Nociceptors are needed to guide tooth development,function,repair,and regeneration
Sarah B.Peters,Joshua J.Emrick
Toothaches have received widespread attention and commentary dating back to ancient times,appearing in Egypti an texts from over 3000 years ago and the writings of Hippocrates,Aristotle,and Galen.Scientific studies of the innervation of dental pulp and dentin date back to at least the 1800’s,yet our understanding of the basis for tooth pain is sti ll limited.Standard treatments for toothache conti nue to be drastic and irreversible,including removal of the dental pulp or extraction of the tooth.While the trigeminal sensory innervation of the tooth pulp is commonly the origin of toothache pain,it is also indispensable for normal function and physiology,and should be retained as an integral part of the repaired tooth.Repair or regeneration of the dental pulp and its innervation could represent the next step in restorative denti stry,but a better understanding of the roles of the tooth’s sensory nerve supply and the mechanisms underlying its development and repair is needed.
During the development of primary and permanent teeth,the dental mesenchyme secretes neurotrophic factors to guide the timing and patterning of axonal extensions into and throughout the dental pulp.These signals include neurotrophins to encourage neurite outgrowth as well as guidance cues to ensure correct localization.A recent report found that the innervation and the differentiation and mineralization capacity of the dental pulp cells are inti mately linked,suggesting that the signaling between the mesenchyme and innervation is bidirectional (Stanwick et al.,2022).Supporting this relationship,it was shown that prior to the exfoliation of a primary tooth,the pulpal tissue,including the innervation,undergoes degeneration that results in a progressive loss of tooth sensation.This is followed by a typically painless loss of the tooth.Understanding the mechanisms associated with denervation and the loss of primary teeth,as well as the subsequent eruption and innervation of the permanent teeth,may reveal the molecular signals required for pulp regeneration.
It is generally believed that the dental pulp tissue in primary dentition retains its ability to heal and sense unti l the advanced stages of root resorption.Recent studies have detailed the histological changes that occur in the pulp tissue,including neurofilament fragmentation,increased apoptosis in the Schwann cells,and a gradual loss of myelinated axons.Investigations into what is driving this Wallerian degradation should provide useful information about nerve repair and regulation that could contribute to improved clinical treatments for primary denti tion (Suzuki et al.2015;Xiao et al.2022).While the replacement permanent tooth (i.e.,succedaneous tooth) will ultimately be innervated,it is currently unclear whether the afferents are repurposed from the primary tooth afferents,appropriated from the surrounding periodontal tissue,or arise de novo(Figure 1A).It is plausible that primary axon degeneration represents loss of only a portion of the neurons’ collateral axons,and the others are retained to reinnervate the permanent denti tion.Interestingly,the observation that axonal degeneration in the spinal trigeminal nucleus in the brainstem coincides with tooth exfoliation(Westrum and Canfield 1979),suggests that the central projections of this circuit also undergo remodeling during transitions.Further studies are necessary to understand the transitions between primary and permanent denti tion and its innervation.
It is already known that there are differences between the innervation of permanent and primary teeth.For example,in innervated permanent dentition,sensory afferents branch into a denser distribution of terminals than in primary teeth (Kaj and Gibbs,2014).This difference in morphology (Figure 1AandB),as well as changes in transcription,might contribute to the increased sensation clinically reported in adult dentition (Kim et al.,2014).To date,transcriptomic comparisons of the primary and permanent denti tion have focused exclusively on the pulp tissues.It is still unknown whether the transcriptomes and functions of tooth-innervating neurons differ between these teeth despite serving an overtly equivalent role.Analyses of diphyodont models (i.e.,animals that transition through two sets of teeth) will be needed to investigate how innervation strategies conserve the sensory capacity of teeth.Ulti mately,if toothinnervating neurons are retained when the pulp or the enti re tooth is lost,there may be a capacity for regenerative therapies to re-attract afferents using the process(es) found to be involved in tooth development.
Teeth normally function without any perceptible output from their innervation.Odontogenic pain(toothache) occurs as a result of alteration(s)in the tooth’s sensory system.Nociceptive and inflammatory pain in the tooth and oral cavity stems from the trigeminal sensory neurons and their afferents.When triggered by environmental sti muli,these neuron terminals relay information via electrical signaling to higher order structures in the nervous system.These signals are the neural substrate for integration and the resulting perceptions.Sensory neurons stimulated by noxious or damaging stimuli are defined as nociceptors,and the associated perception is pain.The human experience leads us to expect that the activation of tooth afferents and sensory neurons results in pain.This is a reasonable association considering a toothache can become supremely intense and lead to work absenteeism,reduced producti vity,and reduced quality of life.However,the focus on acute dental pain associated with damaged teeth may conceal beneficial functions of these neurons in healthy teeth.
Recent work has provided important insights into the full complement of gene expression in the trigeminal sensory neurons that innervate the dental pulp.These data indicate that a substanti al proportion of the neurons that target the dental pulp expressed the voltage-gated sodium channel Nav1.8 (Scn10a) associated with pain signaling,indicating that these neurons likely detect noxious stimuli (i.e.,they are nociceptors).However,the expression profile of these neurons also indicates that they are large,myelinated,and have the capacity to respond to mechanical sti muli (Emrick et al.,2020).These latter data might indicate that tooth innervation serves as a sentinel that protects the dentition,not just produces painper se.It is possible that tooth-innervating neurons are capable of detecting untoward tooth movements or excessive physical forces that would damage tooth integrity.These electrical impulses,by virtue of myelinated axons,rapidly enter the brainstem for processing and lead to a protective response,which is essenti al for ensuring the longterm use of the tooth.While tooth pain research has generally focused on neuronal activation and transcriptional responses,assays including behavioral and reflexive responses could deepen our understanding of how the innervating afferents protect tooth integrity.
Once a tooth is damaged by trauma or dental caries (i.e.,an infection),the dental pulp develops inflammation (pulpitis),which is associated with severe pain that does not resolve without surgical intervention.This process lacks clear evolutionary benefit considering the paucity of mammalian dentists,outside of humans.Several groups have proposed that this pain is due to an inflammation-induced upregulation of the expression of nociceptor receptors in the ganglion,such as transient receptor potenti al cation channel subfamily V member 1 (Trpv1) (Luo et al.,2021).Retrograde tracing of tooth-innervating neurons(Emrick et al.,2020),as well as monitoring and recording from these neurons in the trigeminal ganglionin vivo,will be essenti al for determining the particular triggers for these neurons,discerning their transcriptional changes after damage,and identi fying which neurons contribute to pulpitis.Because pulpitis has a diverse eti ology,it would also be prudent to evaluate whether the mechanisms and pathways involved in the process differ based on the type of insult.Understanding these processes may reveal targets to attenuate tooth pain and/or reverse it,which could allow for supplementary treatments to promote dental pulp repair.
When acute trauma or infection of the tooth results in irreversible pulpitis,the current clinical therapy relies on pulp extirpation,sterilization,and obturation with dental materials (i.e.,traditional root canal therapy) in conjunction with analgesics.For moderate pain,clinicians prescribe nonsteroidal anti-inflammatory drugs (NSAIDs).Severe pain is often treated with opioid analgesics,which has contributed to the opioid addiction crisis worldwide.Thus,there is an urgent need to better understand the role(s) of nociception,with a goal of developing specifically-targeted,effective analgesics with a low potential for abuse.Notably,the denti tion receives substanti al innervation by nociceptors (Emrick et al.,2020)and is readily available forin vivostudies due to its exposed nature in the oral cavity.Preclinical research using the tooth as a pain model can help to develop a better understanding of the utility of nociception and pain,and how it regulates healing and subsequent functions.Ultimately,it is anticipated that other forms of pain may share common mechanisms with toothache,and the insights gained from dental research could prove to be broadly useful in various fields of medicine.
Beyond their established sensory roles,nociceptors contribute to both normal pulp physiology and healing after injury.For instance,shallow tooth injuries have been shown to induce peptidergic neurite outgrowth during dentin regeneration(Taylor et al.,1988;Figure 1BandC).Additionally,denervation of teeth and/or nociceptor ablation has been shown to accelerate necrosis in the dental pulp (Diogenes 2020) and surrounding periodonti um (Austah et al.,2022) after injury.In both of these studies,the sprouting sensory fibers demonstrated increased levels of calcitonin generelated peptide (CGRP),a neuropeptide that has been used to define pepti dergic nociceptors.CGRP also regulates blood pressure and inflammatory responses within the tooth unit.A recent study demonstrated upregulation of the gene,the gene of the receptor and the expression of CGRP during irreversible pulpitis correlative with increased pain sensiti vity (Casti llo-Silva et al.,2019).We believe it is essenti al to explore dental pulp injury/infection and healing in rodent models with knock-out of nociceptive receptors,as well as pharmacological inhibition,to determine the dependence of healing and regeneration on nociceptors,nociceptor sensory signaling,and pain.Antagonism may be useful to circumvent undesirable effects of genetic deletion of receptors,if any are noted.Importantly,medical research and clinical practice suggest that pharmacological inhibition is capable of providing pain relief without delaying or preventing healing or regeneration.For example,NSAIDs and opioid analgesics are often prescribed post-surgically without overt effects on tissue healing.Strategies to uncouple nociceptor activation and pain from repair could be an essential component of restorative or regenerative therapies.
Regenerative medicine and tissue engineering represent the next frontier in dentistry,with the current emphasis being placed on the application of stem cell therapies toward dental tissue regeneration.Dental researchers and clinicians exploring tooth structure and function have traditionally focused their attention on the predominantly collagenous matrix mineralized with hydroxyapati te crystals.However,regenerated dental hard tissues are more fibrous or bone-like in nature,lacking tubular structure and sensiti vity(Diogenes 2020).We predict that a multicellular environment is needed to produce synthetic dentin that more closely approximatesin vivonatural structures.Thus,regenerated pulp tissue should ideally include soft tissue components of the tooth,including vasculature and innervation with proper localization.The tissue would also have the capacity to control blood flow,respond to re-infection,and provide normal sensation.In other words,the regenerated pulp would be capable of performing the normal roles required to maintain the function and health of the denti tion.
Here,we provided a brief summary of the current understanding of the development and function of dental pulp innervation.While tooth innervation is associated with the severe pain,it also provides essential sensory and physiological cues for the maintenance of healthy denti tion.Studying tooth innervation may help us to understand pain affecting various physiological sites and facilitate the development of specific and effective nonopioid analgesics.We propose an array of studies that address the limits of our knowledge,would contribute to our fundamental understanding of tooth pulp development and neurophysiology,and ultimately facilitate dental pulp repair and regeneration.With this,we believe that pulp therapies could be steered away from removal and replacement toward repair and regenerative therapies that preserve tooth vitality throughout all stages of life (summarized inFigure 1D–F).
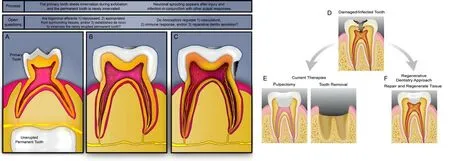
Figure 1|Tooth innervation and treatments at varying stages of tooth life.
This work was supported by grants from NIH K99/R00DE027706 (to SBP) and NIH K22DE029779 (to JJE).
Sarah B.Peters*,Joshua J.Emrick
Division of Biosciences,College of Denti stry,The Ohio State University,Columbus,OH,USA(Peters SB)
Department of Biologic and Materials Sciences &Prosthodontics,School of Denti stry,University of Michigan,Ann Arbor,MI,USA (Emrick JJ)
*Correspondence to:Sarah B.Peters,MS,PhD,peters.1026@osu.edu.
https://orcid.org/0000-0003-3384-5109(Sarah B.Peters)
https://orcid.org/0000-0001-5619-1078(Joshua J.Emrick)
Date of submission:July 7,2022
Date of decision:September 14,2022
Date of acceptance:October 20,2022
Date of web publication:November 9,2022
https://doi.org/10.4103/1673-5374.360280
How to cite this article:Peters SB,Emrick JJ(2023) Nociceptors are needed to guide tooth development,function,repair,and regeneration.Neural Regen Res 18(7):1503-1504.
Open access statement:This is an open access journal,and articles are distributedunder the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Bystanders or not? Microglia and lymphocytes in aging and stroke
- Alzheimer’s disease risk after COVID-19: a view from the perspective of the infecti ous hypothesis of neurodegeneration
- Serine and arginine rich splicing factor 1: a potenti al target for neuroprotection and other diseases
- Can glial cells save neurons in epilepsy?
- Lights for epilepsy: can photobiomodulation reduce seizures and offer neuroprotection?
- The landscape of cognitive impairment in superoxide dismutase 1-amyotrophic lateral sclerosis

