Generating oligodendroglia from adult mesenchymal cells for transplantation: cell reprogramming or direct lineage conversion?
Jorge Pascual-Guerra,José A.Rodríguez-Navarro,Carlos L.Paíno
Cell replacement therapy has long been proposed as a treatment for the damaged nervous system.One of the most challenging aspects of such a strategy,however,is finding sources of donor cells for transplantation.Autologous neural cells are rarely an option as every cell in the nervous system has a defined function that would be lost if that cell was to be removed.One possibility would be sourcing precursor or differenti ated cells from fetal tissues;however,aside from ethical issues,heterologous cells are at risk of immunological rejection in the long term.Methodological improvements over the past 15 years have led to the possibility that autologous non-neural cells could be used for cell transplantation through their conversion into neural derivatives.
Cell reprogramming as a source of neural cells:Neural cells can be generated by reprogramming somatic cells into embryonic-like pluripotent cells—the so-called induced pluripotent stem cells(iPSCs)—and then guiding their differenti ation first into neural stem cells (NSCs) and then into glial precursors or neurons of differing phenotypes(Figure 1).This procedure is laborious and involves the application of a precise sequence of inducing factors,morphogens,and chemical activators or inhibitors,among other agents.The reprogramming of adult somatic cells implies the remodeling of their epigenetic traits,such as patterns of DNA methylation,histone modification,noncoding RNA expression,and chromatin compaction,to erase those that restrict the expression of characteristics of differenti ated cells and create those that enable pluripotent stemness (Wang et al.,2017;Basu and Tiwari,2021).Reprogramming to iPSCs can be achieved through the expression of specific transcription factors (Takahashi and Yamanaka,2006),including Oct4,Sox2,Klf4,and Nanog,as well as DNA methylation modifiers (Basu and Tiwari,2021).These transcription factors are highly expressed in embryonic stem cells but their expression becomes downregulated as the cells lose their pluripotency.Accordingly,they must be exogenously applied to somatic cells to generate iPSCs.
The procedures for the reliable generation of iPSCs and their subsequent conversion to neural cells are conti nuously being improved.Two important challenges limiting the usefulness of this procedure for cell therapy are the heterogeneity of the produced iPSC and NSC cultures and the complexity of the multi-step protocols needed to generate the neural cells.Nevertheless,the main challenge associated with the use of iPSCderived cells for transplantation lies in that these preparations must be devoid of PSCs when transplanted as iPSCs are highly teratogenic.
Direct lineage conversion of adult somatic cells:An alternative procedure for generating neural cells involves the direct phenotypic conversion of somatic cells (also known as transdifferenti ation).This can be achieved via the ectopic expression of cell lineage-specific genes,leading to the generation of different types of neurons or glia(Figure 1).
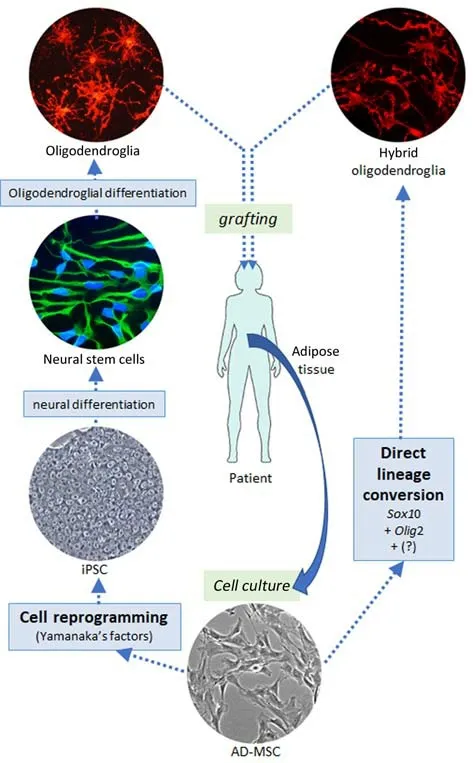
Figure 1|Schematic illustration of the procedures for generating oligodendroglia from adult adipose tissue.
Epigenetic remodeling in direct lineage conversion:When a set of lineage-specifying transcription factors known to induce a functional phenotypic conversion is transfected into an adult somatic cell,many epigenetic traits of the original cell may not be erased,and the converted cell may exhibit a hybrid phenotype;thus,it cannot be considered a reprogrammed cell.Relatively few studies on lineage conversion have assessed whether the acquired phenotype is permanent and self-sustained and if the traits of the donor cell have disappeared.Some transcription factors,such as the basic-helix-loop-helix protein NeuroD1,regulate both transcriptional and epigenetic networks.Accordingly,when NeuroD1 is transduced into microglia,it promotes the acquisition of neuronal characteristics and,subsequently,at least a part of the microglial epigenetic signature in promoter and enhancer regions is erased (Matsuda et al.,2019).So-called“pioneer transcription factors”,such as NeuroD1,can access chromatin sites that are not accessible to non-pioneer transcription factors and also render chromatin competent for the binding of other factors (Zaret and Carroll,2011).Pioneering activity on the epigenetic signature must be demonstrated both for the combinations of transcription factors and in the source cells used for lineage conversion.For instance,NeuroD1 appears to be inefficient at inducing the neuronal transdifferenti ation of cultured astrocytes (Brulet et al.,2017;Wang et al.,2021).Additionally,Ascl1,another well-stablished neuronal conversion inducer,shows notable pioneering activity in human dermal fibroblasts,but is less effective at accessing its cognate sites in normal human keratinocytes (Wapinski et al.,2013).Thus,the level of reprogramming of somatic cells achieved by direct lineage conversion relies on the properti es of the transcription factors used as well as the type and level of differenti ation of the source cells.
Differences between fetal and adult somatic cells for direct lineage conversion:A large variety of protocols for direct lineage conversion have been published,most of which employ fetal fibroblasts as source cells.Although the use of fetal cells may facilitate research progress,the obtained results may not be directly extrapolatable to adult somatic cells,which are the cells that would be used for autologous transplantation.Fetal “fibroblast”cultures comprise cell subtype-rich populations at low differentiation stages.In contrast,adult somatic cells,including adult stem or progenitor cells,exhibit a more restricted differentiation potential,i.e.,they contain a greater number of epigenetic modifications.This difference may affect the speed,efficiency,and completeness of functional conversion.Moreover,the conversion of adult somatic cells may even require the expression of a different set of transcription factors or accompanying chemicals compared with fetal fibroblasts.For instance,it has been reported that the ectopic expression ofSox10,Olig2,andZfp536(Yang et al.,2013) orNkx6.2(Najm et al.,2013)induced the direct functional conversion of rodent fetal fibroblasts into oligodendrocyte precursor cells (OPCs) in only a few weeks.When the OPCs were co-cultured with neurons or transplanted into myelin-deficient mice,compact myelin processes could be observed.The conversion of human fetal/neonatal fibroblasts into oligodendrocyte-like cells through the expression ofSox10,Olig2,andNkx6.2has also been reported (Chanoumidou et al.,2021).Similar procedures were used for adult rat adipose tissue-derived mesenchymal stromal cells (AD-MSCs);however,in this case,only theSox10,Olig2,andZfp536combination induced the conversion into oligodendroglia-like cells with processes capable of wrapping axons (Vellosillo et al.,2022).It took longer for these cells to acquire the oligodendroglial phenotype and compacted myelin surrounding co-cultured axons could not be shown.The conversion of adult human AD-MSCs into oligodendrocyte-like cells was achieved usingSox10,Olig2,andNkx6.1transgene expression;however,conversion required 5 months of culture.Additionally,the procedure showed low consistency,while the oligodendrocyte-like cells exhibited reduced proliferative capacity (Paíno,unpublished data).These differences suggest that the outcomes of lineage conversion of fetal cells may not be directly extrapolatable to similar cells of adults,even when the AD-MSCs show the multi potenti al capabilities of adult stem cells.
Direct lineage conversion does not mean reprogramming:The incomplete reprogramming of converted neural cells would have important implications regarding their possible use for cell therapy.In the above-mentioned study by Vellosillo et al.(2022),adult rat AD-MSCs were lentivirally transduced with tetracycline-inducibleSox10,Olig2,andZfp536transgenes,such that these genes were only expressed when the tetracycline analog,doxycycline,was added to the culture medium.This commonly employed experimental setting allowed to test the irreversibility of the conversion through the withdrawal of doxycycline from the medium once the oligodendroglial phenotype had been completely established.Under these conditions,the molecular signature and axon-ensheathing capacity of the oligodendroglia were lost after 7 to 10 days,and the cells again showed the characteristics of MSCs.These observations suggested that the transgenes had to be continuously expressed to maintain the converted phenotype.In addition,some mesenchymal markers,such as CD73 or smooth muscle actin (SMA),were detected in the converted oligodendroglia,indicating that the cells had not been completely reprogrammed and retained some properti es of AD-MSCs.These results implied that the procedure for generating oligodendroglia from adult AD-MSCs had been unsuccessful;however,it is possible that these hybrid cells may possess qualities that pure oligodendrocytes do not.AD-MSCs have been reported to show neurotrophic,anti -inflammatory,and immunomodulatory qualities (Samadi et al.,2021).Furthermore,they integrate well after intracerebral transplantation without undergoing tumorigenic growth.Transplanting OPCs into multiple sclerosis lesions would likely not succeed as the immunological and cytokinetic environment that damage the host brain would also impair the grafted cells.In contrast,oligodendroglia-like cells converted from AD-MSCs might overcome this non-permissive environment,thus allowing axonal maintenance and ensheathment.Evidently,when using these cells in regenerative medicine,the tetracycline-inducible promoter employed for transgene expression should be replaced.For instance,a self-regulated promoter,such as the SMA,endoglin,or CD73 promoter,would be expected to activate transgene expression as long as MSC traits were maintained,thereby providing a self-regulatory mechanism.Another possibility would be to use the promoter of CNPase,which is expressed in both AD-MSCs and oligodendrocytes,thereby allowing converted cells to maintain a stable phenotype.
Adult MSCs as a source of cells for direct lineage conversion into oligodendroglia-like cells:We propose the use of AD-MSCs for generating oligodendroglia by direct lineage conversion owing to their ease of obtainment in the adult through a small biopsy of subcutaneous fat and their potential therapeutical properties after transplantation.As mentioned above,the efficient lineage conversion of adult human AD-MSCs has yet to be achieved,whereas in rats the procedure is 100% efficient,yielding large populations of oligodendroglial-like cells after only 2 months.Additional transcription factors,small molecules,epigenetic remodelers,growth factors,and other agents must be tested.One possible alternative would be to use MSCs obtained from cranial neural crest-derived adipose tissue,such as ear lobe fat,so that the source cells would have an epigenetic signature closer to that of neural cells.However,we have tried to convert MSCs from dental pulp,also a cranial neural crest derivative,but without success.Eventually,another option might be to use non-autologous but immunologically compatible human umbilical cord MSCs for testing direct lineage conversion given that these cells have a low level of differentiation and,consequently,fewer epigenetic constraints.Further studies are needed to evaluate the potenti al of these strategic perspectives.
This work was supported by projects NDG09/014 and Proyecto VEXEM (to CLP),European Social Fund,YEI,(Nos.PEJ16/MED/AI-1153 and PEJD-2018-PRE/SAL-8532) of the Community of Madrid,Spain;SAF-2016-78666-R,CP19-0010,and PID 2020-113014RB-I00 funded by MCIN/AEI/10.13039/501100011033 (to JARN).
Jorge Pascual-Guerra,
José A.Rodríguez-Navarro,
Carlos L.Paíno*
Service of Neurobiology-Research,Ramón y Cajal University Hospital– IRYCIS,Madrid,Spain(Pascual-Guerra J,Rodríguez-Navarro JA,Paíno CL)Department of Cellular Biology,Complutense University,Madrid,Spain (Rodríguez-Navarro JA)
*Correspondence to:Carlos L.Paíno,PhD,carlos.paino@hrc.es
https://orcid.org/0000-0001-6667-3310(Jorge Pascual-Guerra)
https://orcid.org/0000-0002-0741-6338(José A.Rodríguez-Navarro)
https://orcid.org/0000-0003-4245-592X(Carlos L.Paíno)
Date of submission:June 30,2022
Date of decision:September 21,2022
Date of acceptance:October 19,2022
Date of web publication:November 9,2022
https://doi.org/10.4103/1673-5374.360278
How to cite this article:Pascual-Guerra J,Rodríguez-Navarro JA,Paíno CL (2023) Generating oligodendroglia from adult mesenchymal cellsfor transplantation: cell reprogramming or direct lineage conversion? Neural Regen Res 18(7):1493-1494.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Bystanders or not? Microglia and lymphocytes in aging and stroke
- Alzheimer’s disease risk after COVID-19: a view from the perspective of the infecti ous hypothesis of neurodegeneration
- Serine and arginine rich splicing factor 1: a potenti al target for neuroprotection and other diseases
- Can glial cells save neurons in epilepsy?
- Lights for epilepsy: can photobiomodulation reduce seizures and offer neuroprotection?
- The landscape of cognitive impairment in superoxide dismutase 1-amyotrophic lateral sclerosis

