Molecular mechanisms underlying the neuroprotection of environmental enrichment in Parkinson’s disease
Tamara Andrea Alarcón ,Sarah Marti ns Presti -Silva, ,Ana Paula Toniato Simões ,Fabiola Mara Ribeiro,,Rita Gomes Wanderley Pires,
Abstract Parkinson’s disease is the most common movement disorder,affecting about 1% of the population over the age of 60 years.Parkinson’s disease is characterized clinically by resting tremor,bradykinesia,rigidity and postural instability,as a result of the progressive loss of nigrostriatal dopaminergic neurons.In addition to this neuronal cell loss,Parkinson’s disease is characterized by the accumulation of intracellular protein aggregates,Lewy bodies and Lewy neurites,composed primarily of the protein α-synuclein.Although it was first described almost 200 years ago,there are no diseasemodifying drugs to treat patients with Parkinson’s disease.In addition to conventional therapies,non-pharmacological treatment strategies are under investigation in patients and animal models of neurodegenerative disorders.Among such strategies,environmental enrichment,comprising physical exercise,cognitive stimulus,and social interactions,has been assessed in preclinical models of Parkinson’s disease.Environmental enrichment can cause structural and functional changes in the brain and promote neurogenesis and dendritic growth by modifying gene expression,enhancing the expression of neurotrophic factors and modulating neurotransmission.In this review article,we focus on the current knowledge about the molecular mechanisms underlying environmental enrichment neuroprotection in Parkinson’s disease,highlighting its influence on the dopaminergic,cholinergic,glutamatergic and GABAergic systems,as well as the involvement of neurotrophic factors.We describe experimental pre-clinical data showing how environmental enrichment can act as a modulator in a neurochemical and behavioral context in different animal models of Parkinson’s disease,highlighting the potential of environmental enrichment as an additional strategy in the management and prevention of this complex disease.
Key Words:acetylcholine;brain-derived neurotrophic factor;dopamine;environment enrichment;gamma-aminobutyric acid;glial cell line-derived neurotrophic factor;glutamate;molecular mechanisms;Parkinson’s disease
Introduction
Parkinson’s disease (PD) is the most frequently observed movement disorder,affecting around 1% of the population over the age of 60 (Sprenger and Poewe,2013).The increase in life expectancy due to medical advances has led to a substanti al increase in the number of people affected by this disease (Kalia and Lang,2016).PD is characterized primarily by motor impairments such as tremor,rigidity,and bradykinesia,as a result of a complex mechanism that involves a slow and progressive loss of nigrostriatal dopaminergic neurons(Hirsch et al.,1988).Yet,other dopaminergic and non-dopaminergic pathways are also affected,albeit to a lesser extent (Fox,2013).In addition to the loss of dopaminergic neurons,this neurological disorder is characterized by a second pathological marker,which is the accumulation of α-synuclein protein that leads to the formation of inclusions named intracellular Lewy bodies or Lewy neurites.Lewy bodies consist mainly of misfolded protein aggregates that may arise from an ascending gradient that originates at the bottom of the brain stem,expands to the basal ganglia,and ends in the cerebral cortex(Braak et al.,2003).
Although PD was first described almost 200 years ago,despite all intensive research,the cause of neuronal loss remains unclear (McDonald et al.,2018a).The current view is that PD eti ology involves a complex interaction between genetic and environmental factors (Oczkowska et al.,2013).Several genes play a role in the development of PD,such as those coding for α-synuclein,Parkinson protein 7,parkin,and phosphatase and tensin homologue-induced kinase 1 (PINK1) (Oczkowska et al.,2013).Data from genetic studies highlight the role of mutations in the α-synuclein and parkin genes in the pathogenesis of PD;however,genetic defects account for only a small fraction of PD cases (Oczkowska et al.,2013).Regarding the biological factors involved in PD etiology,several biochemical key events have been consistently investigated inpostmortemhuman tissues,in vitrohuman cell lines,human brain organoids,and in animal models of PD,including mitochondrial dysfunction,impairment of protein clearance(involving ubiquitin-proteasome and autophagy-lysosomal systems),neuroinflammation,and oxidative stress (Fox et al.,2018).
At present,PD is incurable,causing irreversible neuronal damage associated with symptoms that worsen progressively.There are a few therapeutic options to treat PD patients (Smith et al.,2012).The gold standard treatment is based on dopamine-replacement therapies through supplementation with the biochemical precursor of dopamine,levodopa.
Levodopa is the most effective drug in the management of PD when associated with carbidopa or benserazide,aromatic acid decarboxylase inhibitors that prevent its peripheral metabolism and markedly reduce the risk of nausea (Trenkwalder et al.,2019).However,over time,patients start to notice a loss of effect and an increase in the dose of levodopa is sorely needed.To further complicate matters,levodopa is closely associated with several side effects,including irreversible levodopa-induced dyskinesia(Pandey and Srivanitchapoom,2017).Moreover,levodopa does not modify PD progression,which highlights the need for novel therapies to treat PD patients.
Effective therapy for PD patients should encompass an individualized multidisciplinary approach (Fox et al.,2018).In addition to conventional therapies,non-pharmacological treatment strategies such as exercise,rehabilitation,and social support are under investigation in patients and animal models of neurodegenerative disorders,if they act synergistically when associated with pharmacological therapeutic agents (Ball et al.,2019).Among such strategies,environmental enrichment (EE),comprising physical exercise,cognitive sti mulus,and social interactions,has been widely assessed in preclinical studies.In addition,several studies have investigated the effect of EE exposure in animal models of PD (Fischer,2016).
In this review,we focus on the current knowledge about the molecular mechanisms underlying the neuroprotection of EE in Parkinson’s disease.We describe experimental pre-clinical data showing how EE can act as a modulator in a neurochemical and behavioral context in different animal models of PD,highlighting the potenti al of EE as an additional strategy in the management and prevention of this complex disease.
Search Strategy
The PubMed database was used to search available literature,using the following combinations of keywords to select articles: environmental enrichment AND Parkinson’s disease AND neurotransmitters;environmental enrichment AND Parkinson’s disease AND neurotrophins;environmental enrichment AND Parkinson’s disease AND α-synuclein.Search was done between January and September 2022.
Environmental Enrichment and Parkinson’s Disease
The environment is the surrounding conditions and elements with which a living being interacts,which may affect metabolism and behavior.Indeed,when Donald Hebb compared the cognitive behavior of rats raised at home as pets to that of laboratory animals,he observed that the former performed better in tests requiring problem-solving abilities than the latter,giving rise to the concept of the enriched environment as cited in (McDonald et al.,2018b).Although originally described as a “combination of inanimate and social stimulation” (Rosenzweig et al.,1978),EE currently refers to “housing conditions that enhance sensory,cognitive,and motor stimulation”(Nithianantharajah and Hannan,2006).In an animal context,these conditions include larger cages,with several objects such as tunnels,stairs,hiding places,seesaws,and a wheel;which are changed periodically to stimulate curiosity and exploration (Nithianantharajah and Hannan,2006).There are several protocols for EE,which vary in terms of the type of box cage,kind,and number of objects,and type of nest [for a detailed discussion,see (Simpson and Kelly,2011;Bayne,2018)].Although the benefits of EE exposure are clear,there is no agreement on which protocol is the most efficient.Moreover,strain (Abramov et al.,2008),age (Chandler et al.,2020),and duration of EE exposure (Leger et al.,2015) may affect the outcome.As to humans,changes in the environment usually include family,friendship,hobbies,socioeconomic status,and schooling among others (Sale,2018),with EE being,therefore,characterized by the association of these elements.
EE exposure is a promising strategy for the treatment of neurodegenerative diseases,as it can cause structural and functional changes in the brain,promoting neurogenesis (Grońska-Pęski et al.,2021;Gresita et al.,2022) and dendritic growth (Rizzi and Tan,2017;Ztaou and Amalric,2019).It does so by inducing alterations in gene expression levels (Zhang et al.,2016;Griñán-Ferré et al.,2018) and by enhancing neurotrophic factor expression (Gualti eri et al.,2017;Santoso et al.,2020).In addition,it increases neurotransmission modulation (Ragu Varman and Rajan,2015;Arroyo et al.,2020) and may influence the immune system tone (Singhal et al.,2014;Xiao et al.,2019).
For instance,some studies have shown that dance movement and physical therapy counteract both movement and cognitive impairment,improving the quality of life of PD patients (Stuckenschneider et al.,2019;Lihala et al.,2021).On another front,virtual reality-based EE,an approach in which a computer produces an environment that users can feel and interact with through multiple sensory channels,much like they would in a physical setting,emerges as a powerful tool to manage neurodegenerative diseases.Virtual reality-based EE is shown to be potenti ally effective in the long-term support of older adults with mild cognitive impairment and mild dementia (Riaz et al.,2021),in addition to acting as an aid in the rehabilitation of patients with other neurological diseases (Parsons et al.,2017).More to the point,virtual reality-based EE has been shown to improve the quality of life of PD patients (Pazzaglia et al.,2020;Triegaardt et al.,2020).Currently,game-based interventions are gaining attention because of the facilitation of rewarding experiences through the deployment of multi sensory stimulation (Janssen et al.,2017) displaying a favorable effect on PD patients (Garcia-Agundez et al.,2019;Chua et al.,2021).
Additionally,over the last several years,large studies have noticed the longterm effects of aerobic exercise on motor and non-motor parameters in select human trials (Alberts et al.,2020).Uc et al.(2014) reported that a 6-month walking program increases aerobic fitness,motor skills,fatigue,mood and quality of life in patients.On the other hand,the SPARX2 study examined the effects of long-term high-intensity aerobic exercise in slowing PD progression inde-novopatients (Moore et al.,2013).Recently,a randomized clinical trial showed that aerobic exercise could improve functional connectivity of the anterior putamen with the sensorimotor cortex relative to the posterior putamen and stabilizes motor progression and enhances cognitive performance in individuals with PD (Johansson et al.,2021).
Although EE has been shown to improve the symptoms and quality of life of PD patients (Feng et al.,2020),with benefits having been described also in animal models of PD (Jungling et al.,2018),the molecular mechanisms involved remain unclear.It has been reported that EE exposure reverts the motor impairment induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine(MPTP) in mice (Hilario et al.,2016) and 6-hydroxydopamine (6-OHDA) in rats (Jungling et al.,2017),both animal models of PD.Additionally,as well as neurotoxin models,the genetic models are suitable to decipher the impact of environmental interventions on PD.Given the central role of α-synuclein in PD pathogenesis,recent studies have further highlighted the role of EE in mice overexpressing human α-synuclein mutant (Seo et al.,2020;Kim et al 2021).Seo et al.(2020) found that EE ameliorated motor impairments in an α-synuclein transgenic mouse.A recent study revealed that EE has also a physiological role in non-motor symptoms of PD.In this study,EE ameliorates hyperacti vity and anxiety in a hA53T α-synuclein overexpression mouse and reduces aggregated α-synuclein levels and the interaction between α-synuclein and vesicle-associated member protein 2 in the nucleus accumbens (Kim et al.,2021).In another study with mice that overexpress human A53T α-synuclein,the EE ameliorated olfactory dysfunction and decreases oxidative stress and levels of nitrated α-synuclein (Wi et al.,2018).The mechanism by which EE impacts the aggregate α-synuclein in animal models of synucleinopathies is still unclear.Wassouf et al.(2018)compared wildtype to transgenic mice overexpressing human α-synuclein,housing them in a standard and enriched environment from weaning to 12 months of age.The investigators profiled their hippocampal transcriptome using RNA sequencing and found that a long-term enriched environment is capable of preventing α-synuclein-induced disturbances in the hippocampal transcriptome including disturbances in microglia and astrocytes.These preventive effects were accompanied by sustained activation of a group of immediate early genes,including the transcription factors EGR1 and NURR1/ NR4A2,and an increase in the astrocytic marker GFAP (Wassouf et al.,2018).The effect of EE exposure on non-motor symptoms of PD has also been investigated and a few studies have reported an improvement in cognitive (Campêlo et al.,2017;Yuan et al.,2018) and psychological damage(Kim et al.,2021).It has been proposed that these beneficial effects might result from decreased dopaminergic neuronal death in the substanti a nigra pars compacta (SNpc) (Jungling et al.,2017;Cho and Kang,2020),increased dopamine turnover (Hilario et al.,2016),reduced oxidative stress (Barker et al.,2020),and decreased intestinal inflammation due to the modulation of anti-inflammatory gut bacteria (Singh et al.,2019),or even through the modulation of neurotrophins (Campêlo et al.,2017;Cho and Kang,2020).This complex scenario highlights the need for further studies to elucidate the mechanisms behind the beneficial effects of EE in PD.
Molecular Mechanisms Underlying the Neuroprotection of Environmental Enrichment in Parkinson’s Disease
Dopaminergic system
Since the neurotransmitter dopamine was discovered in 1960 by Arvid Carlsson (Carlonsson,1993),its central role in PD has been well established(Glenthøj and Fibiger,2019).Dopamine plays an important role in several body functions,such as movement and coordination.A deficiency in dopamine levels may disturb the nigrostriatal pathway and alter neuron firing patterns,which,in turn,can cause movement disorders.Evidence suggests that patients diagnosed with PD will have lost at least 60–80% of dopamine-producing neurons in the SNpc by the time symptoms appear(Masato et al.,2019).A frequent strategy in the field of PD is to assess the impact of new approaches like EE in the severity of the lesion in the nigral dopaminergic system,evaluating the loss of dopamine-producing cells,as well as neostriatum changes.Evidence in mouse and rat models of PD suggests that EE affects various processes,including dopamine metabolism,enzymes involved in dopamine synthesis and degradation,dopamine receptors,and dopamine storage into vesicles.Each of these processes will be discussed next(Jüngling et al.,2018).
MPTP-treated mice,which exhibit decreased levels of the dopamine synthesis enzyme tyrosine hydroxylase (TH),show an improvement in TH mRNA expression levels,increased numbers of TH-immunoreactive neurons,and improved percentage of entries and time spent in the open arms of the elevated plus maze test when exposed to EE for 3 months (Yuan et al.,2009).Similarly,Bezard et al.(2003) observed that mice exposed to EE for 2 months are protected against MPTP-induced neurotoxicity by increasing the number of TH-positive midbrain dopaminergic neurons,as revealed through immunohistochemistry analysis (Goldberg et al.,2011).
A number of studies analyzed the impact of EE at the molecular level on dopamine transporter (DAT) and investigated how this strategy affects the transport of monoamines in toxin-induced models of PD (Faherty et al.,2005;Yuan et al.,2009;Goldberg et al.,2011).DAT plays a key role in the initial events of MPTP toxicity,since the toxic metabolite MPP+is a high-affinity substrate for this transporter (Giros and Caron,1993).Bezard et al.(2003)demonstrated that a 2-month exposure to EE prevents the loss of dopamine neurons in the SNpc of MPTP-treated mice.In line with these results,it has been shown through densitometric analysis that EE downregulates the striatal levels of DAT mRNA and decreases the DAT-binding capacity in the rostral caudate-putamen of MPTP-treated mice when compared with animals exposed to a standard environment (Bezard et al.,2003).Regarding DAT,it has been suggested that the protection elicited by EE may be mediated,at least in part,by increased levels of growth factors such as brain-derived neurotrophic factor (BDNF) (Bezard et al.,2003;Yuan et al.,2009).
In a previous study,our research group reported that EE affects dopamine metabolism.In mice treated with sub-chronic doses of MPTP,EE restores the 3,4-dihidroxylphenylacetic acid and homovanillic acid depletion and increases dopamine turnover,which was associated with the prevention of MPTPinduced hyperlocomotion (Hilario et al.,2016).However,EE does not prevent the dopaminergic depletion in the striatum induced by MPTP (Hilario et al.,2016).
Based on the studies summarized above,it is safe to assume that EE triggers neurochemical and behavioral changes that counteract the damage exhibited by animal models of PD (Figure 1).Although the dopaminergic system clearly plays a major role in PD,it is common knowledge that non-dopaminergic,non-catecholaminergic,and non-motor systems are involved as well(Miguélez Palomo et al.,2020).In fact,the progressive loss of dopaminergic neurons may impact other pathways and cells.The role played by each brain neurotransmitter in the neuroprotective effects elicited by EE described in the literature will be discussed next.
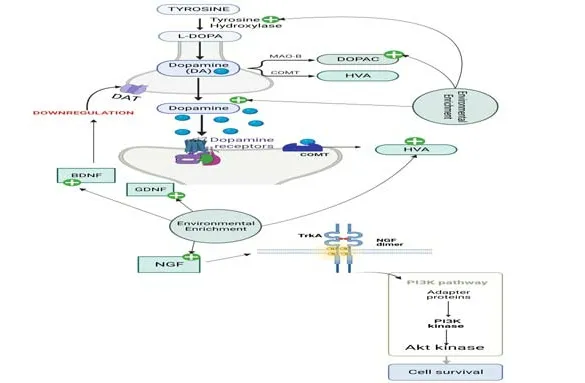
Figure 1|Effects of environmental enrichment on the dopaminergic system.
Cholinergic system
The cholinergic system is affected in PD and is probably involved in cognitive impairments (Hall et al.,2014;Bohnen et al.,2015).In fact,PD patients have a higher risk of developing dementi a,which is known as Parkinson’s disease dementi a (Pedersen et al.,2017),with afflicted subjects displaying reduced choline acetyltransferase (ChAT) activity and acetylcholine (ACh) density in the neocortex (Colloby et al.,2016).In addition,the cortical projection of the cholinergic system has been associated with several cognitive functions that might be correlated with gait and fall problems,disturbing quality of life(Morris et al.,2019).
The cholinergic system is important for mobility control.In fact,1–2% of the striatal cells are cholinergic interneurons,which exert reciprocal modulation on the dopaminergic system.Dopaminergic neurons of the SNpc influence striatal neurons because of their spontaneous acti vity and extensive terminal arborization (Ztaou and Amalric,2019).Moreover,cholinergic interneurons express dopamine receptors (D2) postsynaptically,decreasing autonomous firing and diminishing cholinergic signaling (Chuhma et al.,2014).In turn,cholinergic receptors are expressed in dopaminergic terminals,modulating dopamine release.While the presence of postsynaptic nicoti nic ACh receptors(nAChRs) increases dopamine release (Koranda et al.,2014),presynaptic muscarinic ACh receptors (mAChRs) reduce it (Yorgason et al.,2017).In PD patients,ACh predominates over dopamine,which contributes to the motor deficit (McKinley et al.,2019).Indeed,some studies have reported that dopamine and ACh exert opposing effects on striatal circuits (Rizzi and Tan,2017).
The effect of EE on other neurotransmitters involved in PD,such as ACh,gamma-aminobutyric acid (GABA),and glutamate,has been poorly investigated,as reviewed by (Jungling et al.,2018).Nonetheless,Hilario et al.(2016) showed that EE exposure attenuates the decrease in gene expression of choline acetyltransferase while preventing the MPTP-mediated increase in muscarinic ACh receptor expression in mouse midbrain.Interestingly,it has been reported that lifelong EE increases choline acetyltransferase protein expression in the basal forebrain and striatum of aged rats when compared with those raised in a standard environment (Harati et al.,2011,2013).These data support the assumption that EE has a neuroprotective effect.
The prodromal stage of PD as a restraint stress leads to an increase in acetylcholinesterase acti vity,which might cause neuroinflammation (Pavlov et al.,2009).In this context,Nawaz et al.(2018) showed that EE exposure restores the acetylcholinesterase acti vity levels that had been increased by restraint stress,which could lessen neuroinflammation (Nawaz et al.,2018).Last but not least,Pelosin et al.(2020) showed that treadmill training combined with non-immersive virtual reality,though not treadmill training alone,induces changes in the cortical cholinergic system through shortlatency afferent inhibition,a transcranial magnetic stimulation paradigm used to assess cholinergic acti vity,thereby promoting functional gait improvements in PD patients and older adults (Pelosin et al.,2020).
All the information discussed above highlights the major involvement of the cholinergic system in PD and the importance of further studies to elucidate the mechanism by which EE counteracts this disease (Figure 2).
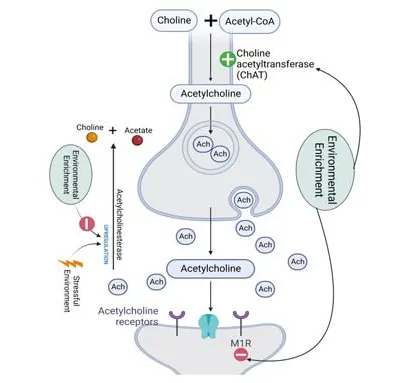
Figure 2|Effects of environmental enrichment on the cholinergic system.
Glutamatergic system
Glutamate is an abundant excitatory neurotransmitter widely distributed in the central nervous system (Zhou and Danbolt,2014).In mammals,it is esti mated that in every 1 kg of brain tissue there is 5–10 mmol of glutamate(Schousboe,1981).This amino acid is a thousand times more abundant in the brain than many other important neurotransmitters,such as dopamine,serotonin,and norepinephrine (Shen et al.,1999).
There is evidence that the glutamatergic system is involved in the development of PD,as glutamate-mediated excitotoxicity induces a variety of cellular insults that lead to degeneration in the SNpc (Johnson et al.,2009).As the dopaminergic tone is lost in the striatal region of the putamen in these conditions,other pathways become more active in a compensatory way.For instance,an increase in the glutamatergic tone from the motor cortex has been reported (Bergman et al.,1994).However,a balance in brain glutamate concentration is necessary to maintain a balance in brain function,and an excess of glutamate beyond normal limits leads to excitotoxic neuronal death(Bergman et al.,1994).
In the striatal regions,the N-methyl-D-aspartate (NMDA) glutamate receptors are composed mostly of NR1,NR2A,and NR2B subunits (Küppenbender et al.,2000).Enhancement in glutamatergic signaling in the striatum is associated with the hyper-phosphorylation of these subunits and is responsible for the development of levodopa-induced dyskinesia in both 6-OHDA-and MPTPinduced animal models of PD (Calon et al.,2002).Moreover,it causes excitotoxic events that contribute to the neurodegenerative process in the nigrostriatal regions (Calon et al.,2002).
Although the EE paradigm has been studied in PD models since 2000,few studies have addressed the possible effects of this approach on the neurochemistry of glutamate and its receptors in this context.On the other hand,the effect of EE in glutamatergic pathways in other brain conditions,such as aging (Segovia et al.,2006) and schizophrenia (Burrows et al.,2015),has been more thoroughly evaluated.For instance,Segovia et al.(2006)showed through microdialysis analysis that EE changes and stimulates the basal levels of glutamate in the hippocampus of aged rats,having pointed out that this could be a compensatory mechanism against the deterioration that normally occurs in the aging process.Environmental manipulation leads to pyramidal cell dendritic branching and increased levels of BDNF protein in the hippocampus,while these changes are blocked in a mGluR5 KO mouse,showing that mGluR5 may be associated with these responses (Burrows et al.,2015).
Additionally,enriched experience enhances learning and memory processes in mice in the novel object recognition task and contextual and cues conditioning.It does so by upregulating the expression of the glutamatergic subunits GluR1,NR2B,and NR2A,indicating that both AMPA and NMDA receptors are affected by the enriched experience (Tang et al.,2001).In this context,EE significantly facilitates the formation of long-term recognition memory by enhancing glutamatergic signaling in the hippocampus (Tang et al.,2001;Burrows et al.,2015).Of note,the aforementioned beneficial effects of glutamate induced by EE are on hippocampal circuits,while little is currently known about the role played by EE in the glutamatergic system in basal ganglia regions,particularly regarding the effects on indirect and direct pathways that control the coordination of movement (Figure 3).Therefore,the exact molecular impacts of EE in the glutamatergic alterations observed in PD remain poorly explored.

Figure 3|Effects of environmental enrichment on the glutamatergic system.
GABAergic system
The dopamine depletion observed in the SNpc in PD affects the normal function of the GABAergic system (Calabresi et al.,2014).Moreover,GABA plays a modulatory role in PD eti ology,which is independent of dopaminergic neurons.HPLC measurements indicate that GABA levels are higher in the striatum of MPTP-treated monkeys than in that of control ones (Huang et al.,2019),which is consistent with the GABA concentrations observed in the striatum of PD patients (Emir et al.,2012).Furthermore,cholinergic interneurons release both ACh and GABA,thus creating a balance between them.In addition to the increased excitatory acti vity observed in cholinergic interneurons in PD,there is a decrease in their inhibitory action through GABAergic neurotransmission,caused by high intracellular Cl-concentrations(Lozovaya et al.,2018).
A few studies have shown that EE can modulate the GABAergic system.This approach decreases GABAergic inhibition,reversing cognitive impairment in a mouse model of Down syndrome (Begenisic et al.,2011).Furthermore,EE exposure decreases basal GABA concentrations in the hippocampus of aged rats (Segovia et al.,2006).In addition,post-weaning,30-day EE exposure reverts the cognitive impairments caused by prenatal stress in rats,which are abolished with the administration of muscimol,a GABAergic agonist.Also,EE exposure restores hippocampal synaptic plasticity impairment and GABAergic agonists suppress this effect (Aghighi Bidgoli et al.,2020).These data suggest that EE may have a beneficial effect through GABAergic modulation (Aghighi Bidgoli et al.,2020).Furthermore,EE raises the immunoreacti vity of glutamic acid decarboxylase 67,an enzyme that catalyzes the synthesis of GABA,in the hippocampus of rats treated with an NMDA receptor antagonist when compared to control animals (Murueta-Goyena et al.,2018;Figure 4).There are no studies about the effect of EE exposure on the GABAergic system in PD.However,in the Pink1–/–rat model of PD,it has been observed an upregulation of all GABA-related genes in vocal-and sham-exercised animals,in addition to an increase in glutamic acid decarboxylase levels revealed by western blotting,suggesting that alternative therapy may play a key role in shaping vocal sign,an early non-motor symptom of PD (Stevenson et al.,2019).Thus,further studies are needed to delineate the mechanism by which EE modulates the GABAergic system in PD.
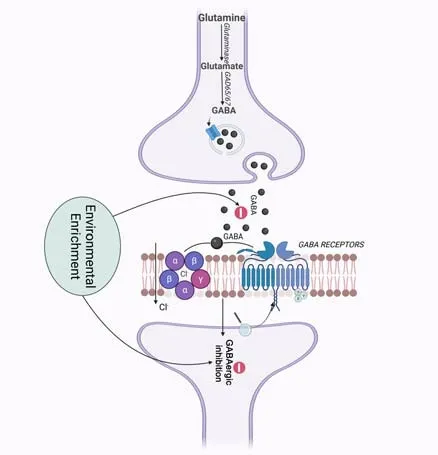
Figure 4|Effects of environmental enrichment on the GABAergic system.
Brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor
Until a few years ago,it was believed that the brain could not regenerate and repair.However,knowledge on the subject has improved and there is now solid evidence that neurotrophic and growth factors regulate brain development and play an important role in neural regeneration (Colucci-D’Amato et al.,2020).
BDNF,a member of the nerve growth factor gene family,acts in a number of neuronal pathways,especially in the nigrostriatal system (Mogi et al.,1999).BDNF signaling is activated through its binding to tyrosine kinase receptors as well as through the p75 receptor.Both BDNF and tyrosine kinase receptor are expressed by neurons that produce dopamine (Okazawa et al.,1992).Postmortem analyses of PD patients show through different techniques– e.g.,in situhybridization,immunohistochemistry,and western blot– decreased levels of BDNF in dopaminergic regions of the afflicted brain,which correlates with the prognosis of the disease (Mogi et al.,1999;Parain et al.,1999).
Glial cell line-derived neurotrophic factor (GDNF) is a powerful neurotrophin that plays a major role in the survival of dopaminergic nigrostriatal neurons(Choi-Lundberg et al.,1997).Current clinical trials suggest GDNF as a potenti al therapy for patients with PD (Barker et al.,2020).Pre-clinical studies also point out that the exogenous administration of GDNF protects animals against MPTP neurotoxicity.These results were reproduced in different species,including C57Bl/6 mice,monkeys,and marmosets (Barker et al.,2020).Moreover,GDNF administration was also protective against the cell loss induced by 6-OHDA in rats (Li et al.,2013).
Experimental evidence suggests that the beneficial effects of the EE paradigm may be mediated by an increased expression of the neurotrophins BDNF and GDNF in both 6-OHDA and MPTP PD animal models (Ickes et al.,2000;Campêlo et al.,2017).Interestingly,similar results were observed in different PD models and protocols (Zhu et al.,2015).The literature shows that the prolonged exposure to EE for 3 months in a progressive PD mouse model,induced by repeated treatment with low doses of reserpine,leads to increased BDNF levels in the striatum (Fernandes et al.,2012).These data are in line with those observed by Faherty et al.(2005),which also indicate that previous exposure to EE avoids neuronal death and increases BDNF in animals receiving MPTP 20 mg/kg/2 h intervals.Similar results were reported by Bezard et al.(2003) who observed an environmentally driven increase in BDNF expression.This upregulation may,in turn,affect dopamine neurons,especially regarding DAT downregulation and the subsequent resistance to MPTP and cocaine toxicity displayed by mice exposed to EE (Bezard et al.,2003).In addition,physical training restores BDNF levels in the striatum of 6-OHDA model animals (Kim et al.,2014).
In line with these results,other studies have reported reduced neurogenesis in response to MPTP and also in extranigral regions,such as the hippocampus(Zhu et al.,2015).However,when mice are subjected to EE,there is a reversal in the MPTP-induced neurogenesis deprivation along with a better performance in behavior tasks requiring motor skills (Neeper et al.,1995;Kim et al.,2014;Koo and Cho,2017).
At the same time,the exogenous administration or stimulus-induced expression of neurotrophic factors through physical exercise may increase the brain’s resilience to insults and neuronal impairment (Gash et al.,1996;Björklund and Lindvall,2000;Zhao et al.,2014).In addition,EEper se,without an established neurodegenerative condition,can change BDNF levels in animals exposed to it when compared with those submitted to standard housing conditions,corroborating the evidence that environmental stimulation modulates the expression of neurotrophins (Faherty et al.,2005).The molecular mechanism by which EE affects neurotrophin expression is not well established.Nevertheless,a transcriptome analysis approach identified that EE induced 41 differentially expressed genes (23 upregulated and 18 downregulated),terms involved in morphogenesis,neuron differentiation,cognition,learning,and memory as well upregulated differenti ally expressed genes attributed to pyramidal neurons (Wassouf et al.,2018).
Faherty et al.(2005) demonstrated that mice exposed to EE unti l adulthood showed a 350% increase in the mean mRNA expression for GDNF in the SNpc compared with animals raised in standard housing conditions.These effects were also observed as a result of exercise training after MPTP-induced neurotoxicity (Faherty et al.,2005).
All the aforementioned findings strengthen the hypothesis that new nonpharmacological and preventive approaches can prevent the motor and functional cognitive deterioration observed in PD,being,at least in part,mediated by the neurochemical action of neurotrophins.However,the actual mechanisms involved in this phenomenon are poorly explored and must be investigated.
Nerve growth factor
Nerve growth factor (NGF) is a polypeptide of the neurotrophin family involved in neuronal growth,development and survival,transmitter synthesis,and apoptosis.NGF levels are reduced in PD (Mogi et al.,1999) and this neurotrophin has neuroprotective effects by alleviating dopaminergic neuronal death (Chaturvedi et al.,2006).Moreover,NGF modulates cholinergic innervation in the hippocampus and reduces apoptosis in a toxin PD model via PI3K/Akt pathway (Liu et al.,2020).
Exercise,a factor in EE,is effective in preventing NGF alterations.Mohammadi et al.(2019) showed that progressive exercise has a better protective effect than mild-intensity treadmill running,decreasing PD severity by preventing dopamine and tyrosine hydroxylase loss,and increasing neurotrophins as NGF in the striatum of a 6-OHDA-induced model of PD in rats.
EE was also shown to increase NGF expression in the dentate gyrus and hippocampus of healthy rats after,respectively,6 and 8 weeks of exposure(Birch et al.,2013;Santoso et al.,2020).Though not stati stically significant,NGF mRNA levels are upregulated by EE exposure in MTPT-treated mice,while the levels of proNGF and p75 neurotrophin receptor are significantly downregulated,suggesting that EE has neuroprotective effects via diminishing the activation of the p75 neurotrophin receptor in this PD model (Cho and Kang,2020).These results assert even further that non-pharmacological approaches might be useful as therapeutic interventions to facilitate recovery or prevent PD progression.
Conclusions
The neuroprotective effects of EE in neurodegenerative diseases such as PD have been well established by many studies,although the molecular mechanisms underlying such effects are not yet completely understood.Moreover,as most studies focus mainly on the dopamine system,further studies are needed to fully elucidate the effects of EE exposure on the other neurotransmission systems known to be involved in the pathology of PD.
Acknowledgments:Authors would like to thank Dr.Fabiana V.Campos for the great contribution with English review and text format.
Author contributions:TAA,SMP,APTS,FMR and RGWP were responsible for the manuscript writing,proofreading and editing.APTS was responsible for figure drawing.All authors approved the final version of the manuscript.
Conflicts of interest:The authors declare that there is no conflict of interest regarding the publication of this paper.
Availability of data and materials:All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:This is an open access journal,andarticles are distributed under the terms of the Creative CommonsAttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Bystanders or not? Microglia and lymphocytes in aging and stroke
- Alzheimer’s disease risk after COVID-19: a view from the perspective of the infecti ous hypothesis of neurodegeneration
- Serine and arginine rich splicing factor 1: a potenti al target for neuroprotection and other diseases
- Can glial cells save neurons in epilepsy?
- Lights for epilepsy: can photobiomodulation reduce seizures and offer neuroprotection?
- The landscape of cognitive impairment in superoxide dismutase 1-amyotrophic lateral sclerosis

