Mitochondria in Huntington’s disease: implications in pathogenesis and mitochondrial-targeted therapeutic strategies
Anamaria Jurcau ,Carolina Maria Jurcau
Abstract Huntington’s disease is a genetic disease caused by expanded CAG repeats on exon 1 of the huntingtin gene located on chromosome 4.Compelling evidence implicates impaired mitochondrial energetics,altered mitochondrial biogenesis and quality control,disturbed mitochondrial trafficking,oxidative stress and mitochondrial calcium dyshomeostasis in the pathogenesis of the disorder.Unfortunately,conventional mitochondrial-targeted molecules,such as cysteamine,creatine,coenzyme Q10,or triheptanoin,yielded negative or inconclusive results.However,future therapeutic strategies,aiming to restore mitochondrial biogenesis,improving the fission/fusion balance,and improving mitochondrial trafficking,could prove useful tools in improving the phenotype of Huntington’s disease and,used in combination with genome-editing methods,could lead to a cure for the disease.
Key Words:antioxidants;calcium homeostasis;Huntington’s disease;mitochondrial biogenesis;mitochondrial fission/fusion;mitochondrial trafficking;oxidative phosphorylation;oxidative stress;SS peptides;therapeutic intervention
Introduction
Huntington’s disease (HD) is a neurodegenerative disease with a global prevalence ranging between 0.02 and 17.27/100,000 inhabitants,which tends to increase in most regions due to increasing mutation rate,increasing life expectancy,greater availability of genetic testing,and access to symptomatic treatment (Rawlins et al.,2016).It is transmitted as an autosomal dominant disease with complete penetrance,the age of onset varying inversely proportional with the number of CAG repeats above 40 in exon 1 of the huntingtin gene located on chromosome 4 (Julayanont et al.,2020).The clinical picture consists of a variety of involuntary movements,such as chorea,dystonia,bruxism,variable degrees of bradykinesia and rigidity,psychiatric symptoms,and cognitive decline progressing to dementi a (Zielonka,2018).Much research has tried to elucidate how the production of an abnormal protein,mutant huntingtin (mHtt),leads to widespread neuronal loss in the striatum,cortex,and hypothalamus.An intricate complex of vicious cascades involving excitotoxicity,impaired proteostasis,mitochondrial dysfunction,oxidative stress,transcriptional dysregulation,reduced bioavailability of growth factors,as well as astrocytic dysfunction and neuroinflammation,has been found to contribute to the different stages of the disease (Jurcau,2022).The present review will focus on the involvement of mitochondrial dysfunction in the pathogenesis of HD and discuss some therapeutic targets.
Search Strategy and Selection Criteria
The references cited in this review have been obtained from the PubMed and Google Scholar databases.We referenced full-text review articles,randomized control trials,and meta-analyses published between 1995 and 2023.
Normal Functions of the Mitochondria
Neurons are cells with very high energy demands and which,unlike other cells in the body,have strict aerobic metabolism.The large amounts of energy are required for generation of action potenti als and postsynaptic potenti als as well as for membrane repolarization,neurotransmitter synthesis,vesicle recycling,axoplasmic transport,and synaptic plasticity.ATP is generated in neurons mainly by the mitochondrial oxidative phosphorylation (OXPHOS)(Deitmer et al.,2019),a process during which complexes I–IV located on the inner mitochondrial membrane (IMM) pump protons from the matrix to the intermembrane space using the electrons removed from the Krebs cycle by reduced nicotinamide adenine dinucleoti de and flavin adenine dinucleoti de(FADH2).This results in a potenti al gradient across the IMM (–150 to–180 mV),which is used in the final step of OXPHOS by complex V to synthesize ATP(Jurcau,2021).
To meet the cellular energy requirements,mitochondria number,shape,and size must be ti ghtly regulated,a process called mitochondrial dynamics.
Mitochondrial biogenesis is a complex process,involving transcription and translation of both mitochondrial DNA (mtDNA) and nuclear DNA-encoded proteins.The latter are synthesized in the cytosol as precursors,maintained in an unfolded conformation by forming complexes with chaperones,such as heat shock protein (HSP) 70 or HSP90,and imported to the mitochondria through cooperation of translocase of the outer mitochondrial membrane(TOM) and translocase of the inner mitochondrial membrane (TIM) 23(Bausewein et al.,2017).A key role in this process is played by peroxisome proliferator-activated receptor (PPAR)γ-coactivator 1 α (PGC-1α),which regulates the expression of nuclear-encoded subunits of the electron transport chain complexes (ETC),of the mitochondrial transcription factor A(TFAM),of anti oxidant defence proteins,and of nuclear respiratory factors 1 and 2 (Nrf1 Nrf2) (Panes et al.,2022).
For mitochondrial fission,a process that also allows for generation of new mitochondria,dynamin-related/-like protein 1 (Drp1) must be bound to the outer mitochondrial membrane (OMM) by adaptor proteins such as mitochondrial dynamics proteins 49 and 51 (MiD49 and MiD51) and mitochondrial fission factor,followed by recruitment of dynamin 2 (Dnm2),a GTPase which completes the process (Jurcau,2021).
Fusion allows for repair of degraded organelles and sharing of essential components,and is regulated by Mfn1 and Mfn2,which tether the OMM of adjacent mitochondria,after which optic atrophy 1 (OPA1) mediates the fusion of the IMM (Adebayo et al.,2021).
Irreversibly damaged mitochondria are disposed through mitophagy,which involves the formation of an isolation membrane,followed by activation of the pre-initiation complex,containing Unc-51-like kinase 1,autophagyrelated protein 13 and 101,and focal adhesion kinase family interacting partner 200 (FIP200;Swerdlow and Wilkins,2020).Subsequent recruitment of phosphatidyl inositide 3-kinase (PI3K),autophagy-related protein 14,beclin1,autophagy and beclin 1 regulator 1,and vascular sorting protein 34 and 15 (Vsp 34 and 15) produce phosphatidyl inositol 3-phosphate,also called the initiation complex (Hurley and Young,2017).Atg4 mediates cleavage of pro-light chain 3 (LC3) to LC3-I,which is further transformed by phosphatidylethanolamine to LC3-II,resulting in elongation and closure of the isolation membrane.The subsequent fusion of the autophagosome with lysosomes is mediated by Rab7 and lysosome-associated membrane protein 2.Recognition of the damaged mitochondria occurs through phosphatase and tensin homologue (PTEN)-induced putative kinase 1 (PINK1) and Parkin,an E3 ubiquitin ligase (Jurcau,2021).
Due to the particular shape of the neurons,mitochondria must be trafficked along the neural outgrowths.The process is mediated by a protein complex consisting of the heavy chain of kinesin-1,Miro 1 and 2 (known in humans as mitochondrial Rho GTPase -RhoT1 and RhoT2),attached to the outer surface of the mitochondrion,milton (or TRAK1 and TRAK2 in humans),which links kinesin and Miro (Melkov and Abdu,2018;Fenton et al.,2021),and dynein.While anterograde transport is mediated by kinesin-1,dynein is involved in retrograde trafficking.Calcium signals (transient elevations of cytosolic Ca2+),slow down or stop mitochondrial trafficking (Jurcau,2021).
Mitochondria also contribute to maintenance of cellular calcium homeostasis.Normally,cytosolic calcium concentrations are nanomolar (50–300 nM),while extracellular calcium concentrations are milimolar (Jurcau,2021).Elevated cytosolic Ca2+concentration is rapidly restored through the activity of the Ca2+-ATPase,which hydrolyses ATP to pump Ca2+against the concentration gradient,and of the Na+/Ca2+exchanger,which extrudes calcium in exchange for sodium.Cytosolic Ca2+also binds to Ca2+-binding proteins,or is taken up by mitochondria or the endoplasmic reticulum (ER) through the ATPdependent sarco-endoplasmic reticulum Ca2+-ATPase (Primeau et al.,2018).In mitochondria,Ca2+is transferred into the intermembrane space by voltagedependent anion-selective channels (VDACs),and into the mitochondrial matrix by the mitochondrial Ca2+uniporter (MCU),where it activates ATP production by stimulating the acti vity of dehydrogenases in the Krebs cycle and the acti vity of complex I of the ETC (Naia et al.,2017).Once inside the mitochondria,calcium is either converted to insoluble salts,or extruded by the Na+/Ca2+anti porter,powered by the Na+gradient resulting from the acti vity of the Na+/H+anti porter (MacAskill and Kittler,2010).Mitochondriaassociated membranes (MAMs) are microdomains enriched in inositol 1,4,5-trisphosphate (IP3) receptors (IP3Rs),ryanodine receptors (RyRs),and molecular chaperones,such as glucose-regulated protein 75,which generate functional complexes with voltage-dependent anion-selective channels to mediate Ca2+transfer from the ER to mitochondria (Eysert et al.,2020).However,increases in mitochondrial Ca2+concentrations cause mitochondrial depolarization and trigger the opening of the mitochondrial permeability transition pore (MPTP),resulting in release of cytochrome c and neuronal apoptosis (Jurcau and Ardelean,2021).
Research has shown differences between synaptic and nonsynaptic mitochondria in their calcium-buffering ability.Synaptic mitochondria display faster rates of Ca2+uptake and release to sustain neurotransmitter exocytosis(Naia et al.,2017).Furthermore,calcium influx evoked by N-methyl-Daspartate (NMDA) receptor (NMDAR) activation,through the mitochondrial Rho-GTPase Miro acting as a calcium sensor,leads to postsynaptic translocation of mitochondria,calcium overload,mitochondrial dysfunction and excitotoxic cell death (Naia et al.,2017).
Mitochondria in Huntington’s Disease
Structural abnormalities in mitochondrial morphology were observed already in 1978 in post-mortem cortical tissue from HD patients (Zuccato et al.,2010),and evaluation of the cellular bioenergetics in HD-derived neurons showed an altered glycolytic capacity (The HD iPSC Consorti um,2020).In addition,one of the first HD models was obtained through administration of 3-nitropropionic acid,which irreversibly inhibits the mitochondrial complex II (Zuccato et al.,2010).These findings point toward a crucial role of mitochondria in HD pathogenesis.
Impaired mitochondrial energy production
Research has shown reduced glucose consumption,especially in the basal ganglia even in presymptomatic carriers of the HD mutation (Antonini et al.,1996),and increased lactate levels in the caudate and occipital cortex of HD patients (Jenkins et al.,1998).Due to the ubiquitous nature of huntingtin,the defective energy production affects peripheral tissues as well,such as lymphoblasts,muscle fibres,or adipose tissue,leading to weight loss in most patients with HD (Silva et al.,2013).
Normal mitochondrial ATP synthesis requires the mitochondrial transmembrane potenti al to be maintained at 80–90 % of its maximal value(–150 to–180 mV) (Carmo et al.,2018).The decrease in the acti vity of the mitochondrial complexes (mainly complexes II -succinate dehydrogenase,and III -ubiquinol-cytochrome c oxidoreductase),together with the interaction of the N-terminal fragment of mHtt with TIM23 and inhibition of mitochondrial protein import,synergistically decrease the mitochondrial transmembrane potential (Wright et al.,2017),leading to mitochondrial dysfunction which is directly correlated with the increased number of glutamine repeats.In addition,elongated CAG repeats in the mutated huntingtin gene promote age-dependent expansion of pathogenic mtDNA heteroplasmies (coexistence of wild-type and mutated mtDNA),which accelerate disease progression,as shown in a longitudinal study on HD lymphoblasts (Wang et al.,2021).
Furthermore,mHtt prevents the CREB/ TATA-binding protein-associated factor 4 (TAF4) complex from binding to the PGC-1α promoter and the transcription of PGC-1α,a key regulator of glucose metabolism and β-oxidation of fatty acids (Jesse et al.,2017).Pyruvate dehydrogenase,the enzyme that catalyses the oxidative decarboxylation of pyruvate to acetyl-CoA in the matrix,was decreased in the putamen and caudate of HD patients (Carmo et al.,2018),as were other enzymes of the Krebs cycle,such as aconitase (detailed further) or succinate dehydrogenase (Benchoua et al.,2006).
Glycolysis is also impaired,mainly due to the abnormal function of glyceraldehyde-3-phosphate dehydrogenase (GAPDH),the enzyme mediating the sixth step of glycolysis.GAPDH preferentially interacts with cleaved polyglutamine (polyQ) domains,leading to enhanced nuclear translocation of mHtt via the ubiquiti n-E3 ligase Siah1 and increasing its cytotoxicity,as well as to GAPDH sequestration in mHtt aggregates (Bae et al.,2006).
Increased oxidative stress
Mitochondria are one of the main generators of reactive oxygen species (ROS).About 1–2% of the consumed oxygen will generate superoxide anion by electrons leaking at the level of complexes I and III (Carmo et al.,2018) even in normal conditions.Despite neuronal and mitochondrial anti oxidant defence systems (Mn-SOD,glutathione peroxidases,the cytosolic Cu,Zn-SOD or SOD1,catalase,and peroxiredoxins),the high rate of ROS production overwhelms these anti oxidants and leads to oxidative stress,which impairs OXPHOS and energy production (Jurcau and Ardelean,2021).Aconitase is particularly suscepti ble to ROS-induced impairment (Chen et al.,2017),its acti vity being reduced by 70–90% in the caudate and putamen of HD patients.Other ROSproducing enzymes,such as NADPH oxidase,also show augmented acti vity in cortical and striatal post-mortem samples from HD patients (Valencia et al.,2013).
The generated ROS oxidize proteins (including mitochondrial enzymes),membrane lipids (Manoharan et al.,2016),as well as nuclear and mitochondrial DNA (Askeland et al.,2018),having a crucial role in HD pathogenesis (Onyango et al.,2021).In addition,mHtt-induced transcriptional dysregulation interferes with the Nrf2/ARE pathway,which normally upregulates the expression of anti oxidant enzymes (Moretti et al.,2021) in a vicious cascade.
Impaired mitochondrial quality control
The constant renewal of the mitochondrial network is modulated by a series of transcription factors,such as cAMP responsive element-binding protein (CREB)binding protein or TAF4.The interaction of mHtt with CREB binding protein or TAF4 and the indirect impairment of the expression of PGC-1α results in severely downregulated expression of nuclear-encoded subunits of the electron transport chain and of genes involved in anti oxidant response (Johri et al.,2013;Jesse et al.,2017).In addition,PGC-1α regulates the expression of TFAM,the key regulator of mitochondrial DNA transcription (Palikaras and Tavernarakis,2014),as well as the transcription of ATP synthase and manganese superoxide dismutase (Johri et al.,2013).In HD mice,there was a 6-fold decrease in PGC-1α in the medium spiny neurons of the caudate nucleus compared to wild-type mice,due to interactions of mHtt with the abovementioned signalling pathways and to direct binding to the PGC-1α promoter(Cui et al.,2006).Overall,72 genes involved in cellular metabolism,nervous system development,or apoptosis regulation have down-regulated microRNAs(miRNAs),while only 19 genes have up-regulated miRNAs (Dubois et al.,2021).Table 1summarizes some of the down-and up-regulated miRNAs described by researchers in HD,with relation to mitochondrial dysfunction.Mitochondrial morphology and dynamics are also significantly impaired by the direct interaction of the N-terminus fragment of mHtt with Mfn2,inhibiting its mitochondrial elongation-promoting action (Jurcau,2022).Furthermore,the expression of Mfn1,Mfn2,and OPA1 were shown to decrease,paralleling the stage of HD (Machiela et al.,2021).As for mitochondrial fission,mHtt accumulates on mitochondria and increases the recruitment of Drp1.Activated calcineurin,together with mitogen-activated protein kinase 1,dephosphorylates Drp1,thereby potenti ating Drp1 recruitment (Roe and Qi,2018).Calcineurin also activates the striatal enriched tyrosine phosphatase(STEP),through which it leads to reduced synaptic NMDA receptor expression(Braithwaite et al.,2006).Inhibition of the calcineurin-Drp1 pathway proved neuroprotective against striatal degeneration,as did OPA1 overexpression(Machiela et al.,2021).
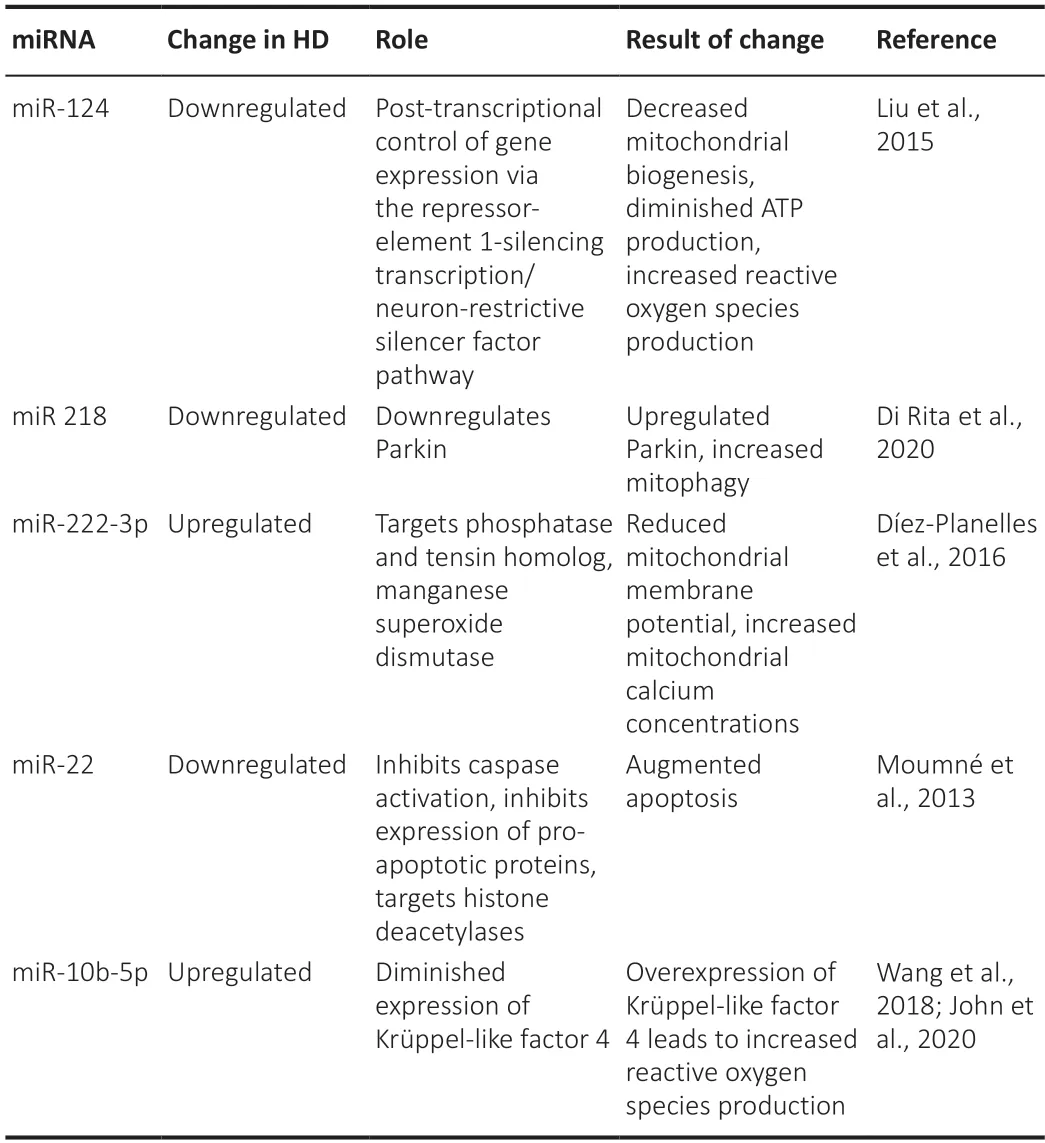
Table 1|Changes in miRNAs described in Huntington's disease and their effect on mitochondrial function
In depolarized mitochondria,PINK1 stabilizes at the OMM and is no longer imported to the IMM,favouring mitochondrial Parkin translocation(Kazlauskaite et al.,2015).Parkin molecules recruit ubiquitin chains to the OMM,recognized by autophagy adaptors such as p62.Fibroblasts from patients with juvenile HD showed increased Parkin levels and enhanced proteasomal degradation of Mfn1 (Aladdin et al.,2019),while PINK1 overexpression proved protective in HD fly models (Khalil et al.,2015).Wildtype Htt aids p62 to associate with LC-3.This scaffolding function is lost by mHtt,leading to impaired mitophagy (Rui et al.,2015).Htt,together with huntingti n-associated protein 1 (HAP1),have also an important role in controlling the dynamics of autophagosomes through regulation of kinesin and dynein.
The result of all these alterations is impaired mitochondrial quality control,with a reduced number and altered morphology shift ing towards increased fragmentation of mitochondria due to increased levels of Drp1 and mitochondrial fission 1 protein (Fis 1) and decreased levels of Mfn1,Mfn2,and OPA1 (Sawant et al.,2021;Panti ya et al.,2020).
Impaired mitochondrial trafficking
Mitochondrial trafficking is critical in neurons,which need energy for regular bioenergetic homeostasis,as well as for synaptic transmission.As shown above,the process involves a complex machinery of motor proteins which attach to the organelles and transport them in an anterograde or retrograde direction (towards the cell soma).HAP1 interacts with both kinesin and dynein,regulating cargo transport on microtubules (Franco-Iborra et al.,2018),while Htt interacts with HAP1 and recruits GAPDH for producing the necessary amount of ATP.Further,the Htt–HAP1 complex serves as an adaptor protein,binding to both kinesin-1 and dynein as well as to the dynactin subunit p150glued (Twelvetrees et al.,2019).In HD,GADPH,HAP1 and dynamin are sequestered into mHtt aggregates (Qin et al.,2004).Further,huntingti n-interacting protein 1 (HIP1) contributes to the assembly of cytoskeletal structures,a function which is disturbed in HD due to a weaker interaction of HIP1 with mHtt.In addition,PINK1 and Parkin accumulate on the OMM and phosphorylate the GTPases RhoT1 and RhoT2,leading to a detachment of kinesin from mitochondria and arrest of the organelle (Jurcau,2021).The impaired mitochondrial trafficking results in accumulation of fragmented mitochondria in the cell bodies,further augmenting the energetic disturbances and leading to synaptic loss and neuronal degeneration.
Impaired mitochondrial calcium handling
The soluble N-terminal fragment of mHtt interacts directly with the OMM and,via TIM23,with the IMM,disturbing protein import and leading to mitochondrial depolarization (Schrank et al.,2020).In addition,polyQ tracts are able to form proton-selective ion channels in the mitochondrial membrane,further diminishing the electrochemical proton gradient across the IMM (Monoi et al.,2020).These abnormalities interfere with the mitochondrial ability to buffer excess cytosolic Ca2+.The consequence of mitochondrial depolarization is opening of the MPTP at lower Ca2+concentrations,leading to cytochrome c release and mitochondrial swelling(Jurcau,2022).
The carboxy-terminus of mHtt also interacts with the IP3 receptors (IP3Rs)in a complex with HAP1,increasing their responsiveness and causing ER Ca2+release at lower IP3 concentrations (Mackay et al.,2018).In addition,mHttdiminishes the expression of sigma-1 receptor (Sig-1R),an ER protein localized at MAMs which forms a chaperone complex with immunoglobulin protein/glucose-regulated protein 78.Upon ER Ca2+depletion,Sig-1R dissociates from BiP and stabilizes IP3Rs,prolonging Ca2+signalling (Delprat et al.,2020).The RyRs have been less extensively studied in HD,but the finding that inhibition of RyRs with dantrolene protects cortical and striatal neurons expressing polyQ stretches from degeneration clearly implicate these receptors as well in the dysfunction of MAMs and calcium leakage from the ER (Sun and Wei,2021).Figure 1summarizes the alterations in cellular calcium homeostasis in HD.

Figure 1|Impaired calcium homeostasis in Huntington’s disease leads to apoptosis.
Mitochondria-dependent apoptosis
Following the opening of the MPTP and cytosolic release of cytochrome c,the latter forms with deoxy-ATP,Apaf-1 and caspase 9 the apoptosome that activates downstream targets such as caspases 3,6,and 7,initi ating caspasedependent apoptosis (Jurcau and Ardelean,2021).Other mitochondrial proteins,such as apoptosis-inducing factor or second mitochondrion-derived activator of caspase/direct inhibitor of apoptosis-binding protein with low pI(SMAC/DIABLO) are released as well.Apoptosis-inducing factor translocates to the nucleus and causes DNA fragmentation and poly ADP ribose polymerase inhibition,accelerating cellular damage and destruction.SMAC/DIABLO binds to X chromosome-linked inhibitor-of-apoptosis protein and triggers apoptosis by suppressing the anti -apoptotic acti vity of X chromosome-linked inhibitorof-apoptosis protein (Jurcau and Ardelean,2022).Caspase-2 can cleave mHtt and generate toxic N-terminal fragments which cause degeneration of neurites,while caspase-7,preferentially expressed in MSNs,interacts with mHtt and triggers the acti vity of other caspases,accelerating apoptosis (Carmo et al,2018).Increased cytosolic Ca2+activates calpains and caspase 8,leading to cleavage and activation of Bcl-2 (B-cell lymphoma 2) interacting domain,which in turn induces conformational changes in other proapoptotic proteins,such as Bax,Bad,Bcl-XS,and inactivate anti -apoptotic proteins like Bcl-2 or Bcl-XL (Stevens and Oltean,2019),thereby inducing caspase-independent apoptosis.In addition,mHtt binds more efficiently to p53,a major transcription factor,leading to upregulation of nuclear p53 and increasing the levels of target genes such as Bax and p53 upregulated modulator of apoptosis (Schapira et al.,2014).Furthermore,with increasing length of the polyQ tracts,the interaction of mHtt with HIP1 decreases,allowing HIP1 to interact with apoptotic proteins such as procaspase 8 and potentiating the extrinsic apoptotic pathway (Choi et al.,2006).
Therapeutic Strategies Targeting Mitochondrial Dysfunction
Although the most logical approach in a disease caused by transcription of an abnormal protein would be to stop the transcription of that protein,clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9(CRISPR/Cas9) or RNA interference approaches are sti ll in their infancy,require usually invasive modes of administration,and target the transcription of mHtt only in cerebral tissue (Jurcau and Jurcau,2022).Given the widespread presence of mitochondrial dysfunction,and the fact that diagnosis is often established late,in symptomatic patients,the search for means to efficiently counterbalance these impairments is worth pursuing.Many molecules have been tested in cell lines or animal models of HD,but few of them have advanced to clinical testing.
Conventional mitochondria-targeted therapies
Melatonin,a hormone produced by the pineal gland,inhibited mHtt-induced mitochondrial depolarization and the release of pro-apoptotic factorsin vitro(Wang et al.,2011).It also exhibits anti-inflammatory effects by inhibiting the binding of nuclear factor kappa-light-chain-enhancer of activated B cells(NF-κB) to nuclear DNA and the production of pro-inflammatory cytokines,up-regulated Nrf2 and sirtuin 1 (SIRT1),upregulated γ-aminobutyric acid activity and prevented excitotoxicity (Cardinali,2019).A pilot clinical trial(NCT04421339) is currently recruiting HD gene carriers to evaluate efficacy in improving sleep and quality of life (https://www.clinicaltrials.gov).
A series of natural compounds act as anti oxidants and improve mitochondrial function by interfering with various pathways.The dietary flavonoid quercetin acts as a free radical scavenger and induces the Nrf2/ARE pathway,upregulating SOD,glutathione transferase,glutathione peroxidase,catalase and thioredoxin.It showed potent anti oxidant effect in a rat chemical model of HD (Grewal et al.,2021).Resveratrol,a dietary polyphenol,activates SIRT1 and enhances the PI3K/protein kinase B pathway and the nuclear translocation of Nrf2,exhibiting also anti-inflammatory effects through inhibition of the NF-κB and MAPK pathways (Hannan et al.,2020).In YAC128 mice,resveratrol improved motor function by increasing the expression of mitochondrial ETC complexes and of mitochondrial-related transcription factors such as TFAM and PGC-1α (Naia et al.,2017b).Another natural compound,β-lapachone,also increased SIRT 1,PGC-1α deacetylation and CREB levels,reducing the formation of ROS and leading to phenotypic improvement in R6/2 mice (Lee et al.,2018).Curcumin interferes with the NF-κB,signal transducer and activator of transcription 3 and Nrf2 pathways,acting as an antioxidant and anti-inflammatory molecule,being also able to suppress protein aggregation (Labanca et al.,2021).It showed beneficial effects in R6/2 mice (Elifani et al,2019).Unfortunately,dietary antioxidant molecules have poor blood brain barrier penetrance,but nanotechnologybased delivery methods,such as nanomaterials,nanozymes and drug-loaded nanosystems,could help overcome this drawback (González et al.,2021).
Other anti oxidants have also been shown to improve mitochondrial function.Nicotinamide mononucleoti de adenylyl transferase reduced mHtt aggregation and restored ATP levels in a fly model of HD (Zhu et al.,2019),N-acetylcysteine restored the activities of complexes II,IV,and V in 3-nitropropionic acidtreated mice (Sandhir et al.,2012),while insulin-like growth factor 1 restored mitochondrial function by upregulating the PI3K/protein kinase B signalling pathway (Ribeiro et al.,2014).Bezafibrate is an activator of PGC-1α expression,which in R6/2 and full-length mHtt mouse models of HD reduced striatal atrophy and improved the disease phenotype (Johri et al.,2012).Alpha-lipoic acid and acetyl-L-carnitine,two other anti oxidants,improved the lipid composition and structure of mitochondria,leading to improvement of memory impairments in chemical HD models (Mehrotra et al.,2015).
However,a series of clinical trials with antioxidants failed or yielded minor improvements.Cysteamine,shown to improve motor dysfunctions and increase life expectancy in R6/2 HD mice,failed in a phase 2 clinical trial(NCT02101957) (Verny et al.,2017).Coenzyme Q10,shown in preclinical settings to diminish ROS production and improve mitochondrial bioenergetics,also yielded negative results in a phase 3 clinical trial on 609 patients with early-stage HD (NCT00608881,2CARE) (McGarry et al.,2017).Similar results were obtained for creatine,shown to stimulate mitochondrial respiration,in the CREST-E study (NCT00712426) (Hersch et al.,2017) and ethyleicosapentaenoic acid,shown to bind to mitochondrial PPAR,inhibit caspases,and downregulate the c-Jun N-terminal kinase pathway,in the TREND-HD trial(NCT00146211) (Huntington Study Group TREND-HD Investigators,2008).The effect of resveratrol was evaluated in a phase 3 clinical trial (REVHD,NCT02336633),but the results have not been published so far (https://clinicaltrials.gov).
Potential targets proved to be the Sig-1 receptors.Pridopidine,initially used as a dopamine stabilizer,was subsequently shown to interact with Sig-1 receptors and improve neuronal calcium dyshomeostasis (Grachev et al.,2021).Pridopidine showed modest beneficial effects in clinical trials(NCT02006472,Open-PRIDE-HD-NCT02494778).In addition,through restoring calcium levels in the ER and preventing excessive Ca2+release,pridopidine may also be synaptoprotective by stabilizing dendritic spines (Wu et al.,2018).Another molecule,known as PRE-084,improves the expression of Sig-1 receptors and of antioxidants such as Cu,Zn-SOD,Mn-SOD,or thioredoxins,diminishes caspase-3 cleavage and activates the NF-κB pathway(Motawe et al.,2020) in preclinical research.
Olesoxime is a small cholesterol-like molecule able to accumulate in mitochondria and target to the voltage dependent anion channels.It showed beneficial effects in a BACHD rat model of HD by improving mitochondrial bioenergetics,enhancing the expression of OMM transport proteins,and inhibiting the MPTP opening (Clemens et al.,2015).
Future potenti al therapeutic strategies
A series of novel molecules,which are able to more efficiently restore normal mitochondrial functions,are in research and development.
The mitochondrial fission/fusion process can be regulated with fission inhibitors.Currently,three molecules are evaluated (Kalra,2023).Mdivi-1 has been explored in rodent models for epilepsy and shown to inhibit mitochondrial fission,also acting as a ROS scavenger (Kim et al.,2016).Dynasore acts as a dynamin GTPase inhibitor (Kalra,2023),while P110 appears to block mitochondrial fission through inhibiting the enzymatic acti vity of Drp1 (Qi et al.,2013).
Conjugating conventional mitochondrial-targeted antioxidants,such as coenzyme Q10 or vitamin E,with the lipophilic triphenylphosphonium cation significantly increases the mitochondrial availability of these antioxidants,delivered as MitoQ or MitoVitE (Kalra,2023). MitoQ (mitoquinone mesylate)was shown to penetrate to the IMM and improve healthy mitochondrial biogenesis in striatal progenitor neural cells by increasing the expression of PGC-1α and TFAM (Yin et al.,2016).
Szeto-Schiller pepti des (SS pepti des) are small cell-permeable tetrapepti des which penetrate to the IMM and prevent ROS formation and ROS-mediated damage (Ding et al.,2021),being also able to inhibit mitochondrial depolarization and MPTP opening.SS-31 (also known as Elamipreti de,MTP-131,or Bendavia) increased ATP production by upregulating the mRNA levels of complex I,IV,and V,as well as the mRNA levels of mitochondrial biogenesis regulators (PGC-1α,TFAM).In addition,it improved the mitochondrial fission/fusion balance by upregulating Mfn1,Mfn2,and OPA1 and downregulating Drp1 and Fis1 in striatal HD progenitor neurons (Yin et al.,2016).Mitochondrial trafficking,impaired by tubulin acetylation in HD and Alzheimer’s disease,was shown to improve after histone deacetylase 6 inhibition with tubastatin A,which also increased mitochondrial fusion in striatal neurons (Guedes-Dias et al.,2015).
Another emerging strategy for various diseases in which mitochondrial dysfunction plays a key role is mitochondrial transplantation (or mitochondrial replacement therapy).Although still in development,research has shown that systemically delivered mitochondria can improve neurotoxin-induced Parkinson’s disease in mice (Shi et al.,2017).Increased availability of mitochondria can be obtained through pepti de-mediated delivery or using synaptosomes as natural vehicles (Picone et al.,2021).However,isolation of mitochondria can prove difficult,needing to be completed quickly and at low temperature,and methods for long-term storage of the organelles are not yet available (Park et al.,2021).
Conclusions
Despite compelling evidence for the contribution of mitochondrial dysfunction in the pathogenesis of HD,therapeutic attempts aiming to restore normal mitochondrial function have so far yielded disappointing results in clinical trials,as opposed to encouraging results in animal models.Various reasons may contribute to this discrepancy.
First,a variety of natural compounds,such as resveratrol,quercetin,curcumin,can improve mitochondrial dysfunction by acting on multiple pathways,but they have poor bioavailability.Nanotechnology-based delivery methods could significantly improve our ability to use these compounds and more efficiently rescue mitochondrial function.
Second,a fine balance between mitochondrial fission and fusion is difficult to achieve,while mitochondrial trafficking may be impaired by the disturbed function of the motor proteins and the mutant huntingtin protein(mHtt) aggregates.In addition,in many cases the diagnosis is made in the symptomatic stage,when the vicious cascades involved in HD pathogenesis have already led to significant neuronal loss and synaptic dysfunction,making therapeutic success difficult to achieve.
Nonetheless,in our opinion,the future holds promise.Widespread genetic testing in the families of affected patients could improve diagnosis in presymptomatic stages.The further refinement of genetic editing tools could silence the expression of the defect gene and stop the transcription of the mutant protein while preserving transcription of the wild-type allele.A multimodal approach,enhancing the clearance of mHtt and administering drugs to improve mitochondrial dysfunction,could improve neuronal survival;in extreme cases mitochondrial transplantation could be offered,while stem cell therapies could replace lost cells.
Author contributions:Both authors reviewed the literature.AJ wrote theiniti al draft,while CMJ drew the figures.Both authors approved the submitted version of the manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Open access statement:This is an open access journal,andarticles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Bystanders or not? Microglia and lymphocytes in aging and stroke
- Alzheimer’s disease risk after COVID-19: a view from the perspective of the infecti ous hypothesis of neurodegeneration
- Serine and arginine rich splicing factor 1: a potenti al target for neuroprotection and other diseases
- Can glial cells save neurons in epilepsy?
- Lights for epilepsy: can photobiomodulation reduce seizures and offer neuroprotection?
- The landscape of cognitive impairment in superoxide dismutase 1-amyotrophic lateral sclerosis

