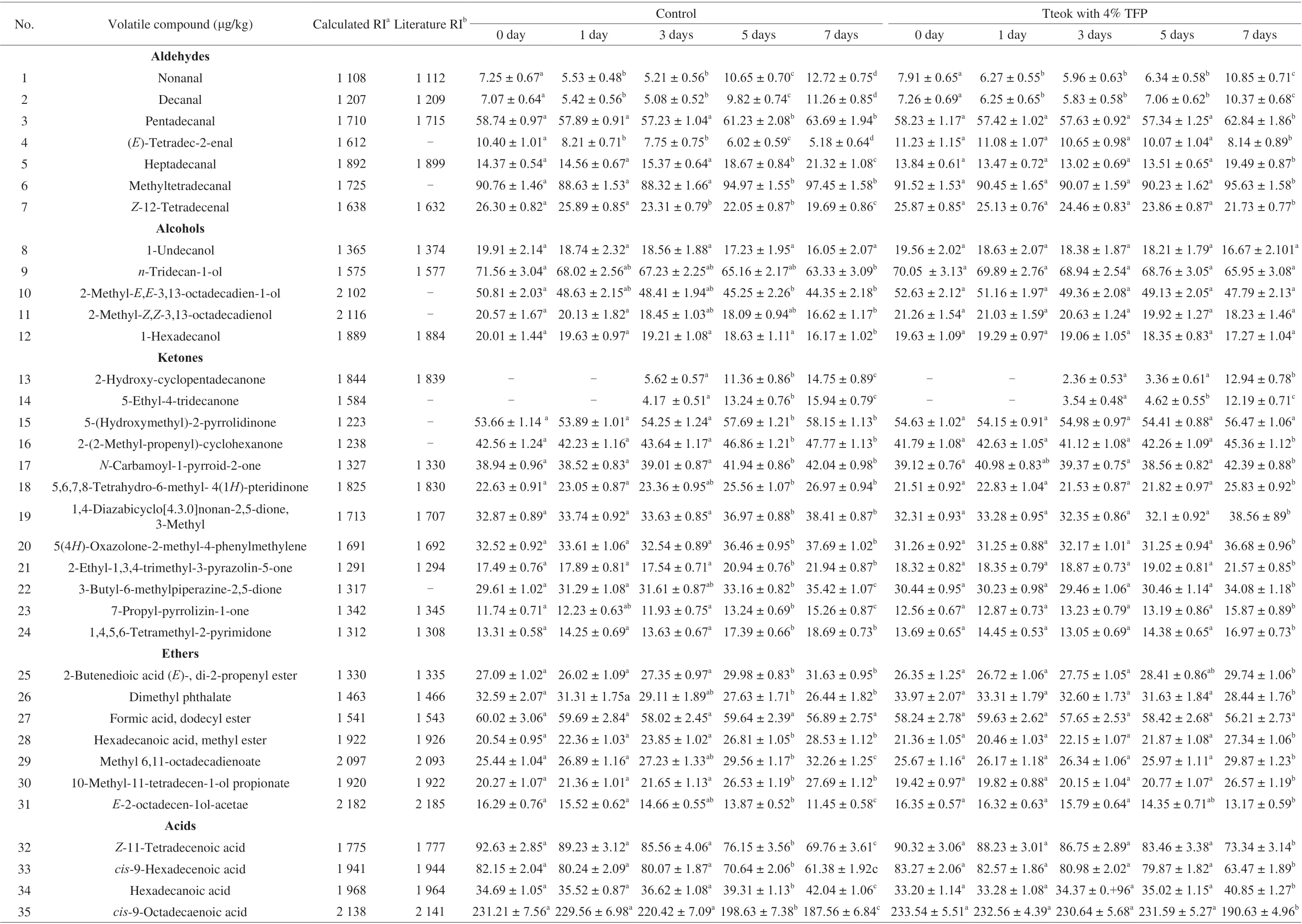
Table 6 Comparison of the volatile compounds of the control tteok and the tteok with 4% TFP during storage.
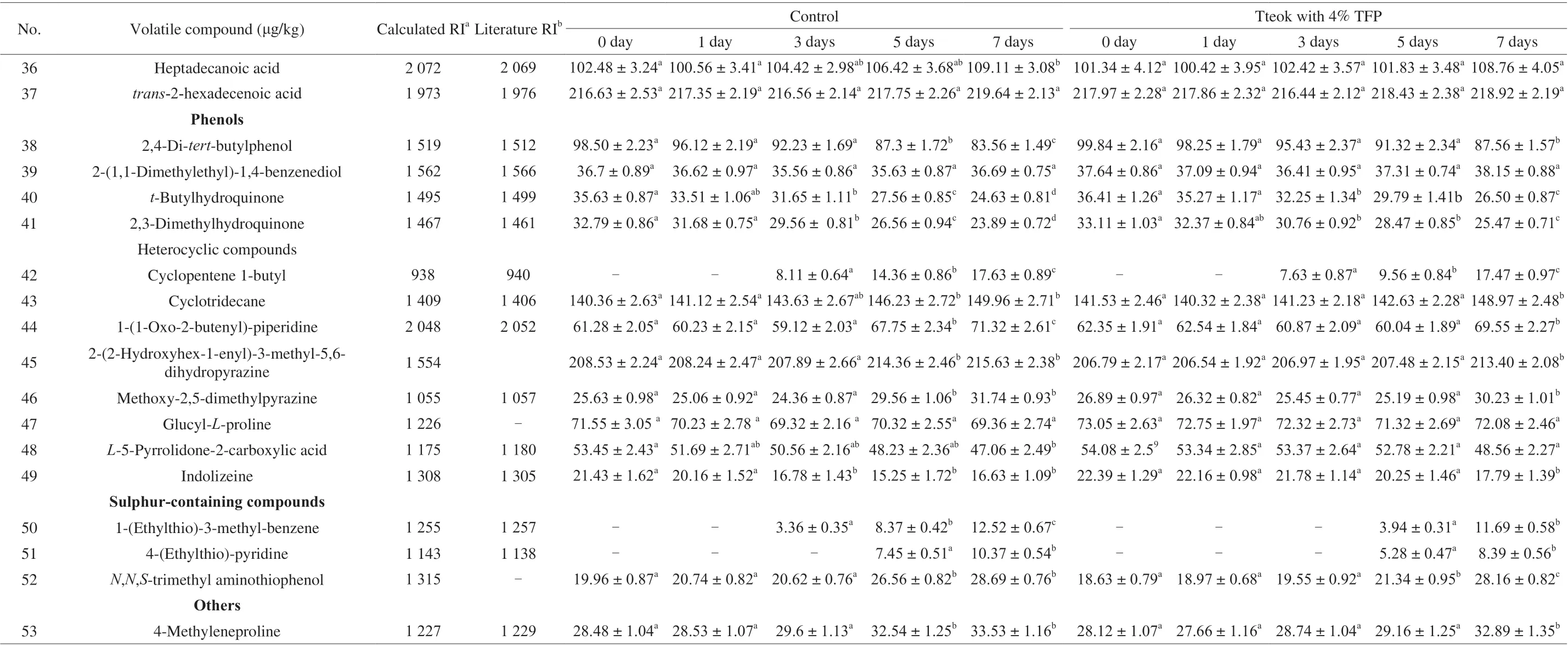
Table 6 (Continued)
As shown in Table 6, the contents of 2,4-di-tert-butylphenol,t-butylhydroquinone and 2,3-dimethylhydroquinone in the tteoks decreased with the advancement of storage period. These phenolic compounds can be used as antioxidants for lipid peroxidation [55,56].Besides,t-butylhydroquinone contributes slightly sweet flavor [57].After 7 days of storage, the contents of phenolic compounds in the tteok with 4% TFP was significantly higher than those of the control tteok, indicating that addition of TFP was helpful for the retention of antioxidant compounds in the tteok, which could result in low concentrations of lipid-oxidation-derived volatile compounds and thus was helpful for the retardation of tteok flavor deterioration.
3.6.3 Sensory evaluation of tteoks
Sensory evaluation of tteok samples including a control and the tteok with 4% TFP during storage was performed and the results are summarized in Table 7. It can be observed that there was no significant difference between the sensory scores of the control tteok and the tteok with 4% TFP when they were freshely produced (on day 0), indicating that addition of 4% TFP did not bring negative effect on the flavor of tteok. In addition, with the increase of storage time, the flavor deteriorated, the acceptability of tteoks decreased,and the sensory scores decreased, which might be due to unpleasant volatile compounds that formed and accumulated during the storage of tteok. The sensory score of the control tteok were significantly higher than the tteok with 4% TFP in late storage periods (day 5 to day 7). The result indicated that addition of 4% TFP could delay the deterioration of tteok flavor and contribute to preserving the flavor of tteok during storage.
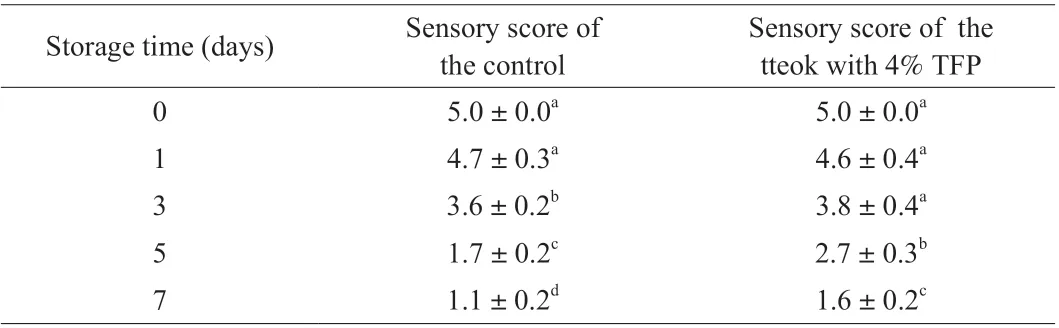
Table 7 Sensory score of the control tteok and the tteok with 4% TFP during storage.
4. Conclusions
This study investigated the effect of TFP on the retrogradation property and aroma profile change of tteok during storage. The addition of TFP to tteok significantly decreased short-term retrogradation, delayed hardness increase and reduced retrogradation enthalpy of tteok during storage, but had no significant effect on the amylopectin chain length of tteok. Such retardation of starch retrogradation effect was dependent upon the addition amount of TFP.The possible mechanism for retarding starch retrogradation is related to the interaction between TFP and starch chains that interfered with the alignment of starch chains. Analysis of electronic nose and GC-MS indicated that the difference in the concentrations of volatile compounds and fatty acids of tteoks at different storage time gradually increased with the advancement of storage period. The addition of TFP to tteok inhibited the development of unpleasant volatile compounds, probably by delaying the oxidation and decomposition of lipids and preserving the antioxidant phenolic compounds in tteok,thus slowing down the flavor deterioration of tteok and contributing to flavor maintainace. This study thus provides new insights into the impact of TFP on the anti-staling of tteok and could help food manufacturers to choose a high-effetive and natural polysaccharide to control the retrogradation rate and flavor loss of tteok, contributing to the industrialscale processing of tteok with high quality.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgement
This work was financially supported by the Key Projects of the National Research and Development Program of China(2018YFD0400103-6).
- 食品科学与人类健康(英文)的其它文章
- Wine, beer and Chinese Baijiu in relation to cardiovascular health:the impact of moderate drinking
- Comparative analysis of physicochemical properties, ginsenosides content and α-amylase inhibitory effects in white ginseng and red ginsen
- Monitoring and identif ication of spoilage-related microorganisms in braised chicken with modif ied atmosphere packaging during refrigerated storage
- Effect of cooking processes on tilapia aroma and potential umami perception
- Formation mechanisms of ethyl acetate and organic acids in Kluyveromyces marxianus L1-1 in Chinese acid rice soup
- Volatile prof ile and multivariant analysis of Sanhuang chicken breast in combination with Chinese 5-spice blend and garam masala

