From dolphins to dogs: new opportunities to understand the role of P2X4 receptors in spinal cord injury and neuropathic pain
Reece A.Sophocleous,Ronald Sluyter
The P2X4 receptor belongs to the P2X receptor family of trimeric ligand-gated ion channels and was the first member of this family for which a crystal structure was obtained (Kawate et al.,2009).This structure confirmed the trimeric stoichiometry of P2X receptors and subsequent studies from the same group revealed the orthosteric binding site of the natural ligand adenosine 5′-triphosphate (ATP) in a cleft between each adjacent subunit (Hattori and Gouaux,2012).Now synonymous with structural descriptions of P2X receptors,these original studies described the structure of each P2X4 receptor subunit as that resembling a dolphin,with the two transmembrane domains representing the fluke and the extracellular loop representing the upper body,including the head,dorsal fin,and left and right flippers.Along with subsequent structures of other P2X receptor members,obtained by either X-ray crystallography or cryo-electron microscopy,these studies have helped to further elucidate the agonist binding sites and conformational states during activation,as well as orthosteric and allosteric binding sites of P2X receptors (Mansoor,2022).Transfer of this knowledge to the design of novel P2X4 receptor antagonists remains scant,with most antagonists described to date arising from the screening of chemical libraries and none advancing to clinical trials beyond NC-2600 (Inoue,2021).Nevertheless,given the roles of the P2X4 receptor within the nervous system,this receptor remains an attractive therapeutic target in the treatment of a range of neurological disorders including pain (Sophocleous et al.,2022).In this regard,the screening of chemical libraries identified the anti-depressants,duloxetine and paroxetine as P2X4 receptor antagonists.This suggests that the historical use of such drugs to alleviate pain in people may have been,at least in part,due to P2X4 receptor inhibition and may warrant future trials for their potential use in humans to safely inhibit P2X4 receptor activity(Kohno and Tsuda,2021).
Further to its potential roles in pain generally,the P2X4 receptor has an established role in neuropathic pain following spinal cord injury.Neuropathic pain results from damage due to a lesion or disease to cause dysfunction of the somatosensory system and can include spontaneous pain,hyperalgesia and allodynia(Kohno and Tsuda,2021).The study of rodent models of spinal cord injury and neuropathic pain has helped established a working model for the role of this receptor in these events and has highlighted the potential benefits of selectively targeting these receptors to improve clinical outcomes in people (Inoue,2021;Kohno and Tsuda,2021;Figure 1).In this model,peripheral nerve injury results in the upregulation of fibronectin,chemokine (C-C) motif ligand 21 and colony-stimulating factor 1,which in turn increases cell-surface P2X4 receptor expression on microglia in the dorsal horn.ATP released from spinal dorsal horn neurons then activates P2X4 receptors to mediate the release of brain-derived neurotrophic factor.Brain-derived neurotrophic factor then binds to tropomyosin-related kinase B on spinal dorsal horn neurons to downregulate the K+-Cl–cotransporter,leading to an increase in intracellular Cl–.This in turn increases the propensity of these neurons to undergo depolarization being excitatory to γ-aminobutyric acid (GABA) or glycine,rendering the individual susceptible to neuropathic pain including allodynia.
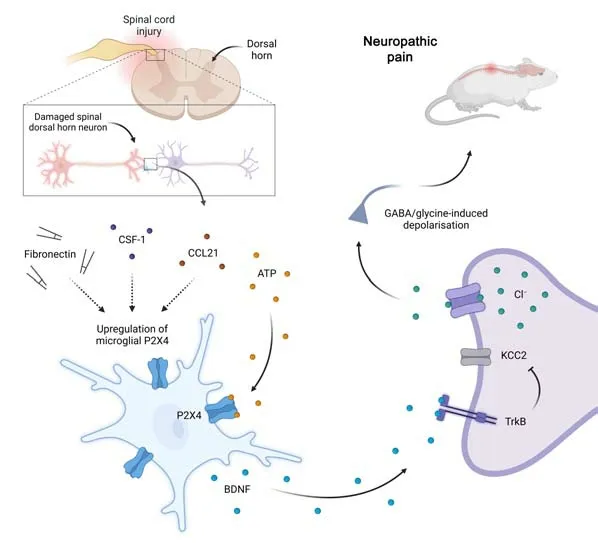
Figure 1|Proposed role of microglia P2X4 receptor activation in neuropathic pain following spinal cord injury.
The clinical implications of spinal cord injury and neuropathic pain are not limited to humans,with such conditions extending to other mammalian and non-mammalian species,some of which serve as models of this condition (Stefanova and Scott,2022).Moreover,spinal cord injury and neuropathic pain are important health issues in many species including dogs (Canis familiaris)(Moore,2016).To this end,our group has recently begun characterizing the canine P2X4 receptor.Comparisons of a recombinant form of this receptor with the recombinant human P2X4 receptor have revealed similar pharmacological profiles (Sophocleous et al.,2020a).ATP activates the canine and human P2X4 receptors with a halfmaximal concentration of 260 nM and 190 nM,respectively.The synthetic ATP analogue 2′(3′)-O-(4-benzoylbenzoyl) ATP is a partial agonist of both receptors,although the half-maximal concentration for the canine P2X4 receptor (360µM) is greater than that for the human P2X4 receptor (10 µM).Neither receptor respond to adenosine 5′-diphosphate.Moreover,ATP-induced activation of canine and human receptors could be positively modulated by ivermectin,reducing the half-maximal concentration of ATP to 100 and 120 nM,respectively.Likewise,the antagonist profiles are similar for canine and human P2X4 receptors,being impaired by the same five antagonists tested and with similar half-maximal inhibitory concentrations for the non-selective P2X antagonist,2,4,6-trinitrophenol-ATP (8 and 4µM,respectively),the selective P2X4 antagonists 5-BDBD (6 and 5 µM,respectively) and BX 430 (8 and 2 µM,respectively),and the anti -depressants duloxetine (15 and 17 µM,respectively) and paroxetine (13 and 78 µM,respectively).Finally,this study revealed that the gene (P2RX4) encoding the P2X4 receptor is highly conserved between dog breeds,suggesting that this receptor is an ideal candidate for drug development for use in dogs.
In addition to the study of recombinant canine P2X4 receptors above,our group has also revealed the presence of native canine P2X4 receptors in cells,with the detection of P2X4 receptor in canine DH82 macrophages (Sophocleous et al.,2020b).Furthermore,this study revealed that ATP or 2′(3′)-O-(4-benzoylbenzoyl) ATP,in part,could induce a Ca2+influx into these cells and that the ATP response was positively modulated by ivermectin and impaired by 2,4,6-trinitrophenol-ATP,5-BDBD or paroxetine.It remains to be determined if P2X4 receptor activation in these cells can mediate events downstream of P2X4 receptor activation such as prostaglandin or cytokine release or whether primary canine macrophages,including microglia,express this receptor.It should be noted that this study revealed that the P2Y2receptor,belonging to the P2Y family of G proteincoupled receptors,was the main purinergic receptor responsible for Ca2+mobilization in DH82 macrophages,which may complicate the future use of this cell line to study native canine P2X4 receptors.
Despite observations of neuropathic pain in numerous species (Stefanova and Scott,2022),this type of pain is an underappreciated health problem in dogs.Neuropathic pain in dogs can arise from a variety of causes including syringomyelia and Chiari-like malformation,radiculopathy (from chronic cervical or lumbosacral disc disease),polyneuropathies,chronic osteoarthritis,stroke and intervertebral disc disease (Moore,2016) with the latter a common cause of spinal cord injury in dogs (Olby and Tipold,2021).The most commonly used drugs for the treatment of neuropathic pain in dogs include the GABA analogues,gabapentin and pregabalin (which appear to act independently of GABAergic action),and to a lesser extent the tricyclic antidepressant amitriptyline,and the N-methyl-D-aspartate receptor antagonist amantadine.The use of these drugs in neuropathic pain in dogs however is limited to a small number of case reports rather than case-controlled studies(Moore,2016).As such,management of canine neuropathic pain remains sub-optimal,but the identification of functional canine P2X4 receptors opens a new area of investigation to understand this disorder and how to best treat it in dogs.Moreover,given the various causes of neuropathic pain in these animals,dogs provide an opportunity to explore the wider role of P2X4 receptors in this disorder and to determine if the role of this receptor in neuropathic pain extends beyond spinal cord injury.In this regard,dogs may serve as a useful source of nervous tissue,which is not as readily available from humans,to investigate the expression and distribution of P2X4 receptors in the various conditions that lead to neuropathic pain development and to determine if such pain extends beyond spinal cord injury.Moreover,with the ongoing development of P2X4 receptor antagonists,including the potential use of the anti-depressants duloxetine and paroxetine as P2X4 receptor antagonists,that display acceptable safety in dogs (Fitzgerald and Bronstein,2013),these animals provide a model of drug testing for neuropathic pain for the potenti al use of such antagonists in people and dogs therapeutically.
In conclusion,P2X4 receptors are an emerging drug target for the treatment of neuropathic pain and other neurologic disorders in people.Although there has been limited progress in the generation of clinically suitable drugs,the well-established structure of the P2X4 receptors,with the shape of its subunits akin to that of the dolphin,conti nues to afford new opportunities for drug design and discovery.The identification of functional P2X4 receptors in dogs suggests that these and other companion animals may also benefit from the use of drugs targeting these receptors.However,the expression of P2X4 receptors within the nervous system of dogs,including changes in receptor expression in response to spinal cord injury or other causes of neuropathic pain,remains unknown.Nonetheless,cell and tissue samples from dogs will provide a valuable resource for exploring the role of P2X4 receptors in spinal cord injury and neuropathic pain with potenti al benefits for humans and dogs alike.
Reece A.Sophocleous,Ronald Sluyter*
Illawarra Health and Medical Research Institute,Wollongong,NSW,Australia (Sophocleous RA,Sluyter R)
Molecular Horizons and School of Chemistry and Molecular Bioscience,University of Wollongong,Wollongong,NSW,Australia (Sophocleous RA,Sluyter R)
*Correspondence to:Ronald Sluyter,PhD,rsluyter@uow.edu.au.
https://orcid.org/0000-0002-8339-9090(Reece A.Sophocleous)
https://orcid.org/0000-0003-4909-686X(Ronald Sluyter)
Date of submission:August 26,2022
Date of decision:October 11,2022
Date of acceptance:October 19,2022
Date of web publication:November 9,2022
https://doi.org/10.4103/1673-5374.360294
How to cite this article:Sophocleous RA,Sluyter R(2023) From dolphins to dogs: new opportunities to understand the role of P2X4 receptors in spinal cord injury and neuropathic pain.Neural Regen Res 18(7):1497-1498.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Bystanders or not? Microglia and lymphocytes in aging and stroke
- Alzheimer’s disease risk after COVID-19: a view from the perspective of the infecti ous hypothesis of neurodegeneration
- Serine and arginine rich splicing factor 1: a potenti al target for neuroprotection and other diseases
- Can glial cells save neurons in epilepsy?
- Lights for epilepsy: can photobiomodulation reduce seizures and offer neuroprotection?
- The landscape of cognitive impairment in superoxide dismutase 1-amyotrophic lateral sclerosis

