Na-Promoted Pt/Hydroxylapatite for CO Oxidation
QlAN Xiaoshuang,MA Zhen
(Department of Environmental Science and Engineering,Fudan University,Shanghai 200438,China)
Abstract:Hydroxylapatite was used as a support to load Pt using Pt(NH3)4(NO3)2 as a Pt source,and the effect of Na species on the performance of the catalyst was studied.It was found that the addition of a suitable amount of NaOH via impregnation can enhance the catalytic activity in CO oxidation.The catalysts were characterized to understand the reasons for the promotional effect.The promoting effect can be ascribed to maintaining the relative proportions of Pt0 and Pt2+species,keeping the concentrations of surface hydroxyls and decreasing the amount of basic sites.
Keywords:Hydroxylapatite;Pt;NaOH;CO oxidation
Supported Pt catalysts are useful for catalytic oxidation and hydrogenation.Most supported Pt catalysts are prepared using metal oxide supports,but metal phosphates and hydroxylapatite have been rarely used to prepare supported Pt catalysts[1-4].In a previous work,we prepared a series of Pt/M-P-O(M=Mg,Al,Ca,Fe,Co,Zn,La)catalysts using H2PtCl6·6H2O as the Pt precursor,and found that Pt/Ca-P-O showed the highest activity in CO oxidation[2].Flytzani-Stephanopoulos and co-workers added Na species into Pt/SiO2prepared using P t(NH3)4(NO3)2as a Pt precursor[5].They found that the catalytic activity in water-gas shift was enhanced due to the formation of Pt-Na-Ox(OH)y.In the literature,the influence of alkali metals on the performance of metal oxide-supported Pt catalysts has been documented[5-10].However,reports on the influence of alkali metals on the performance of metal phosphate-supported or hydroxylapatite-supported Pt catalysts are scarce.
Herein,we loaded Pt onto hydroxylapatite using Pt(NH3)4(NO3)2as a Pt precursor,and tested the influence of Na species on the catalytic performance of Pt/Ca-P-O.The resulting catalysts were characterized to understand the reasons for the promotional effect of Na.
1 Experimental
Hydroxylapatite(Ca-P-O)powders(4.00 g,96% purity,purchased from J&K Scientific)was placed in an agate mortar,and 15.9 mL Pt(NH3)4(NO3)2solution(0.01 g/mL)was added into the agate mortar.The mixture was grided using an agate pestle and dried with the help of an infrared light.The dried samples were calcined in static air at 500℃for 2 h to get Pt/Ca-P-O.
Pt/Na/Ca-P-O was prepared using the same impregnation method,except that 0.139 g NaOH(or 0.296 g NaNO3,0.184 g Na2CO3,0.204 g NaCl)was added into the agate mortar before adding the Pt(NH3)4(NO3)2solution.The samples collected after calcination are denoted as Pt/NaOH/Ca-P-O,Pt/Na NO3/Ca-P-O,Pt/Na2CO3/Ca-P-O,and Pt/NaCl/Ca-P-O,respectively.
X-ray diffraction(XRD)patterns were collected on a MSAL XD-2 instrument(PERSEE)with a scanning rate of 4°/min.Elemental analysis was performed using an Optima 800 ICP-OES(PerkinElmer)instrument after dissolving the sample in aquaregia and subsequent dilution.Transmission electron microscopy(TEM)data were recorded on a JEM 2100F(JOEL)instrument according to a standard procedure[2].X-ray photoelectron spectroscopy(XPS)data were recorded using an Axis Ultra Dld(Kratos)instrument.Temperature-programmed desorption of CO2(CO2-TPD)was conducted using a FINESORB-3010(Finetec)instrument according to the procedure describe previously[2].
Catalytic CO oxidation was conducted using a FINESORB-3010(Finetec)instrument.Briefly,0.25 g catalyst was put into a U-shaped tube.The catalyst was flushed by flowing 4% H2/He(50 mL/min),and the temperature was increased linearly(10℃/min)to 300℃while 4% H2/He was still flowing.The catalyst was then retreated in flowing 4% H2/He at 300℃for 2 h,and cooled down.During the reaction testing,flowing 1% CO/air passed through the catalyst,and the temperature was maintained at 25℃for 1 h.Afterwards,the temperature was increased linearly(0.5℃/min)towards 300℃,and the temperature was finally maintained at 300℃for 30 min.The exiting gas was analyzed by a 7890A GC(Agilent)system every 10 min.
2 Results and Discussion
2.1 Catalysis
Pt/Ca-P-O was prepared by impregnating Ca-P-O support with Pt(NH3)4(NO3)2followed by calcination in air at 500℃.We additionally pretreated the catalyst in the reactor by flowing 4% H2(balance He)through the catalyst at 300℃for 2 h.Such a pretreatment is beneficial for enhancing the catalytic activity as the T50(temperature required for 50% conversion)of Pt/Ca-P-O without pretreatment by 4% H2is 102℃,whereas that of Pt/Ca-P-O pretreated by 4% H2at 300℃is 72℃(Fig.1).Thus,in later experiments,we pretreated the catalysts by 4% H2at 300℃before reaction testing.

Fig.1 Effect of H2 pretreatment on CO conversions over Pt/Ca-P-O
The promotional effect of Na is illustrated in Fig.2.Four Pt catalysts with nominal Na contents of 0%,1%,2%,and 4%,respectively,were prepared by impregnatin g Ca-P-O support with NaOH and Pt(NH3)4(NO3)2.The actual Na contents(w(Na))were determined by ICP as 0%,1.09%,1.98% and 3.69%,respectively.The actual Pt contents(w(Pt))were deter mined by ICP as 1.54%,1.85%,1.26% and 1.31%,respectively.As shown in Fig.2,the T50values of these catalysts are 72,62,27,and 62℃,respectively.The data indicate that the activity of catalysts increases with the Na content,becoming the highest when the Na content is 2%,and then decreases as the Na content is further increased.
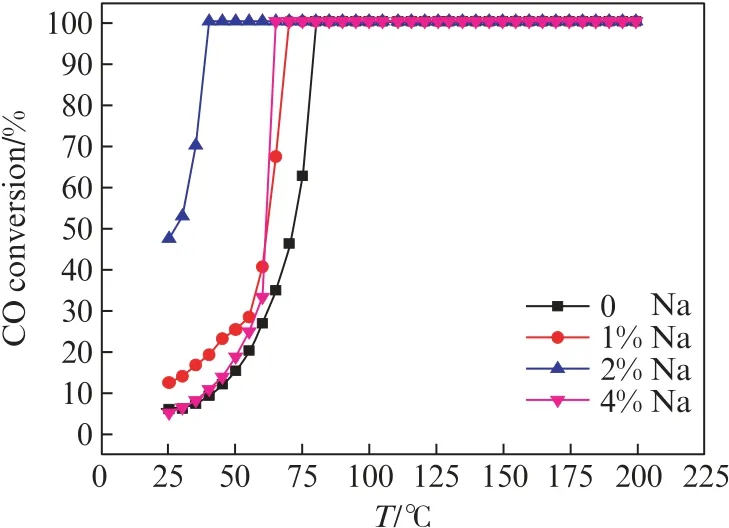
Fig.2 Effect of Na content on the catalytic performance of Pt/NaOH/Ca-P-O
To know whether the promotional effect of Na is general,we prepared several catalysts using different Na precursors,while keeping the same nominal Na content(w(Na)=2%).As shown in Fig.3,Na2CO3and NaOH are the best precursors for making active Pt catalysts.NaNO3stills shows a promotional effect,whereas NaCl has a detrimental effect when the reaction temperature is above 80℃,possibly due to the influence of Cl-.
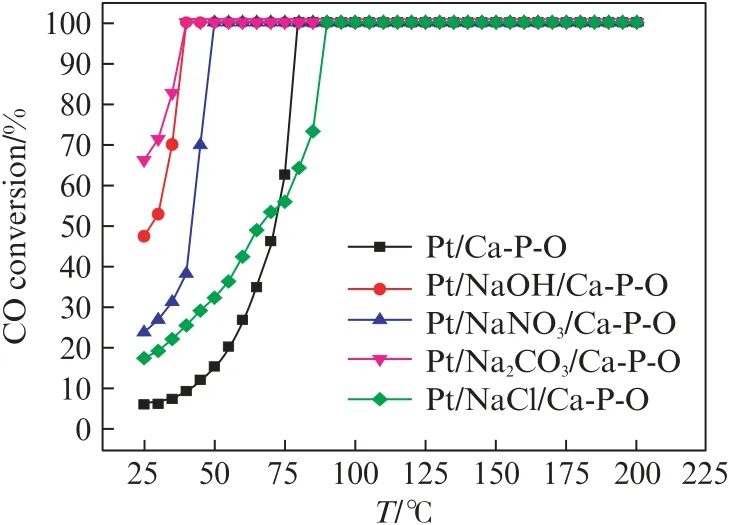
Fig.3 Effect of different Na-containing compounds on the catalytic performance of Na-doped Pt/Ca-P-O
2.2 Characterization
Fig.4 shows the XRD patterns of Pt/Ca-P-O and Pt/NaOH/Ca-P-O collected after reaction testing.
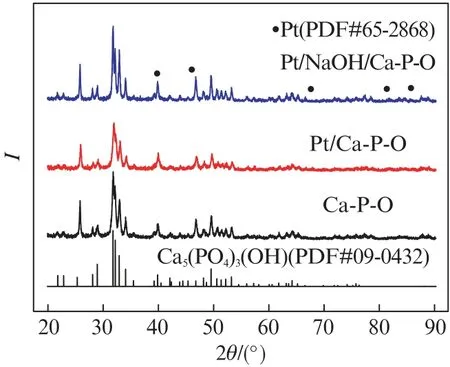
Fig.4 XRD pattern of Ca-P-O calcined at 500℃and supported Pt catalysts collected after reaction testing
The XRD pattern of Ca-P-O obtained by calcining commercial Ca-P-O at 500℃is also shown for comparison.As shown in Fig.4,the XRD patterns of Pt/Ca-P-O and Pt/NaOH/Ca-P-O are similar to that of Ca-P-O.Peaks corresponding to metallic Pt should appear at 2θ=39.7°,46.2°,67.4°,81.2°,and 85.6°(marked using filled circles in the figure)if Pt particles are big enough and the Pt content is high enough.Nevertheless,Pt peaks do not seem to show up for Pt/Ca-P-O and Pt/NaOH/Ca-P-O,possibly due to the low Pt contents and small Pt particles.
Fig.5 shows representative TEM images of Pt/Ca-P-O and Pt/NaOH/Ca-P-O collected after reaction testing.It can be seen that small Pt nanoparticles(1-3 nm)are highly dispersed on both supports.
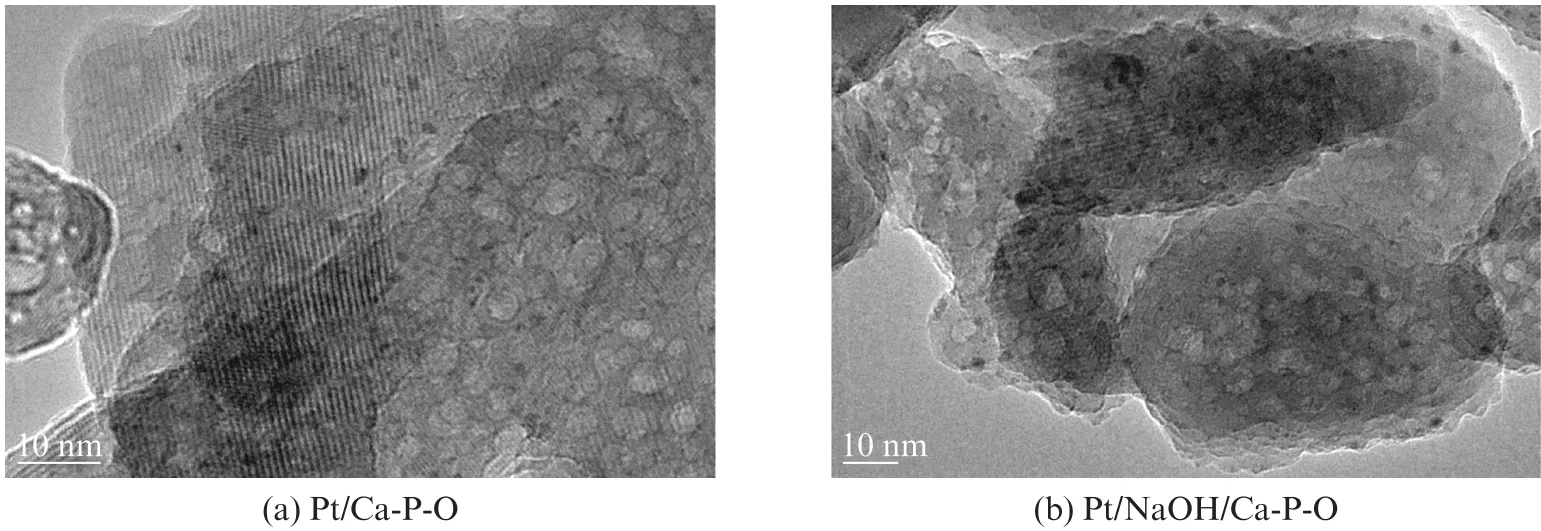
Fig.5 TEM images of Pt/Ca-P-O(a)and Pt/NaOH/Ca-P-O(b)collected after reaction testing
Fig.6 shows the XPS spectra of fresh Pt/Ca-P-O and Pt/NaOH/Ca-P-O(without undergoing reaction testing).The binding energy of Na 1s is located at 1 072.0 e V,and the Auger binding energy of Na is located at 497.4 e V.It is clear from the spectra that both catalysts contain O,Ca,P,and Pt,whereas Pt/NaOH/Ca-P-O additionally contains Na.The Pt peaks are too small to be seen clearly in Fig.6 because the data are obtained by fast scan,so we additionally studied the oxidation states of Pt by detailed XPS scanning.
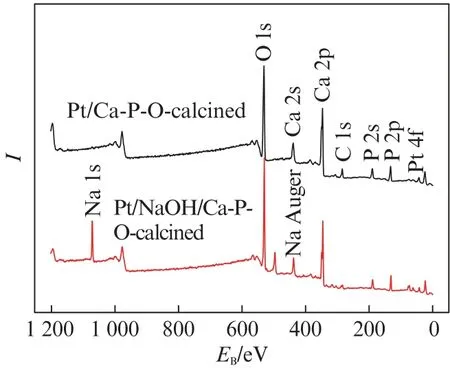
Fig.6 XPS spectra of fresh Pt/Ca-P-O and Pt/NaOH/Ca-P-O
Fig.7 shows Pt 4f XPS spectra of Pt/Ca-P-O collected at differen t stages.The main Pt species on fresh Pt/Ca-P-O(calcined in air at 500℃)are Pt4+(64.7% among all Pt species)and Pt2+(35.3%).No Pt0is detected.When Pt/Ca-P-O is reduced by 4% H2at 300℃,the relative proportions of Pt species become 28.9%(Pt0),38.6%(Pt2+),and 32.5%(Pt4+),indicating the partial reduction of Pt4+to Pt2+and further to Pt0.After undergoing reaction testing,some Pt0is reoxidized,as the relative proportions of Pt species become 19.3%(Pt0),44.5%(Pt2+),and 36.2%(Pt4+).
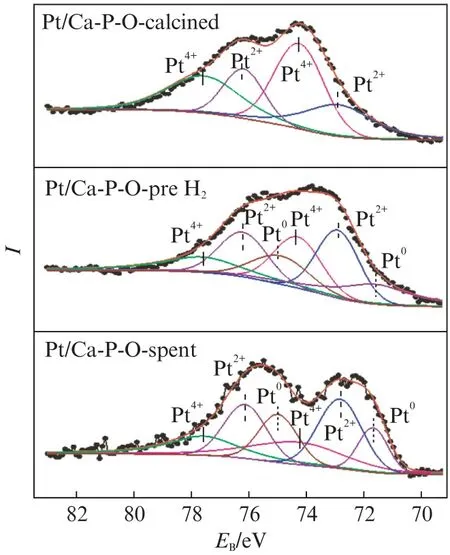
Fig.7 Pt 4f XPS spectra of Pt/Ca-P-O collected at different stages
Fig.8 shows the Pt 4f XPS spectra of Pt/NaOH/Ca-P-O collected at different stages.Fresh Pt/NaOH/Ca-P-O(calcined at 500℃)contains Pt2+only(but no Pt0or Pt4+).The pretreatment in 4% H2at 300℃leads to the reduction of some(43%)Pt2+to Pt0,and the relative proportion of Pt2+among all Pt species becomes 57%.After reaction testing,the relative proportions of Pt0and Pt2+are 34.9% and 65.1%,respectively.
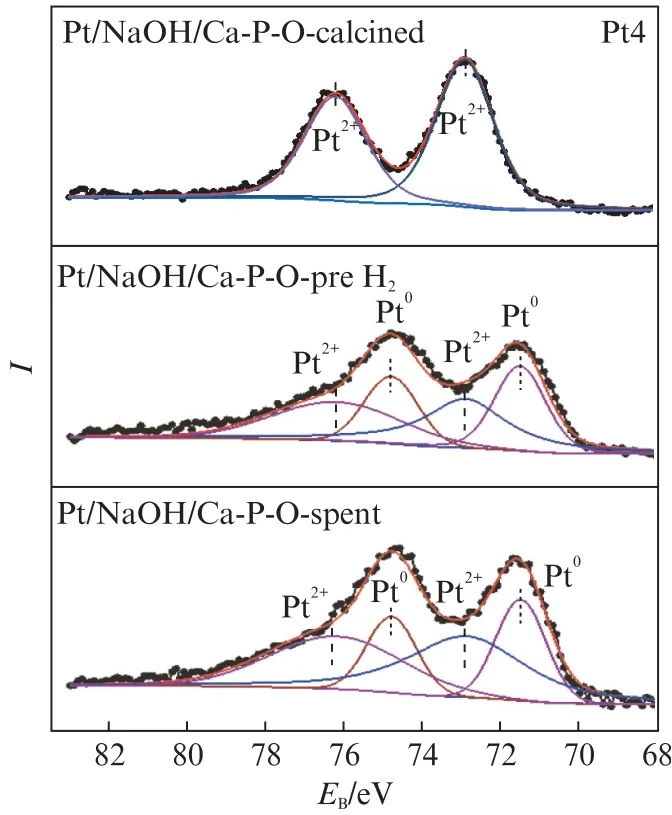
Fig.8 Pt 4f XPS spectra of Pt/NaOH/Ca-P-O collected at different stages
Comparin g the data from Fig.7 and Fig.8,it is generalized that the main Pt species on calcined catalysts are cationic:Pt/Ca-P-O mainly contains Pt4+(relative proportion:64.7%),whereas Pt/NaOH/Ca-P-O contains only Pt2+.The pretreatment in 4% H2at 300℃may reduce some cationic Pt,whereas the reaction testing involving CO and O2from room temperature to 300℃may oxidize some metallic Pt.In addition,the presence of Na in the catalyst may guarantee the presence of large proportions of Pt0and Pt2+(Tab.1).Tanaka et al.found that the addition of Na to Pt Mo/SiO2can decreased the proportion of oxidized Pt on the catalyst'surface[11],whereas others found that the ability of Pt4+for adsorbing CO is weaker than that of Pt0and Pt2+[12-15].Thus,the higher activity of Pt/NaOH/Ca-P-O is justified.
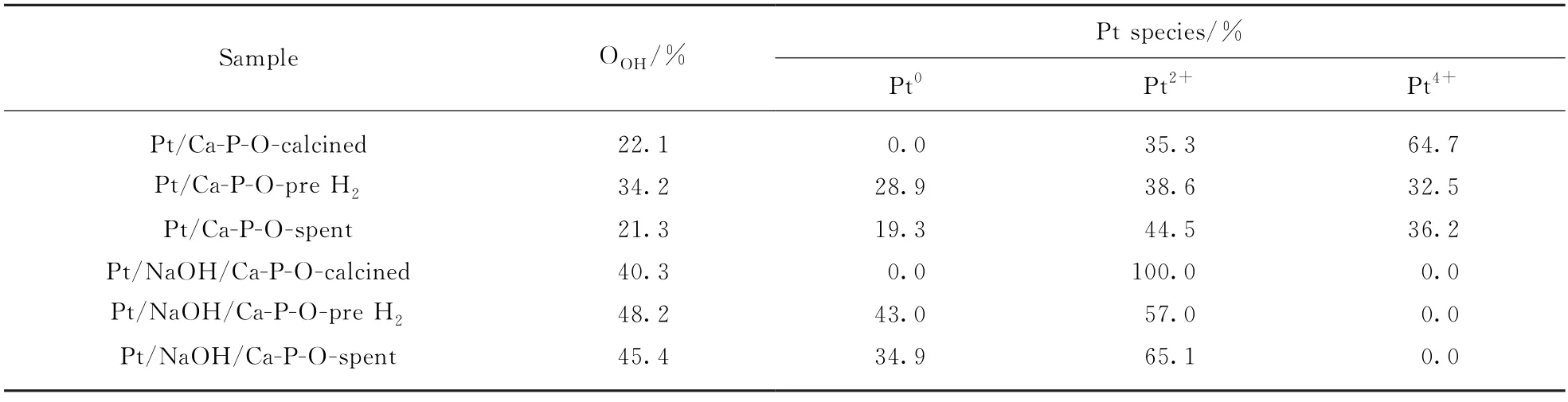
Tab.1 XPSresul ts of Pt/Ca-P-O and Pt/NaOH/Ca-P-Ocatalysts
Fig.9 shows the O1s XPS spectra of Pt/Ca-P-O collected after different stages.The oxygen species on Ca-P-O can be classified as hydroxyl oxygen(—OH)and non-hydroxyl oxygen(oxygen in phosphate).The non-hydroxyl oxygen can be further classified into bridging oxygen(P-O-P)and nonbridging oxygen(P-O).In the literature,the binding energy of O in P-O-P is located at 532-533 e V,that in —OH is located at 531-532 eV,and that in P-O is located at 530-531 eV.As shown in Fig.9,the binding energies of O in P-O-P,—OH,and P-O are (532.7±0.2),531.5,and (530.3±0.2)e V,respectively.The relative proportions of hydroxyl oxygen among all O species for fresh Pt/Ca-P-O,Pt/Ca-P-O collected after reduction in 4% H2,and Pt/Ca-P-O collected after reaction testing are 22.1%,34.2%,and 21.3%,respectively.
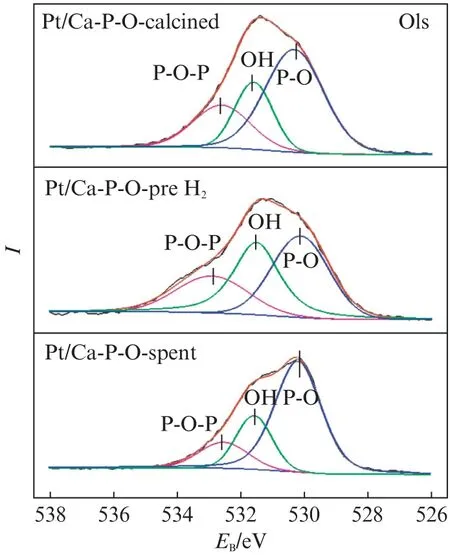
Fig.9 O1s XPS spectra of Pt/Ca-P-O collected after different stages
Fig.10 shows the O1s XPS spectra of Pt/NaOH/Ca-P-O collected after different stages.The relative proportions of hydroxyl oxygen among all O species for fresh Pt/NaOH/Ca-P-O,Pt/NaOH/Ca-P-O collected after reduction in 4% H2,and Pt/NaOH/Ca-P-O collected after reaction testing are 40.3%,48.2%,and 45.4%,respectively.The data indicate that the presence of Na can guarantee the presence of more hydroxyl on catalyst surface.In addition,the relatively proportion of hydroxyl oxygen decreases a little(from 48.2% to 45.4%)after reaction testing for Pt/NaOH/Ca-P-O,whereas it decreases more obviously(from 34.2% to 21.3%)for Pt/Ca-P-O.It was reported that the addition of Na is beneficial for the presence of hydroxyl on catalyst surface[5],whereas hydroxyl can react with adsorbed CO to form CO2[16].Thus,the higher activity of Pt/NaOH/Ca-P-O can be justified.
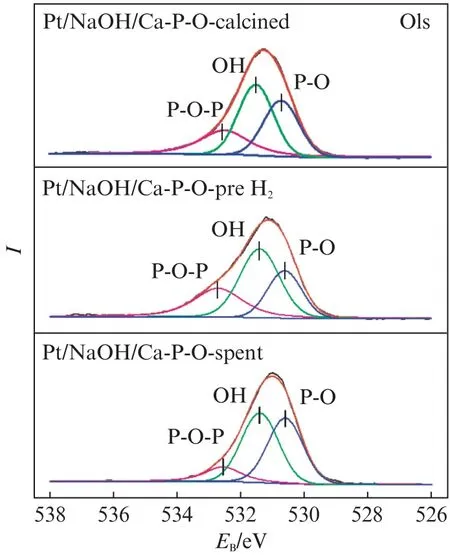
Fig.10 O1s XPS spectra of Pt/NaOH/Ca-P-O collected after different stages
Fig .11 shows the CO2-TPD results.CO2,being an acidic molecule,can adsorb onto basic sites of a solid catalyst and get desorbed as the catalyst is heated.Thus,the desorption peak area can indicate the amount of basic sites on a catalyst,and the peak position can indicate the strength of basic sites.As shown in Fig.11,Ca-P-O shows two peaks at 135 ℃and 180 ℃,respectively,and the total amount of basic sites is determined as 97.3μmol/g.The CO2desorption peaks on Pt/Ca-P-O are at 120 ℃and 196℃,and the total amount of basic sites is 70.4μmol/g.For comparison,CO2desorption peaks on Pt/NaOH/Ca-P-O located at 110 and 201℃are obviously smaller,especially for the peak at 201℃.The total amount of basic sites is 28.8μmol/g.We can see from the CO2-TPR data that the presence of less amount of basic sites on Pt/NaOH/Ca-P-O is advantageous for catalytic CO oxidation because the generated CO2can be desorbed more easily.
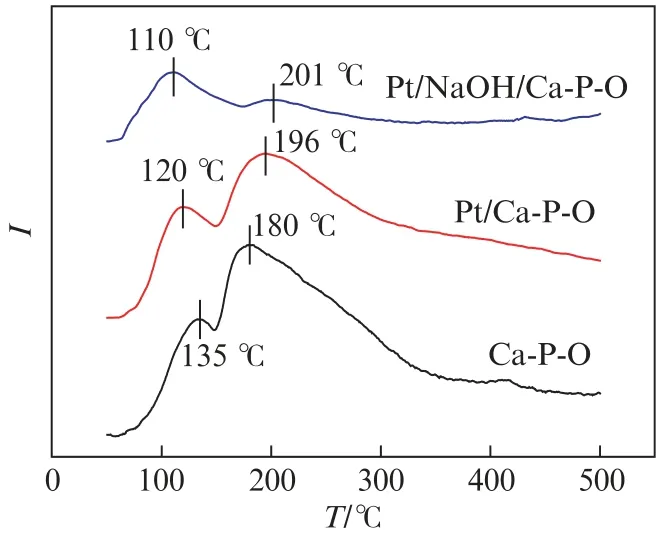
Fig.11 CO2-TPD profiles of the samples
In summary,the presence of a small amount of Na(w(Na)=2%)in Pt/Ca-P-O was found to promote th e catalyst for catalyzing CO oxidation.Both Na2CO3and NaOH are good precursors for making pro moted catalysts.The addition of NaNO3to the catalyst has a medium promotional effect,whereas th e addition of NaCl leads to lower activity.The beneficial roles of adding NaOH are:1)to maintain th e relative proportions of Pt0and Pt2+species,after H2pretreatment and during reaction testing;2)to keep the concentrations of surface hydroxyls,after H2pretreatment and during reaction testing;3)to decrease the amount of basic sites,leading to less adsorption of CO2during the reaction.The factors collectively lead to a more active catalyst for CO oxidation.

