Lights for epilepsy: can photobiomodulation reduce seizures and offer neuroprotection?
Napoleon Torres-Martinez,Stephan Chabardes,John Mitrofanis
Abstract Epilepsy is synonymous with individuals suffering repeated “fits” or seizures.The seizures are triggered by bursts of abnormal neuronal activity,across either the cerebral cortex and/or the hippocampus.In addition,the seizure sites are characterized by considerable neuronal death.Although the factors that generate this abnormal activity and death are not entirely clear,recent evidence indicates that mitochondrial dysfunction plays a central role.Current treatment options include drug therapy,which aims to suppress the abnormal neuronal activity,or surgical intervention,which involves the removal of the brain region generating the seizure activity.However,~30% of patients are unresponsive to the drugs,while the surgery option is invasive and has a morbidity risk.Hence,there is a need for the development of an effective non-pharmacological and non-invasive treatment for this disorder,one that has few side effects.In this review,we consider the effectiveness of a potential new treatment for epilepsy,known as photobiomodulation,the use of red to near-infrared light on body tissues.Recent studies in animal models have shown that photobiomodulation reduces seizure-like activity and improves neuronal survival.Further,it has an excellent safety record,with little or no evidence of side effects,and it is non-invasive.Taken all together,this treatment appears to be an ideal treatment option for patients suffering from epilepsy,which is certainly worthy of further consideration.
Key Words:cell death;gliosis;inflammation;infrared;mitochondria;non-pharmacological;red;seizure
Introduction
Epilepsy is a non-communicable chronic neurological disorder that is characterized by spontaneous recurrent seizures.These seizures can manifest clinically in several ways,for example,as uncontrolled shaking movements involving much of the body with loss of consciousness (tonic-clonic seizure),as shaking movements involving only part of the body with variable levels of consciousness (focal seizure),or as a subtle momentary loss of awareness(absence seizure).Most of these episodes last only a few minutes.The diagnosis of epilepsy is made if there are two unprovoked seizures of unknown cause.By contrast,non-recurring seizures -for example,after alcohol withdrawal or low blood sugar levels -are not considered epilepsy.In most cases,the causes of epilepsy are idiopathic (~60%),but there are instances when the cause is clearer,with either a genetic or acquired (e.g.birth defect,trauma,stroke,tumor,infection) component being evident.Epilepsy can occur at any age but its incidence is higher in the youngest (<1 year old) and oldest (>85 years old) age groups.Overall,it has been esti mated that~50 people per 100,000 per year will be diagnosed with epilepsy (Fisher et al.,2017;Devinsky et al.,2018;Thijs et al.,2019;Beghi,2020;Rho and Boison,2022).
In the sections that follow,we will explore what is known currently of the neural mechanisms that underpin seizures,highlighting the involvement of the mitochondria.We then discuss the current treatments available for the disorder,together with their limitations.Finally,we consider the evidence that photobiomodulation,the use of red to near-infrared light (λ=600–1000 nm) on body tissues,has been shown to improve many of the hallmarks of the disorder,namely the mitochondrial dysfunction leading to abnormal neuronal acti vity and death.We suggest that photobiomodulation can form an effective,safe,non-pharmacological,and non-invasive treatment option for patients with epilepsy.
Search Strategy and Selection Criteria
An electronic search of the PubMed database for literature published up to October 2022 with the key words “epilepsy”,“photobiomodulation”,“low level light therapy” and “low level laser therapy” was performed.The results were further screened by ti tle and abstract to include articles on animal models and patients that were directly relevant to these key search items.The articles that were not specific for the key words were excluded.
The Mechanisms of Seizure
The neural substrate that underpins epileptic seizures centers on an abnormal,synchronous and excessive pattern of neuronal firing in the cerebral cortex,in particular,in regions within,and surrounding,the hippocampus (Figure 1).This abnormal firing can be detected by electroencephalogram (EEG)recordings,either during (ictal) or after the episode (post-ictal).Several contributing factors may trigger this abnormal firing pattern,including a dysregulation of the ion channels in the principal (seizure-generating)neurons,a dysfunction of the local inhibitory interneurons,reactive gliosis,and/or activation of inflammatory pathways (Figure 1;Thijs et al.,2019;Nickels and Noe,2021;Hayatdavoudi et al.,2022).In addition,epilepsy is associated with a considerable amount of neuronal death in and around the site of abnormal neuronal firing (Figure 1;Thijs et al.,2019;Folbergrová and Kunz,2012).There is a selective loss of inhibitory interneurons (or principal neurons driving the interneurons) that manifests ultimately in a decrease of the normal inhibition of the dentate cells (i.e.,principal neurons) in the hippocampus,thus creating the pre-conditions for focal hyper-excitability.Indeed,this neuronal loss is thought to contribute to resistance to drug therapy found in patients (see below) (Folbergrová and Kunz,2012).
There is an ever-increasing body of recent evidence indicating that the abnormal neuronal firing patterns and the neuronal death evident in epilepsy relate closely to a dysfunctional neuronal metabolism,in particular,impaired function of the mitochondria,the so-called engine room of the neurons (Figure 1;Folbergrová and Kunz,2012;Rho and Boison,2022).All body cells depend on adenosine triphosphate (ATP) to perform their required functions.Certain types of cells,such as neurons,have a greater requirement for a ready supply of energy than others.In addition,neurons are terminally differenti ated cells and hence largely lack a capacity for regeneration.These features may explain the vulnerability of the central nervous system to mitochondrial dysfunction and the frequent occurrence of epileptic seizures.The mitochondria provide the energy,in the form of ATP that drives so many intrinsic neuronal functions,together with maintaining internal calcium homeostasis and the generation of reactive oxygen species.Hence,if the mitochondria within neurons become defective,then so many intrinsic functions can be affected,including patterns of neuronal firing and synaptic transmission,together with neuronal homeostasis and survival.Indeed,mitochondrial dysfunction has been reported in sites of seizure focus in patients suffering from epilepsy,as well as in a range of animal models of the disorder (Mueller et al.,2001;Waldbaum and Patel,2010;Folbergrová and Kunz,2012;Wesół-Kucharska et al.,2021;Rho and Boison,2022).Inhibitory interneurons appear to be more suscepti ble than the principal neurons to the effects of mitochondrial-related energy deficiency,particularly complex I deficiency (Kann et al.,2011).
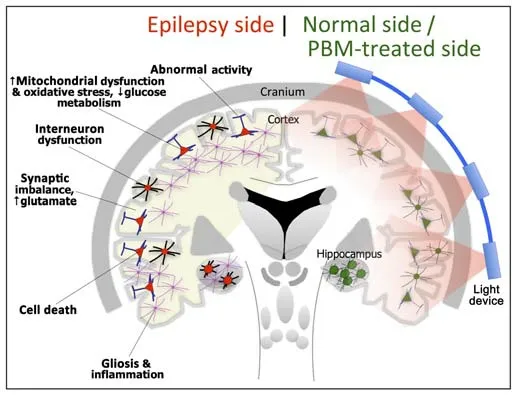
Figure 1|Schematic diagrams of the major abnormalities evident in epilepsy (left side) as compared with normal,and after photobiomodulation treatment (right side).
There is evidence that defects affecting the mitochondrial respiratory chain,as seen in mitochondrial disease,can give rise to diseases affecting the central nervous system.Irrespective of the genetic defect,epilepsy is common.Epileptic seizures may be a presenting or late feature of mitochondrial encephalopathy,and virtually any seizure semiology and combinations can occur.Those patients are at high risk of developing drug-resistant epilepsy or status epilepticus (Bindoff et al.,2012)
When considering the mechanisms that lead to neuronal death after mitochondrial dysfunction,these involve a cascade of events,including a reduction in glucose metabolism and ATP levels,together with the generation of oxidative stress and toxic levels of reactive oxygen species.The precise mechanisms that lead to the abnormal and excessive neuronal firing after mitochondrial dysfunction are less clear,but several have been suggested,including;(1) there is a disruption to the calcium homeostasis within the principal neurons that then leads to abnormal activity patterns (Figure 1;Kann and Kovács,2007);(2) when the local inhibitory neurons are affected,the inhibitory potenti al is reduced,facilitating a spread of excitation across the neuronal network (Figure 1);and (3) there is an increase in the release of glutamate into the extracellular matrix by surrounding astrocytes (Figure 1),as well as a disruption to the glutamate-aspartate transporter system.It should be noted that the neurons suffering mitochondrial dysfunction are at a further disadvantage in that because their means of producing energy has been compromised,they do not have the ATP required to repair themselves after the damage caused by the bouts of excessive firing.These neurons would ultimately become more dysfunctional and distressed,and suffer cell death (Waldbaum and Patel,2010;Folbergrová and Kunz,2012;Wesół-Kucharska et al.,2021;Rho and Boison,2022).
Current Treatments
The current mainstay treatments for epilepsy include both medical and surgical options.In nearly all cases,the first-line treatment for patients is an anti -epileptic drug therapy that aims to suppress the abnormal and excessive firing of neurons.There are a range of different drugs available and these target membrane-bound ion channels and transporters on the neurons,as to reduce excitatory and/or increase inhibitory neurotransmission.For example,some of the most commonly used drugs include those that are involved in sodium channel blockade (carbamazepine,valproate),calcium channel blockade,GABA agonism/potentiation (benzodiazepines,barbiturates,valproate),NMDA receptor blockade (felbamate) and AMPA receptor blockade(topiramate) (Cook and Bensalem-Owen,2011;Devinsky et al.,2018).
However,in approximately 30% of cases,this medical treatment is ineffective in reducing the amount of seizures experienced by patients;further,many patients suffer severe side effects from the drugs,including drowsiness and cognitive impairment.For patients that do not respond to drug therapy and their seizures remain uncontrolled,surgical intervention can be considered.The procedure aims to reduce the amount of seizures by removing the brain region that generates the abnormal neuronal activity.Although it is highly invasive,it remains the gold-standard treatment for focal mesial temporal or neocortical epilepsy;it does however,have limited application in multiple focus epilepsy or when the epileptic zone remains hidden or near eloquent areas.In addition to these two mainstay treatments,some patients respond well to vagal nerve stimulation.This method serves to prevent seizures by sending regular pulses of electrical energy to the brain via the vagus nerve(Devinsky et al.,2018;Thijs et al.,2019;Rho and Boison,2022).In addition,closed loop responsive neurostimulation of areas in the brain and deep brain stimulation of the anterior nucleus of the thalamus can reduce the number of seizures by up to 40%.However,none of these treatments have an effect on the underlying pathological causes.There are also some indications that changes in diet can be beneficial to some patients,in particular a ketogenic diet that is made up of a high-fat and low-protein and carbohydrate daily regime (Rho and Boison,2022).
Notwithstanding these current treatment options available to patients,there remain a large number of patients that are unresponsive to drug therapy and/or seek an effective non-invasive and non-pharmacological treatment to reduce the frequency of their seizures,one that is easy to use and has few or no side effects.Indeed,taken all together,less than 5% of patients remain seizure free using the currently available treatments (Ryvlin et al.,2021).
Photobiomodulation: the Light
In this context,there is a new treatment option that has been receiving considerable interest across the clinical,scientific,and wider communities.Over the last 70 years or so,many previous studies have reported that this treatment has a substantial influence on the functional activity and the survival of neurons.Further,it has an impeccable safety record,with little or no evidence of side effects or toxicity on body cells,it is non-invasive and the devices are easy to use.This treatment is known as photobiomodulation,the use of red to near-infrared light (λ=600–1000 nm) on body tissues (Hamblin,2016),and appears to be an ideal treatment option for epilepsy (Cardoso et al.,2022).
Previous studies have reported that photobiomodulation -across a wide range of animal models of disease,as well as in humans -has a differenti al effect on neurons,dependent on their state of homeostasis,whether they are distressed or healthy (Figure 2).
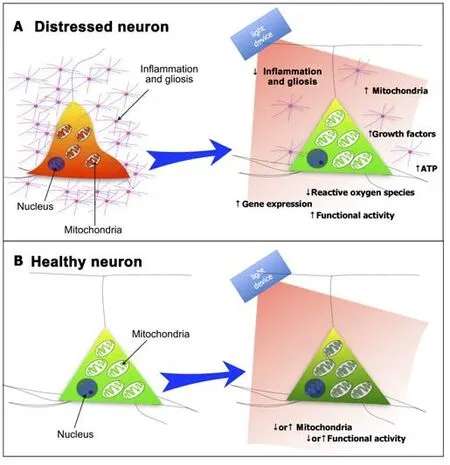
Figure 2|Schematic diagrams of the effect of photobiomodulation on (A) distressed neurons and (B) healthy neurons.
Distressed Neurons
If the neuron is under severe distress,photobiomodulation -after being absorbed by photoreceptors found mainly among the mitochondria,for example,cytochrome c oxidase and/or interfacial nano water -works to stimulate the production of more ATP energy that drives many intrinsic neuronal functions (Figure 2A).One could view light as a drug and the photoreceptors as the receptors to the drug,with the difference being that light is a purely natural phenomenon.In addition to these short-term energy gains,photobiomodulation also induces more long-term cellular changes,by activating the expression of various functional and protective genes.In particular,photobiomodulation prompts the expression of various growth factors,for example,glial-derived neurotrophic factor and brain-derived neurotrophic factor,both of which have been shown to increase the survival of neurons.In essence,photobiomodulation makes the neurons “healthier”,by restoring their function and making them more resistant to distress and disease.Photobiomodulation not only has a direct effect on neurons,but it also has an impact on the hypertrophy and proliferation of the resident glial cells and inflammation (Figure 2A).Many studies have shown that photobiomodulation reduces gliosis and/or inflammation in animal models of disease,trauma,and aging.Through these mechanisms -namely,a stimulation of mitochondria,an increase in ATP levels,and activation of protective genes within neurons,together with a reduction in gliosis and inflammation -photobiomodulation is thought to increase the survival of neurons in distress.Such beneficial outcomes,referred to commonly as disease-modifying or neuroprotective,have been reported in a range of animal models of disease or trauma,from traumatic brain injury to stroke and from multiple sclerosis to Alzheimer’s disease and Parkinson’s disease (Hamblin,2016;Mitrofanis,2019).
Healthy Neurons
When neurons are relatively healthy and functioning normally,not suffering from distress,photobiomodulation tends to have a somewhat different effect;in these cases,there is no need for defense mechanisms -such as more energy and/or the expression of protective genes -to be activated.Many previous studies have reported that photobiomodulation when applied directly to otherwise healthy neurons eitherin vitroorin vivo,can change their functional activity.However,this functional change appears to vary;depending on the cell group and/or system being treated,together with the different experimental conditions (e.g.temperature),photobiomodulation can induce either an increase or a decrease in neuronal acti vity (Figure 2B;Fekete and Zatonyi,2020).There are many reports of photobiomodulation treatment being associated with an increase in functional neuronal acti vity,from bothin vivoandin vitrostudies.For example,transcranial photobiomodulation has been shown to generate an increase in the oscillations of various brain waves,for example,α,β and γ waves (from EEG studies;Vargas et al.,2017;Zomorrodi et al.,2019;Jahan et al.,2019;Shan et al.,2021;Wang et al.,2021),an increase cytochrome c oxidase oxidation and hemoglobin oxygenation (Wang et al.,2017;Saucedo et al.,2021),or an increase in the functional connecti vity of different cortical regions in human subjects (from fMRI studies;Chao,2019;Dmochowski et al.,2020).Further,fromin vitrostudies,photobiomodulation treatment has been shown to increase the acti vity of neurons in thalamocortical slices (Cayce et al.,2010).There are also just as many reports of photobiomodulation treatment being associated with a clear decrease in functional neuronal activity.For example,in the neural pathways associated with pain,photobiomodulation has been shown to inhibit the central translation of nociceptive information,providing something similar to a nerve conduction block.In this system,photobiomodulation generates a decrease in mitochondrial membrane potenti al and ATP levels,and inhibition of fast axonal flow,as well as induces clustering of mitochondria in axonal varicosities,these being clear signs of delayed neural conduction (Chow and Armati,2016).Using transcranial magnetic stimulation,photobiomodulation reduces the size of motor-evoked potentials when applied to the motor cortex (Konstantinovic et al.,2013).With functional magnetic resonance imaging,photobiomodulation has been found to suppress activity in the particular cortical areas activated by certain tasks (i.e.,task-positive),such as finger-tapping (El Khoury et al.,2019) or verbal memory (Vargas et al.,2017).Further,there is evidence that photobiomodulation treatment yields a general increase in the levels of inhibitory (i.e.,γ-aminobutyric acid) and a decrease in the levels of excitatory (e.g.,glutamate) neurotransmitters in the cortex (Ahmed et al.,2008;Feng et al.,2011).One could view this inhibitory effect of photobiomodulation on healthy neurons as neuroprotective,in that it serves to prevent any excessive firing and subsequent excitotoxicity.In addition to all these reports,photobiomodulation has been shown to induce both excitatory and inhibitory effects in the same experimental preparations.In the somatosensory cortex of rats,electrophysiological recordings indicate that photobiomodulation can increase functional activity similar to that evident after tacti le stimulation,but this is followed by a period of inhibition(Cayce et al.,2011).Similar findings to these have been reported in the motor axons of crayfish,where photobiomodulation induces a membrane excitation during the treatment,followed by a period of inhibition (Zhu et al.,2022).
A potential unifying hypothesis,accounting for this differential effect of photobiomodulation -whether excitatory or inhibitory -is that it helps restore the overall balance of function and connectivity across any given system,particularly if it is dysfunctional.To restore the balance,photobiomodulation may inhibit some circuits,while suppressing others (Naeser et al.,2020;Mitrofanis and Henderson,2020).For example,in patients suffering from either traumatic brain injury or Alzheimer’s disease,both of which have abnormal patterns of functional connectivity between cortical regions,transcranial photobiomodulation helps correct these imbalances,restoring the connecti vity between regions to “normal” levels (Chao,2019;Naeser et al.,2020).
Effect of Photobiomodulation in Epilepsy
There have been several previous reports,bothin vivoandin vitro,exploring the effect of photobiomodulation in animal models of epilepsy.In a pentylenetetrazole-induced rat model,one that mimics convulsive status epilepticus (Tsai et al.,2020,2022),photobiomodulation (808 nm) has been shown to reduce the amount of seizure-like activity,the mortality and the patterns of neuronal death within the hippocampus,cortex,hypothalamus,and thalamus.In a pilocarpine-induced rat model,another model that mimics status epilepticus (Radwan et al.,2009),photobiomodulation (830 nm)treatment alters the levels of a range of amino acids,including glutamic acid,glutamine,glycine,aspartate,and taurine in the cortex and hippocampus;all these levels return to normal levels soon after treatment.In a rat model of stroke,one that leads to epileptiform activity in the majority of cases,photobiomodulation (780 nm) has been shown to reduce substantially this activity across the cortex and thalamus (Vogel et al.,2021).In yet another model of epilepsy -using bicuculline methiodide,a selective GABAA antagonist,injected into the hippocampus -photobiomodulation (830 nm)treatment reduces considerably the number of spike potenti als in Mongolian gerbils (Furuyama et al.,2019).Further,anin vitrostudy has reported that light at a wavelength of 1550 nm,one just beyond the wavelengths traditionally associated with photobiomodulation (600–1000 nm),can reduce epileptiform neural activities in rat cortical slices (Xia and Nyberg,2019).Taken all together,these findings fit in well with the observations that sunlight -which contains a broad spectrum of wavelengths,including those of photobiomodulation -imparts many beneficial effects on people with epilepsy(Baxendale,2011).
We suggest that,as a starting point for use in a clinical trial,patients with epilepsy could use,daily,a transcranial photobiomodulation helmet;the daily use of photobiomodulation may act to suppress the onset of abnormal acti vity in the cortex.Several types of helmets have been used successfully in,for example,Alzheimer’s disease (Saltmarche et al.,2017) and Parkinson’s disease (Hamilton et al.,2018,2019) patients;the parameters for these helmets include 670 nm and 810 nm wavelengths,set at a frequency of either 10 Hz or 40 Hz,with power densities of 15–23 mW/cm2(Saltmarche et al.,2017;Hamilton et al.,2018,2019).
We should add that there would be no issue with the light from the photobiomodulation helmet device reaching through to the brain,at least to the superficial layers,including the cerebral cortex.Many previous studies have reported that photobiomodulation can penetrate from 30–50 mm of body tissues and most areas of the cortex are well within that range (~10–15 mm) (Hamblin,2016;Mitrofanis,2019).Further,several studies,using either EEG or fMRI,have shown that transcranial photobiomodulation can change neuronal activity considerably,indicating that the light can certainly reach the brain (see above;Vargas et al.,2017;Chao,2019;El Khoury et al.,2019;Zomorrodi et al.,2019;Jahan et al.,2019;Dmochowski et al.,2020;Shan et al.,2021;Wang et al.,2021).
Conclusions
One of the major issues associated with the treatment of epilepsy is that many patients do not respond to,and/or suffer major side effects from,the drug treatment;further,the surgery option remains highly invasive and has a risk of morbidity.For these patients,there are few effective alternatives,particularly non-pharmacological and non-invasive.In this context,there are some encouraging,early findings in animal models of epilepsy that photobiomodulation treatment -by targeting the key source of the cellular dysfunction in epilepsy,namely the mitochondria -is effective in reducing two of the mainstays of the disorder: that is (1) the abnormal neuronal firing and seizure-like acti vity and (2) the neuronal death.Together with these beneficial outcomes,photobiomodulation is a treatment that is non-pharmacological and non-invasive,and the devices are easy to use;further,it has an excellent safety record with little or no evidence of side effects.When taking all these positive pre-clinical results together,photobiomodulation appears to be an ideal alternative treatment option for patients suffering from epilepsy,and the stage is set for the development of a large-scale clinical trial.
Acknowledgments:We thank Fonds de Dotation (FDD) Clinatec and COVEA France for supporting this work.
Author contributions:All authors contributed to the writing of this manuscript.
Conflicts of interest:None of the authors have a conflict of interest.Editor note: JM is the Section Editor of Neural Regeneration Research.He is not involved in decisions about the paper which he has written himself or has been written by family members or colleagues or whoever relates to products or services in which the editor has an interest.The submission is subject to the journal’s standard procedures,with peer-review handled independently of the relevant editor and their research groups.
Open access statement:This is an open access journal,andarticles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Bystanders or not? Microglia and lymphocytes in aging and stroke
- Alzheimer’s disease risk after COVID-19: a view from the perspective of the infecti ous hypothesis of neurodegeneration
- Serine and arginine rich splicing factor 1: a potenti al target for neuroprotection and other diseases
- Can glial cells save neurons in epilepsy?
- The landscape of cognitive impairment in superoxide dismutase 1-amyotrophic lateral sclerosis
- CMT1A current gene therapy approaches and promising biomarkers

