New unexpected role for Wolfram Syndrome protein WFS1: a novel therapeutic target for Alzheimer’s disease?
Shuo Chen,Diana Acosta,Hongjun Fu
Selective vulnerability of excitatory neurons in Alzheimer’s disease (AD):AD is the most common form of dementia;however,the pathogenesis of AD is largely unknown.One of the characteristic features of AD is the formation of intracellular neurofibrillary tangles (NFTs).NFTs are abnormal accumulates of misfolded tau protein,which may eventually cause neuronal death and neurodegeneration (Jack et al.,2018).In the early stages of AD progression,not all neurons are equally vulnerable to tau aggregates.Previous studies have shown that large pyramidal neurons in the entorhinal cortex (EC) are specifically vulnerable to pathological tau accumulation(Fu et al.,2017).This selective vulnerability of excitatory neurons to tau pathology is one of the fundamental questions needed to be answered in AD research.
Techniques such as single-nucleus RNA sequencing enable us to profile the transcriptomics of cells on a large-scale and allow us to uncover subtypes of excitatory neuronal populations which may play important roles in the onset and progression of AD.For example,one recent study has identified a particular subtype of excitatory neurons expressing the RAR-related Orphan Receptor B gene as more vulnerable in the EC by comparing transcriptomic profiles of postmortem human brains from different AD stages (Leng et al.,2021).Although single-nucleus RNA sequencing allows us to compare differenti ally expressed genes among different subtypes of neurons,thereby allowing us to distinguish vulnerable neurons in AD,we sti ll cannot directly attribute these differences to the presence of pathological components such as NFTs since we are unable to target and profile neurons bearing NFTs at the whole transcriptome level.However,we can validate results from transcriptomic studies by using additional models of AD including tau mouse models,to further inquire about the molecular mechanisms underlying the selective vulnerability of certain excitatory neurons to tau pathology in AD.
Grid cells,which are located in layer II/III of the EC,have been recently found to be preferenti ally vulnerable to tau pathology (Fu et al.,2017).This subtype of excitatory neurons has been identified to regulate animals’ spatial navigation and memory.In EC-tau mice (a tau transgenic mouse model),tau accumulation has been demonstrated to cause grid cell dysfunction and neuronal death(Fu et al.,2017).Interestingly,grid cells also highly express wolframin (WFS1) protein.Delpech et al.(2021) have recently shown WFS1-positive(WFS1+) neurons make up 6.5% of total cells in human EC layer II.Moreover,the proportion of WFS1+neurons dramatically decreased in the postmortem human brain from individuals with clinical dementia rating (CDR) scores over 2(2.5% for CDR2 and 2.7% for CDR3),and PHF1+(pS396/404) p-tau started to accumulate in ECII WFS1+neurons at early stages with mild cognitive decline (Delpech et al.,2021).In summary,the protein level of WFS1 and the number of WFS1+neurons are significantly reduced in both AD-like mouse model brains and human post-mortem AD,suggesting these WFS1+neurons become vulnerable to tau pathology when their WFS1 level decreases.Why WFS1+neurons are particularly vulnerable to tau pathology and the role of WFS1 in tau pathology associated neurodegeneration,however,remains unknown.
Lessons from Wolfram Syndrome: Similarities between Wolfram Syndrome and AD point to a new role for WFS1 in neurodegeneration:WFS1 is an endoplasmic reticulum (ER) membrane protein encoded by theWFS1gene which is highly expressed in the human brain and pancreas.Mutations in the humanWFS1gene have been identified as a causative factor for Wolfram Syndrome,a rare and severe neurodegenerative disease.Though no loss-of-function mutations ofWFS1have been found in AD,both Wolfram Syndrome and AD display similarities in terms of ER Ca2+dyshomeostasis,ER stress,and autophagy dysfunction (Li et al.,2020).It is worth investigating whether WFS1 deficiency in AD also affects Ca2+homeostasis,ER stress,and autophagy in a similar way to that in Wolfram Syndrome.
Since WFS1+cells have been found to colocalize with pathological tau (Delpech et al.,2021),we first investigated whether WFS1 deficiency promotes tau aggregation or inhibits tau degradation and clearance.By knocking out WFS1 in PS19 mice (P301S transgenic tau mice (Yoshiyama et al.,2007)),tau aggregates were found significantly increased in layers II/III of the EC,and the learning and memory of those mice were also impaired.However,tau pathology decreased dramatically when WFS1 was overexpressed in the EC region of PS19 mice three months after the injection of human WFS1 virus,compared with age matched PS19 mice injected with a control GFP virus.Importantly,astrogliosis,synaptic dysfunction and neurodegeneration associated with tau pathology were also reversed in PS19 mice by overexpressing WFS1 (Chen et al.,2022).To further confirm whether the WFS1 protein directly interacts with tau protein in neurons,the Duolink proximity ligation assay was performed.In both the EC-tau mice and human postmortem brain samples,WFS1 was found to interact with total tau (recognized by the Tau46 anti body) and pathological tau (recognized by the AT8,pS202/T205 tau antibody),indicating WFS1 may be involved in tau aggregation,propagation,and degradation by directly interacting with tau protein (Chen et al.,2022).
We next explored the mechanism of how WFS1 deficiency affected tau aggregation and clearance.A previous study of Wolfram Syndrome has shown that WFS1 defects may induce chronic ER stress and further cause the impairment of the ER-associated degradation in pancreatic β-cells (Li et al.,2020).In neurons,we also found evidence linking WFS1 deficiency to chronic ER stress and autophagy-lysosome pathway (ALP)dependent ER-associated degradation.After knocking out WFS1 in PS19 mice,ATF4 and CHOP,two chronic ER stress markers,were significantly increased in the EC.Meanwhile,p62,a critical autophagosome cargo protein in ALP,started to accumulate in the tau aggregated cells.Moreover,autophagy related marker cathepsin D and lysosome biogenesis regulator transcription factor EB were also significantly reduced.On the contrary,overexpressing WFS1 could reverse those phenotypes in PS19 mice.These results suggest that WFS1 deficiency may induce chronic ER stress,and further affect the degradation and clearance of tau aggregates via the ALP pathwayin vivo(Chen et al.,2022).
WFS1: a new regulator of ER stress,and autophagy in AD:ER stress is regulated by three unfolded protein response (UPR) signaling pathways,which are initiated from three ER membrane sensors: protein kinase RNAlike endoplasmic reticulum kinase (PERK),inositol-requiring enzyme 1,and the activating transcription factor 6.In Wolfram Syndrome,WFS1 deficiency has been reported to be involved in the upregulation of all three UPR pathways (Li et al.,2020).Though WFS1 deficiency has been identified to affect the PERK/ATF4/CHOP pathway in AD (Chen et al.,2022),it is unknown whether it is also involved in other two UPR signaling pathways (Figure 1).Moreover,although evidence of ER stress response in AD is apparent,whether it is a causative factor of AD remains in question.Importantly,opposing studies on ER stress response in AD from different groups contribute to this debate.For example,Halliday et al.(2017)observed the neuroprotective effects of PERK pathway inhibitors in both tauopathy and priondiseased mice,while Bruch et al.(2017) found that PERK activation may mitigate tau pathologyin vitroandin vivo.However,considering the potential relationships between WFS1,ER stress,and AD pathogenesis,it will still be of great interest to further investigate the role of WFS1 in ER stress pathways in AD.
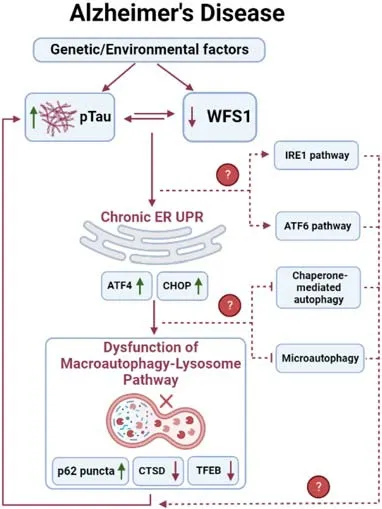
Figure 1|The role of WFS1 in Tau clearance in Alzheimer’s disease.
As discussed above,ER stress may further trigger the ALP dependent ER-associated degradation and downstream autophagy pathway through UPR.Autophagy mainly consists of three categories:macroautophagy,chaperone-mediated autophagy(CMA),and microautophagy.WFS1 deficiency has been found to impair the macroautophagy via chronic ER stress (Chen et al.,2022),however,it is not clear whether WFS1 deficiency is also involved in the dysfunction of CMA and microautophagy in AD and other neurodegenerative diseases.Unlike macroautophagy,CMA selectively degrades cytosolic proteins that contain a KFERQ-like motif which can be recognized by the molecular chaperone heat-shock cognate protein 70.The target protein will then form a complex with heat-shock cognate protein 70 and binds with lysosome-associated membrane protein type 2A on the lysosomal surface for further degradation.Both amyloid precursor protein and tau can be a target of the CMA pathway: amyloid precursor protein contains a KFERQ-like motif (Park et al.,2016),while tau contains QVEVK and KDRVQ motif which can be targeted by CMA pathway(Wang et al.,2009).In contrast,microautophagy has more diverse morphologies and types which directly engulf the cargo by lysosomal action (Oku and Sakai,2018).Mutations in tau could prevent their degradation by microautophagy and in turn become dependent on macroautophagy for degradation of such proteins.Post-translationally modified tau,such as acetylated tau can also undergo microautophagy and if blocked,reroute to macroautophagy (Caballero et al.,2021).However,the role of CMA and microautophagy in amyloid precursor protein/amyloid β or tau clearance is sti ll under investigation.Future studies investigating the role of WFS1 in tau clearance via the CMA or microautophagy pathway are also warranted to reveal their potential relationship(Figure 1).
Conclusions and perspectives:Although we demonstrate WFS1 plays an important role in inhibiting tau pathology,one important question remains.It is sti ll unknown why WFS1+excitatory neurons in EC layer-II are more vulnerable than other types of neurons if WFS1 has a neuroprotective function.In general,selective vulnerability might result from low levels of protective factors or high levels of toxic factors,and both intrinsic and extrinsic factors may contribute to this selective neuronal vulnerability.We hypothesize that there are specific gene signatures and signaling pathways,which may contribute to the selective vulnerability of WFS1+EX neurons to tau pathology in the EC.We will further investigate if WFS1+EX neurons intrinsically express less tau aggregation protector genes and more genes encoding tau co-aggregators and aggregation-prone proteins than WFS1-negative neurons.
Considering the short average life expectancy(30–40 years of age) of Wolfram Syndrome,no Wolfram Syndrome patients have been reported to develop AD,in which aging is the most important known risk factor.However,cognitive disability has been found in 32% of Wolfram Syndrome patients (Li et al.,2020).Both Wolfram Syndrome and AD share common physiopathological signaling pathways (Li et al.,2020).Thus,reduced WFS1 caused by unknown factors may increase the neuronal vulnerability to tau pathology and neurodegeneration in AD pathogenesis,even if Wolfram Syndrome patients do not show AD pathology.
Interestingly,we also found the protein level and the number of WFS1+cells were significantly reduced not only in AD,but also in other neurodegenerative diseases such as frontotemporal lobar degeneration with tau pathology,frontotemporal lobar degeneration with transactive response DNA binding protein,and diffuse Lewy body disease (Chen et al.,2022).This indicates that WFS1+neurons in the EC may be vulnerable in other types of neurodegenerative diseases as well.Although the downstream pathways of WFS1 deficiency remain to be determined for other proteinopathies,WFS1 may be a common therapeutic target in proteinopathies besides AD.
Taken altogether,as an important regulator of both ER stress and autophagy pathways,WFS1 could be a potential target for developing novel drugs to promote the degradation and clearance of misfolded proteins in AD and other neurodegenerative diseases.
The present work was supported by awards K01-AG056673,R56-AG066782-01 and R01-AG075092-01 (to HF) from the National Institute on Aging of the National Institutes of Health.The work was also supported by the award of the W81XWH1910309 (to HF) from the Department of Defense.
Shuo Chen,Diana Acosta,Hongjun Fu*
Department of Neuroscience,Biomedical Sciences Graduate Program,The Ohio State University,Columbus,OH,USA (Chen S)
Department of Neuroscience,The Ohio State University,Columbus,OH,USA (Acosta D,Fu H)
*Correspondence to:Hongjun Fu,PhD,Hongjun.fu@osumc.edu.
https://orcid.org/0000-0001-5346-7075(Hongjun Fu)
Date of submission:August 11,2022
Date of decision:October 20,2022
Date of acceptance:October 29,2022
Date of web publication:November 25,2022
https://doi.org/10.4103/1673-5374.361540
How to cite this article:Chen S,Acosta D,Fu H(2023) New unexpected role for Wolfram Syndrome protein WFS1: a novel therapeutic target for Alzheimer’s disease? Neural Regen Res 18(7):1501-1502.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Bystanders or not? Microglia and lymphocytes in aging and stroke
- Alzheimer’s disease risk after COVID-19: a view from the perspective of the infecti ous hypothesis of neurodegeneration
- Serine and arginine rich splicing factor 1: a potenti al target for neuroprotection and other diseases
- Can glial cells save neurons in epilepsy?
- Lights for epilepsy: can photobiomodulation reduce seizures and offer neuroprotection?
- The landscape of cognitive impairment in superoxide dismutase 1-amyotrophic lateral sclerosis

