Molecular and cellular changes in the post-traumatic spinal cord remodeling after autoinfusion of a genetically-enriched leucoconcentrate in a mini-pig model
Maria Aleksandrovna Davleeva ,Ravil Rasimovich Garifulin ,Farid Vagizovich Bashirov ,Andrei Aleksandrovich Izmailov ,Leniz Faritovich Nurullin, ,Ilnur Ildusovich Salafutdinov,,Dilara Zilbarovna Gatina,Dmitrij Nikolaevich Shcherbinin,Andrei Aleksandrovich Lysenko,Irina Leonidovna Tutykhina,Maksim Mikhailovich Shmarov,Rustem Robertovich Islamov,
Abstract Post-traumatic spinal cord remodeling includes both degenerating and regenerating processes,which affect the potency of the functional recovery after spinal cord injury (SCI).Gene therapy for spinal cord injury is proposed as a promising therapeutic strategy to induce positive changes in remodeling of the affected neural tissue.In our previous studies for delivering the therapeutic genes at the site of spinal cord injury,we developed a new approach using an autologous leucoconcentrate transduced ex vivo with chimeric adenoviruses (Ad5/35) carrying recombinant cDNA.In the present study,the efficacy of the intravenous infusion of an autologous genetically-enriched leucoconcentrate simultaneously producing recombinant vascular endothelial growth factor (VEGF),glial cell line-derived neurotrophic factor (GDNF),and neural cell adhesion molecule (NCAM) was evaluated with regard to the molecular and cellular changes in remodeling of the spinal cord tissue at the site of damage in a model of mini-pigs with moderate spinal cord injury.Experimental animals were randomly divided into two groups of 4 pigs each: the therapeutic (infused with the leucoconcentrate simultaneously transduced with a combination of the three chimeric adenoviral vectors Ad5/35-VEGF165,Ad5/35-GDNF,and Ad5/35-NCAM1) and control groups (infused with intact leucoconcentrate).The morphometric and immunofluorescence analysis of the spinal cord regeneration in the rostral and caudal segments according to the epicenter of the injury in the treated animals compared to the control mini-pigs showed: (1) higher sparing of the grey matter and increased survivability of the spinal cord cells (lower number of Caspase-3-positive cells and decreased expression of Hsp27);(2) recovery of synaptophysin expression;(3) prevention of astrogliosis (lower area of glial fibrillary acidic protein-positive astrocytes and ionized calcium binding adaptor molecule 1-positive microglial cells);(4) higher growth rates of regenerating βIII-tubulinpositive axons accompanied by a higher number of oligodendrocyte transcription factor 2-positive oligodendroglial cells in the lateral corticospinal tract region.These results revealed the efficacy of intravenous infusion of the autologous genetically-enriched leucoconcentrate producing recombinant VEGF,GDNF,and NCAM in the acute phase of spinal cord injury on the positive changes in the post-traumatic remodeling nervous tissue at the site of direct injury.Our data provide a solid platform for a new ex vivo gene therapy for spinal cord injury and will facilitate further translation of regenerative therapies in clinical neurology.
Key Words:autologous genetically-enriched leucoconcentrate;chimeric adenoviral vector;gene therapy;glial cell line-derived neurotrophic factor;mini-pig;neural cell adhesion molecule;spinal cord contusion injury;vascular endothelial growth factor
Introduction
Spinal cord injury (SCI) remains one of the most important problems in fundamental neuroscience and clinical neurology.Post-traumatic spinal cord remodeling includes both degenerating and regenerating processes,which affect the potency of the functional recovery after SCI.The nature of the post-traumatic spinal cord remodelling is well characterized in recent reviews (O’Shea et al.,2017;Alizadeh et al.,2019).In the acute phase,SCI results in the immediate death of brain cells and the rupture of neurites in the grey matter and the spinal tracts of white matter (Beattie et al.,2002;Kerschensteiner et al.,2005;Seif et al.,2007;Almad et al.,2011).The following massive loss of cells associated with hemorrhage,ischemic,and inflammation causes cavities and cysts (Radojicic et al.,2005;Seki and Fehlings,2008).The subsequent regenerative processes lead to remodeling of the SCI lesion (O’Shea et al.,2017).The central area of the mature SCI lesion has a non-neural core,which includes endogenous proliferating fibroblasts,pericytes,endothelial cells,and immigrating from the blood macrophages,neutrophils,and lymphocytes.The non-neural core is bordered by the glial scar consisting of glial progenitors,newly proliferated reactive astrocytes,and microglial cells (Silver and Miller,2004).The glial scar and its associated matrix including certain collagens and chondroitin sulfate proteoglycans prevent not only the spreading of neuroinflammation but also axon regeneration through this region (Alizadeh et al.,2019).The reactive neural tissue surrounding the glial scar is characterized by axon regrowth and sprouting,and restoration of the lost neural connections and neural circuits (Beattie et al.,1997;Krupa et al.,2020).
To date,gene therapy for SCI is proposed as a promising therapeutic strategy for neurorehabilitation (Walthers and Seidlits,2015).Regenerative therapy is aimed to overcome the negative consequences of the SCI lesion and modulate positive remodeling of the post-traumatic spinal cord in terms of prevention of cavitation and scarring and stimulation of neuroregeneration (axon growth,synaptogenesis,and restoration of the functional neural circuits).Delivery of recombinant cDNA encoding neurotrophic (brain-derived neurotrophic factor,ciliary neurotrophic factor,nerve growth factor,and glial cell line-derived neurotrophic factor [GDNF]),vascular (vascular endothelial growth factor[VEGF] and ANG),anti -inflammatory (interleukin-10) and anti -apoptotic (Bcl-2) factors,and neural cell adhesion molecules (L1 and neural cell adhesion molecule [NCAM]) at the epicenter of injury is based onin vivo(direct gene therapy) orex vivo(cell-mediated gene therapy) approaches (Izmailov et al.,2017;Markosyan et al.,2020).
Viral vectors generally used in gene therapy research are based on adenoviruses,adeno-associated viruses,and retroviruses (Abdellatif et al.,2006).Despite the superiority of viral vectors over plasmid vectors in efficacy,their applications are associated with safety problems,such as the risk of insertion mutagenesis and inducing an immune response to viral antigens(Arjmand et al.,2020).
Adenoviruses are widely employed as vaccine and gene therapy vectors(Singh et al.,2019).Due to their lack of ability to integrate into the recipient’s genome,the adenoviral vectors allow a temporary but sufficiently long (for 3 weeks) expression of transgenes.However,there are sti ll problems with the immune response to adenoviral anti gens (Perreau and Kremer,2006;Seregin and Amalfitano,2009).In our previous studies to overcome this issue for delivering the therapeutic genes,we employed autologous white blood cells(WBC) transducedex vivowith adenoviral vectors carrying recombinant cDNA.This approach allows for preventing the negative impact of the adenoviruses,such as immunogenic and toxic effects on the patient organisms,and proposes temporary synthesis and secretion of the recombinant therapeutic molecules by WBC.In this study,to increase the efficacy of WBC transduction,we used a chimeric adenoviral vector (Ad5/35) with a modified fiber,which has a high affinity for the CD46 membrane receptor (Adams et al.,2011).
Another important issue is the translation of the new approaches for SCI treatment,mostly obtained in results with small animals (rodents).Obviously,preclinical studies need to be performed in large animals whose morphological,physiological,and biochemical characteristics are close to human characteristics.Recently,for SCI gene therapy we proposed an effective,simple,and safe method of personalized precisionex vivogene therapy based on intravenous autoinfusion of a genetically-enriched leucoconcentrate (Islamov et al.,2021).Leucoconcentrate obtained from the peripheral blood of mini-pigs was transduced with a chimeric adenoviral vector (Ad5/35) carrying reporter gene encoding an enhanced green fluorescent protein in the blood bag,without any proceduresin vitroand usage of culture medium substances such as antibiotics and biological materials of animal origin.A week after intravenous infusion of the genemodified leucoconcentrate,enhanced green fluorescent protein-producing WBCs were found in the spinal cord at the site of a contusion injury (Islamov et al.,2021).Based on this,we integrated this strategy for tandem delivery of three therapeutic genes encoding VEGF,GDNF,and NCAM with multi site translesional epidural electrical stimulation and demonstrated the positive effect of combined gene therapy and electrotherapy in mini-pigs with SCI(Islamov et al.,2022).However,the impact of cell-mediated gene therapy on post-traumatic spinal cord recovery in the combined approach was obscure.The goal of this research is to evaluate the efficacy of the intravenous infusion of an autologous genetically-enriched leucoconcentrate simultaneously producing recombinant VEGF,GDNF,and NCAM on the remodeling of the spinal cord tissue at the site of damage in mini-pigs with a contusion injury model.
Methods
Animals
Mature 8-month-old miniature Vietnamese pot-bellied female pigs (body weight 25–30 kg;n=11) were obtained from the Kazan State Academy of Veterinary Medicine by N.E.Bauman (Kazan,Russia) and kept separately in a housing area with controlled temperature (24–25°C),air conditioning,12-hour light/dark cycle and organized water/food supply.The experimental procedures were performed in accordance with the standards to minimize animal suffering and the size of the experimental groups.All animal protocols were approved by the Animal Care and Use Committee of Kazan State Medical University (approval No.5) on May 26,2020.
Chimeric adenoviral vectors Ad5/35
For delivery of transgenes into WBC,we constructed chimeric adenoviral vectors based on the human adenovirus serotype 5 with fibers derived from the adenovirus serotype 35 fiber gene (Ad5/35).The 35 fiber has a high affinity for a cluster of differentiation 46 (CD46),which is expressed on all nuclear cells and provides effective transduction of WBC in the blood plastic bag (Islamov et al.,2021).The Ad5/35 adenoviral vectors carrying therapeutic genes encoding VEGF165,GDNF,and NCAM1 were constructed,as described previously (Islamov et al.,2022).The nucleoti de sequences encoding VEGF165(Gene Bank NM_001171626.1),GDNF (Gene Bank NM_000514.4),and NCAM1 (Gene Bank NM_001076682.2) were obtained by chemical synthesis in Evrogen,Moscow,Russia.The titers of the obtained genetic constructs were determined by the plaque formation technique (Baer and Kehn-Hall,2014) in HEK-293 cells (ATCC,293T/17 [HEK 293T/17] CRL-11268TM).
Preparation and analysis of the genetically-enriched leucoconcentrate
In mini-pigs,over a 14–16 hour period before SCI modeling,50 mL of peripheral blood was collected from v.subclavia under anesthesia with an intramuscular (m.trapezius) injection of tiletamine/zolazepam (Zoletil 100,Virbac Laboratoires,Carros,France;10 mg/kg) and xylazine (Interchemie werken ‘De Adelaar’ B.V.,Castenray,the Netherlands;40 mg/kg).The blood was collected from each experimental mini-pig into a plastic blood bag with 35 mL of anticoagulant-preservative solution (CPDA),and the leucoconcentrate was prepared immediately according to our oeonidovnariginal procedure,as described previously (Islamov et al.,2021).The procedure included sedimentation of erythrocytes with 6% hydroxyethyl starch,centrifugation at 34 ×gfor 10 minutes at 10°C (DP-2065 R PLUS,Centrifugal Presvac RV;Presvac,Buenos Aires,Argentina),and washing with saline.The remaining product in the blood bag solution with WBC was considered the leucoconcentrate.
Transduction of the leucoconcentrate with chimeric adenoviral vectors carrying therapeutic genes was performed for 12 hours in the blood bag with a multi plicity of infection (MOI) equal to 10 based on our previous data(Islamov et al.,2022).In this study,transduction of the leucoconcentrate was done according to the WBC count in the leucoconcentrate,the ti ters of the adenoviral vectors,and the equal ratio of each vector correspondingly: 1/3 Ad5/35-VEGF165 (2.0 × 109PFU/mL),1/3 Ad5/35-GDNF (7.0 × 1010PFU/mL),and 1/3 Ad5/35-NCAM1 (5.0 × 1010PFU/mL).Subsequently,after transduction the saline was added to the blood bag,the mixture was centrifuged,and the supernatant was squeezed out of the bag.The solution (30 mL) that remained in the bag was considered as the genetically-enriched leucoconcentrate carryingvegf165,gdnfandncam1.
The efficacy of the WBC transduction was confirmed as described earlier(Islamov et al.,2022).In this study,the samples of the leucoconcentrate simultaneously transduced with a combination of the three chimeric adenoviral vectors Ad5/35-VEGF165,Ad5/35-GDNF,and Ad5/35-NCAM1 were seeded on coverslips,and 72 hours after incubation,immunofluorescence staining with human-specific primary anti bodies to VEGF,GDNF and NCAM was performed.Primary and secondary anti bodies are listed inTable 1.
Spinal cord injury modeling and treatment
All surgical procedures were performed under deep anesthesia induced by intramuscular administration of Zoletil 100 (Virbac Sante Animale,Carros,France) at a dose of 10 mg/kg and maintained using an inhalation apparatus (Minor Vet Optima,Zoomed,Moscow,Russia) with isoflurane(Laboratorios Karizoo,S.A.,Barcelona,Spain) as a 2.0–2.5% mixture with oxygen.A moderate contusion injury of the spinal cord was carried out as described previously (Islamov et al.,2020).Briefly,the operating field was cleaned,shaved,treated with antiseptics (10% solution of povidone-iodine and alcohol solution with chlorhexidine bigluconate 0.05%),and covered with sterile surgical dressing.After laminectomy at the T8–T9 vertebral level,the dura mater was exposed and a 50 g metal rod with a diameter of 9 mm was dropped on the spinal cord from a 50 cm height.Animals were left on the operating table maintaining the body temperature at 38°C,and 4 hours after SCI,the mini-pigs received an intravenous infusion of 30 mL of the autologous leucoconcentrate via the auricular vein.Experimental animals were randomly divided into two groups of 4 pigs each: the therapeutic and control groups.The pigs from the therapeutic group were infused with the leucoconcentrate simultaneously transduced with a combination of the three chimeric adenoviral vectors Ad5/35-VEGF165,Ad5/35-GDNF,and Ad5/35-NCAM1.In the control group,animals received the intact leucoconcentrate (Figure 1A).A silicone 18 Fr urinary Foley catheter (“Vogt medical Verti eb GMBH”,Karlsruhe,Germany) was placed during the surgery and was maintained post-operatively.Intact animals (n=3) were used to collect basic histological data for comparative analysis of the efficacy ofex vivogene therapy for SCI.In the postoperative period,to minimize suffering,experimental animals received proper anti bacterial,analgesic,and vitamin therapies as described previously (Islamov et al.,2020).To maintain a stable state of health,clinical evaluation of the respiratory rate,body temperature,water,and food intake,as well as urine and feces excretion,were performed daily for 60 days of the experiment.The rationality of using female mini-pigs in the study is related to their anatomical characteristics.In female mini-pigs,it is easy to place a urinary catheter and control the bladder emptying in the acute phase after SCI to prevent urinary tract infections which commonly affect the recovery of the experimental animals after surgery.Besides,the selection of female minipigs in the present study is also based on our experience working with minipigs in previous investigations (Islamov et al.,2021,2022).
Samples collection and processing
At 60 days after SCI modeling,mini-pigs from the control and therapeutic groups were intramuscularly injected with Zoleti l 100 (10 mg/kg) and xylazine(40 mg/kg) in the back of the neck.Under deep anesthesia maintained with 2.5% isoflurane,the animals were infused with potassium chloride(150 mg/kg) via the auricular vein.The spinal cords were removed from the vertebral columns,fixed in 4% paraformaldehyde (Sigma,St.Louis,MO,USA)in phosphate-buffered saline (pH 7.4),and cut into three segments: rostral (RS)and caudal (CS) segments 15 mm long each and the epicenter of the injury,which was divided into rostral (ER) and caudal (EC) segments 7 mm long as well (Figure 1B).
Morphometric analysis of grey and white matter preservation
Total cross-sections (20 µm) of the thoracic spinal cord from the RS and CS segments were cut using a cryostat (Microm HM 560,Thermo Fisher Scientific,Waltham,MA,USA) from the sites adjacent to the epicenter of injury.A total of 10 sections were obtained with an interval of 200 µm along a sample with a length of 5 mm from both the RS and CS segments (Figure 1B).Frozen slide-mounted sections were stained with hematoxylin (AbrisPlus,Saint Petersburg,Russia) and eosin (Element,Saint Petersburg,Russia),and digitized images were captured with a 4× objective of the luminescence microscope (Axioscope A1,Carl Zeiss,Germany) and analyzed using ImageJ soft ware (version 1.52u,National Institutes of Health,Bethesda,MD,USA).The area of the pathological cavities in the dorsal horns of the spinal cord relative to the investigated area of the grey matter was measured.The area of the preserved grey matter was presented as a percentage.
Preservation of white matter was analyzed in the lateral columns at the lateral corticospinal tract region.The tissue from ER and EC segments of the epicenter was post-fixed in a 2.5% solution of glutaraldehyde for 4 hours,incubated in a 1% solution of osmium tetroxide (Sigma) for 24 hours,and embedded in EMbed 812 (Electron Microscopy Sciences,Hatfield,PA,USA).The semi-thin cross-sections were cut from both sides of the ER and EC segments adjacent to the RS and CS segments (Figure 1B),correspondingly,using an ultramicrotome (LKB-3;LKB,Sollentuna,Sweden).Totally 10 sections with an interval of 50 µm along a sample with a length of 1 mm from both ER and EC segments were prepared and stained with methylene blue dye(Figure 1B).Digitized images were captured with a 20× objective of the luminescence microscope (Axioscope A1) and analyzed using ImageJ soft ware(NIH).The areas of pathological cavities in the square of 0.2 mm2in the lateral corticospinal tract region from both sides in the ER and EC segments were measured.
Immunofluorescence staining
Free-floating total cross-sections of 20 µm thickness were prepared from the RS and CS segments for immunofluorescence staining with porcine-specific primary anti bodies (Ab) to neural and glial markers in the dorsal horns of the grey matter (the site of the impact rod hit) and the lateral columns (Figure 1B).The sections were collected from RS and CS segments adjacent to the epicenter of injury along a sample with a length of 5 mm together with the sections for hematoxylin and eosin staining (Figure 1B).For each cellular or molecular marker were obtained 10 sections with an interval of 200 µm.Thus in the 200 µm distance,we obtained sections for one set for hematoxylin and eosin,and immunofluorescence staining and ten such settings were prepared.Antibodies to Caspase3 (full-length pro-apoptotic protein 35 kDa and activated fragment 17 kDa) and Hsp27 (heat shock protein 27 kDa) were used to assess the availability of the spinal neural and glial cells.The expression of the synaptic proteins by spinal neurons was analyzed with Ab to postsynaptic density protein 95 kDa (PSD95) and synaptophysin (synaptic vesicle protein).Astrocytes,oligodendroglial and microglial cells were evaluated with Ab to glial fibrillary acidic protein (GFAP),to oligodendrocyte transcription factor 2 (Olig2),and to ionized calcium binding adaptor molecule 1 (Iba1),correspondingly.The longitudinal 20 µm thickness slide-mounted sections were prepared from the ER and EC segments.The sections were collected from both sides of the lateral column.Serial 10 sections obtained from each side were stained with Ab to neuronal-specific tubulin (βIII-tubulin) to identi fy axons in the lateral corticospinal tract region (Figure 1B).Incubation with primary Ab was performed overnight at 4°C.For the detection of the anti gens,the proper secondary anti bodies conjugated with fluorescent dye were applied for 2 hours at room temperature (Table 1).Cell nuclei were visualized with 4′,6-diamidino-2-phenylindole (DAPI;10 µg/mL in PBS,Sigma).Digitized fluorescence images were obtained with confocal (LEICA TCS SP5 MP,Leica Microsystems,Germany) and luminescence microscopy (Axioscope A1).The number of Caspase 3-positive cells and Olig2-positive cells was counted in regard to specific nuclear immunostaining and DAPI-stained nuclei.The expression of synaptic proteins (synaptophysin and PSD95),glial and neural markers (GFAP,Iba1,and βIII-tubulin),and Hsp27 was assessed as the immunopositive area and expressed as a percentage.The pattern of immunoexpression of each target anti gen was evaluated in an area of 0.05 mm2using ImageJ soft ware.
Stati stical analysis
Statistical analysis and data visualization were performed using R 4.1.0 (R Foundation for Statistical Computing,Vienna,Austria).The Kruskal-Wallis test with Dunn’s test as apost hocmethod was used for group comparison.Sample distributions of quantitative values were visualized using box plots.Descriptive statistics are presented as median (1st–3rdquartiles).The small number of experimental animals is not sufficient to provide an acceptable power to reliably evaluation of the recovery after SCI.Thus,the current investigation should be considered an exploratory pilot study.
Results
Expression of transgenes in genetically-enriched leucoconcentrate
Immunofluorescence staining of the mini-pig leucoconcentrate enriched with recombinant genesvegf165,gdnf,andncam1was completed 72 hours after incubation of the samples using specific anti bodies to VEGF,GDNF,and NCAM.Fluorescence microscopy revealed VEGF-,GDNF-,and NCAM-immunopositive WBC (Figure 2).
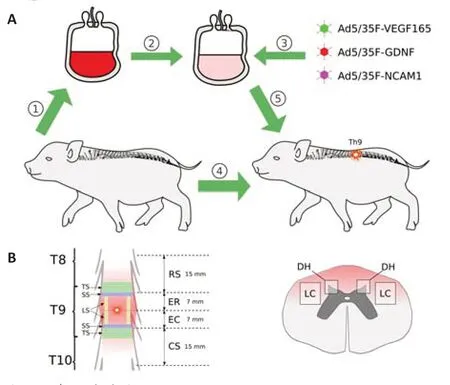
Figure 1|Study design.
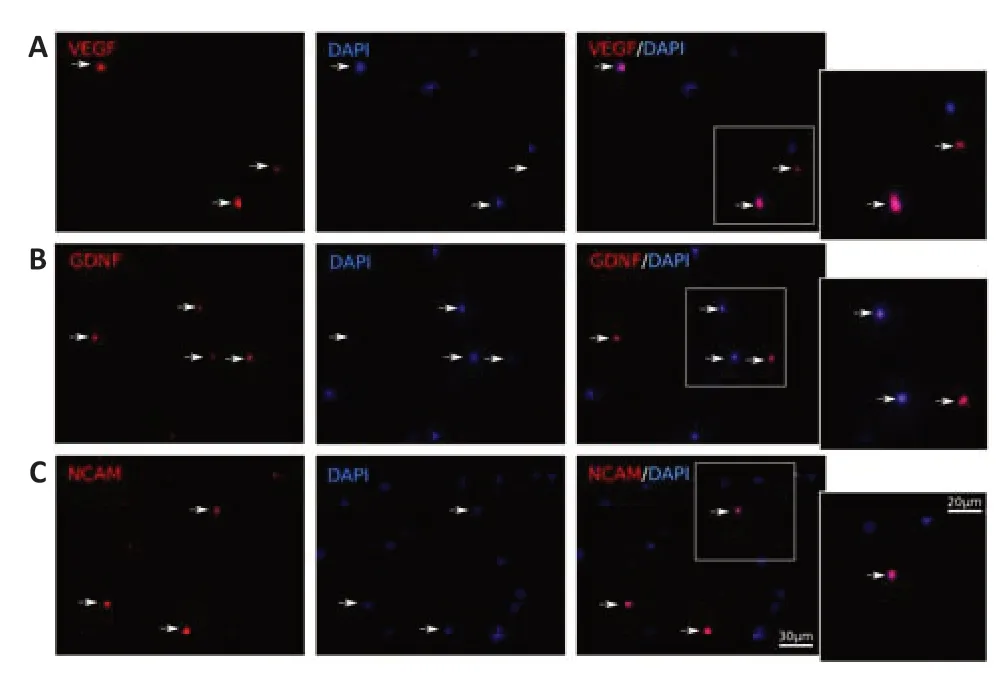
Figure 2|In vitro fluorescence analysis of the recombinant vegf165,gdnf,and ncam1 expression in the genetically-enriched leucoconcentrate 72 hours after transduction of the leucoconcentrate simultaneously transduced with adenoviral vectors Ad5/35-VEGF165,Ad5/35-GDNF,and Ad5/35-NCAM1 in equal ratio at multi plicity of infection=10.
Behavioral performance
One week after SCI animals from both experimental groups demonstrated impaired locomotor activity.The neurological investigation confirmed the central type of paralysis of the hind limbs.Mini-pigs lost any active movements and were unable to use their hind limbs to stand and walk,and had increased tone in the extensor muscles.Urinal incontinence was compensated by bladder emptying every three hours a day in all animals with SCI via the urinary catheter.At 3 weeks after surgery,treated animals demonstrated attempts to draw their hind limbs to the body and there were the first attempts of urination in small volumes.By this time point,no progress in functional recovery and no voluntary voiding were observed in animals in the control group.At 4 weeks after SCI mini-pigs from the therapeutic group were able to stand alone on four legs,while control animals made first efforts to stand on the front legs with help of the investigators and also began occasional voluntary urination.At 5 weeks the treated animals returned to the normal pattern of urination and demonstrated acti vity in the hip joint,where the control mini-pigs were trying to stand up alone on the front legs and showed some improvement in urination.At 7 weeks after SCI,we observed further progress in behavioral performance in mini-pigs from the therapeutic group (more attempts to stand on the four legs,longer being in a standing position,flexion in the knee joint).Recovery of the control animals was noticed by returning to normal urination and the ability to stand on four legs.At 8 weeks after surgery in mini-pigs with infusion of the geneticallyenriched leucoconcentrate successful attempts to stand and walk using hind limbs and the flexion in the hip,knee,and ankle joints were observed,while control mini-pigs were using only forelimbs,dragging the hind limbs to move and no flexion in the joints of the hind limbs were revealed.
Preservation of grey and white matter
In this study,a model of moderate contusion spinal cord injury presumes the hit to the dorsal side of the spinal cord with a metal rod.Thus,the main damage is caused in the dorsal horns and dorsal and lateral columns.On the total cross-sections of the spinal cord in the RS and CS segments,we evaluated the area of the pathological cavities in the grey matter in the dorsal horns.The morphometric analysis revealed the difference between the sparing area of the grey matter in the dorsal horns in the RS and CS segments in experimental animals (Figure 3A).In the RS segment,the sparing area of grey matter did not differ between the control and therapeutic groups.In the CS segment,a more preserved nervous tissue area was shown in the treated mini-pigs compared with that in the control animals (P<0.0001).Important,there was less sparing in CS than in RS in the control group (P=0.0091) and no difference in the therapeutic group.The study of the white matter sparing in the region of the lateral corticospinal tract in the ER and EC segments of the epicenter of the injury revealed pathological cavities as well.Morphometric analysis of the lesion area in the studied regions did not differ in the control and treated mini-pigs (P=0.4397;Figure 3B).
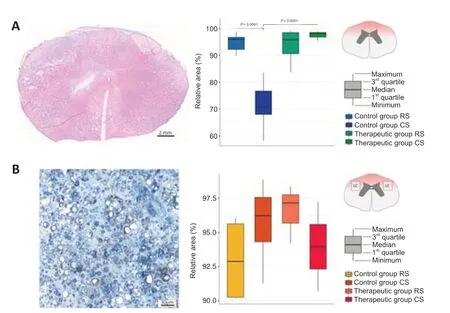
Figure 3|Sparing of the spinal cord tissue in the control and treated animals 60 days after spinal cord contusion injury.
Immunofluorescence analysis results of post-traumatic spinal cord recovery
As was described above in the model of SCI that was used,the main damage occurred in the dorsal region of the spinal cord.Using the immunofluorescence method,we evaluated the molecular and cellular changes in the RS and CS segments of the grey matter in the dorsal horns and the ER and EC segments of the white matter in the lateral columns.
Survivability of spinal cord cells
The number of the pro-apoptotic protein Caspase 3-positive cells in the dorsal horns in the control mini-pigs was significantly higher than that in treated animals in the RS segments (P=0.0033) and did not differ between them in the CS segments (P>0.05;Figure 4A).Evaluation of the cellular stress protein Hsp27-positive relative area demonstrated the significant increase of Hsp27 expression in the control animals in the RS segment (P=0.0004) and the CS segment (P<0.0001) compared with intact mini-pigs.However,the level of Hsp27 expression in the therapeutic group in the RS segment and the CS segment did not differ from intact data (Figure 4B).
Expression of synaptic proteins
Recovery of the synaptic protein expression was evaluated using anti bodies to the synaptic vesicles protein synaptophysin and PSD95.The average immunopositive area for synaptophysin was significantly decreased in the RS and CS segments both in the control (RS,P=0.0173;CS,P<0.0001) and therapeutic (RS,P=0.0259;CS,P=0.0162) groups compared with that in the intact group.However,the synaptophysin-immunopositive area was significantly higher in the CS segment in treated mini-pigs compared with that in the control animals (P=0.0251;Figure 4C).Moreover in the control group in CS,the expression of synaptophysin was significantly lower than in RS (P=0.0251).The analysis of PSD95 expression showed that the PSD95-immunopositive relative area was significantly decreased in the CS segment in the control (P=0.0019) and therapeutic groups (P=0.0043) and did not differ in the RS segment in the control and treated animals compared with intact mini-pigs (Figure 4D).
Astrogliosis
The activation of microglia and astrocytes result in reactive astrogliosis.Analysis of astrocytes in the dorsal horns revealed a higher GFAPimmunopositive area in the control mini-pigs in the RS segment (P=0.0105)and in the CS segment (P=0.0173) in comparison with intact animals (Figure 4E).In the therapeutic group,the GFAP-immunopositive area in the RS and CS segments did not differ from the intact animals.The analysis of microglial cell activation demonstrated the same pattern of changes.The increased Iba1-immunopositive area was observed in the control group in the RS (P=0.0193) and CS (P=0.0014) segments in comparison to intact animals (Figure 4F).In the therapeutic group,the Iba1-immunopositive area in the RS and CS segments was similar to the values of the intact group.
Axon regeneration and myelination
In the longitudinal sections prepared from the rostral (ER) and caudal (EC)segments of the epicenter of injury,axon regrowth was studied in the lateral column at the lateral corticospinal tract region using Ab to neuronal-specific tubulin (βIII-tubulin).The βIII-tubulin-immunopositive area was significantly decreased in the EC segment in the control group (P<0.0001) and did not differ in the therapeutic group in comparison with the intact group.In the ER segment,both in the control and treated animals,the βIII-tubulinimmunopositive area was similar to that in the intact mini-pigs (Figure 4G).The number of myelin-forming oligodendroglial cells was examined in the total cross-sections from the RS and CS segments in both lateral columns in the lateral corticospinal tract region.A significant decrease in the number of Olig2-positive oligodendroglial cells was observed in the control group both in the RS (P=0.0303) and CS (P=0.0016) segments compared with that in the intact group.In the therapeutic group,the number of oligodendroglial cells in the RS (P=0.0531) and CS (P=0.0312) segments was significantly higher than that in the control group and similar to that in the intact group(Figure 4H).
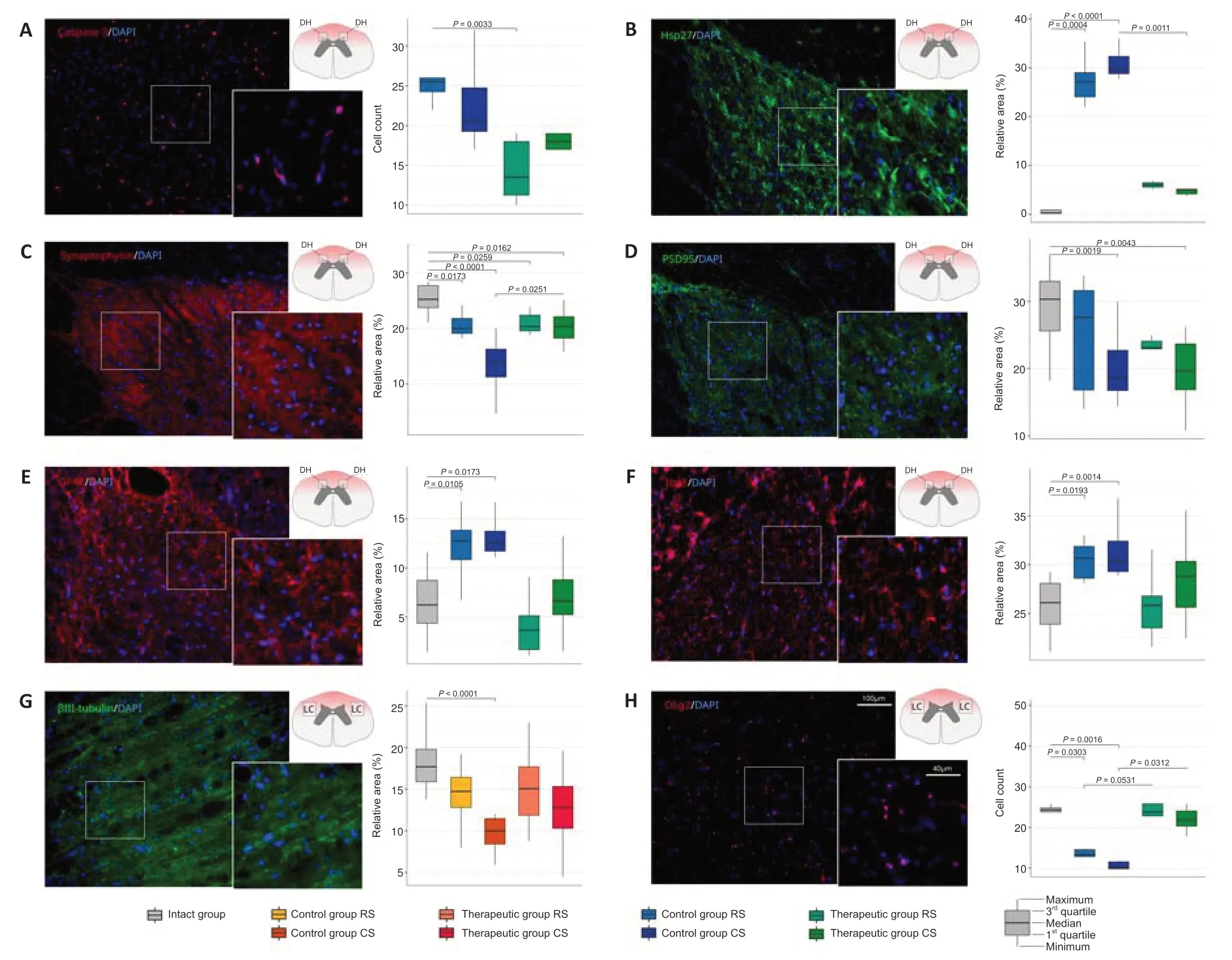
Figure 4|Immunofluorescence staining of the dorsal horns and lateral columns of the spinal cords in the rostral (RS) and caudal (CS) segments and from the rostral (ER) and caudal (EC) segments of the epicenter with Ab to molecular markers of the neural and glial cells in the control and treated animals 60 days after contusion injury.
Discussion
The complexity of pathophysiological and pathomorphological disorders of SCI requires the combination of various strategies to overcome neurodegeneration and stimulate neuroregeneration.In our view,the gene therapy mainly addressed to increase the post-traumatic survivability of neural cells and to stimulate axon regeneration,and restore the disrupted neural connectivity may be effectively used as a complementary therapy of neuromodulation with electrical stimulation and intensive rehabilitation(Siddiqui et al.,2020;Fadeev et al.,2021;Islamov et al.,2022).
To date,for SCI cell-mediated gene therapy,there is an active search for both therapeutic genes and their cell carriers.Cells prepared for transplantation must have predictable and reproducible characteristics,such as maintaining viability,actively migrating to the degeneration region and effectively producing recombinant protein molecules (Thuret et al.,2006).However,many developed methods remain at the level of experiments in laboratories,or (at best) clinical trials have been initiated.Physicians face ethical problems (e.g.,in the case of the embryo or fetal cells),high probability of tumorigenicity of stem cells and immunogenicity to allogeneic cells,contamination of the patient with unknown pathogens from bioproducts of animal origin,and uncontrolled secretion of recombinant molecules.Therefore,an important factor in the strategy of cell-mediated gene therapy is the use of available and safe cellular material for therapeutic gene delivery.
In this study,for delivery of the recombinant cDNA,we used autologous WBC.Recently,we developed a simple,economical,and safe platform for personalized precisionex vivogene therapy based on the infusion of an autologous genetically-enriched leucoconcentrate prepared from a routine unit of a patient’s peripheral blood and chimeric adenoviral vectors (Ad5/35)carrying recombinant therapeutic genes (Islamov et al.,2021).Herein,for cell-mediated gene therapy of SCI,we employed an autologous geneticallyenriched leucoconcentrate expressing the recombinant therapeutic genesvegf165,gdnf,andncam1.Genetically-enriched leucoconcentrate was prepared in a standard blood bag without culturingin vitroand any risks in using animal products and antibiotics.For transduction of the WBC,we employed a chimeric adenoviral vector (Ad5/35) which has a modified fiber with a high affinity for CD46 membrane molecule (cluster of differenti ation 46) expressed on all WBC (Adams et al.,2011).
Intravenous infusion of the autologous genetically-enriched leucoconcentrate carrying transgenes encoding therapeutic molecules (VEGF,GDNF,and NCAM)in mini-pigs 4 hours after modeling of the moderate contusion injury of the spinal cord at the thoracic level demonstrated the positive effect on posttraumatic spinal cord recovery.The morphometric study of grey and white matter sparing confirmed the severe damage caused by a metal rod to the dorsal horns and lateral columns.Based on this,the goal of the present study was to evaluate the molecular and cellular changes in the dorsal horns and lateral columns followingex vivogene therapy at 60 days after SCI.
The analysis of the obtained results revealed better preservation of the grey matter in the dorsal horns of mini-pigs after intravenous infusion of the autologous genetically-enriched leucoconcentrate producing therapeutic molecules (VEGF,GDNF,NCAM) when compared to control animals that received intact autologous leucoconcentrate.Immunofluorescence staining of the dorsal horns in the rostral and caudal segments at the epicenter of the injury in the treated animals showed: (1) increased survivability of the spinal cord cells (lower number of Caspase 3-positive cells and decreased expression of Hsp27);(2) recovery of synaptophysin expression;and (3)prevention of astrogliosis (lower area of GFAP-positive astrocytes and Iba1-positive microglial cells).In the therapeutic group,the study of the white matter in the lateral corticospinal tract region revealed higher growth rates of the regenerating βIII-tubulin-positive axons,accompanied by a higher number of Olig2-positive oligodendroglial cells.The data on the reduction of the immunopositive areas for astrocytes and microglial cells may indicate the decrease of chondroitin sulfate proteoglycans production and enhanced potential for axon regeneration as well.It is worth mentioning that in the control group there were more severe disorders in the caudal segment than in the rostral while in the therapeutic group the differences between rostral and caudal segments were not observed.
The positive effect of the genetically-enriched leucoconcentrate on the posttraumatic spinal cord remodeling may be due to the paracrine and endocrine actions of the recombinant molecules (VEGF,GDNF,and NCAM) with wellknown functions.The crucial role of VEGF in neurogenesis (axon growth and guidance) (Tillo et al.,2012) and in vasculogenesis (Lange et al.,2016)suggests the rationality of usingvegffor stimulation of axon sprouting and angiogenesis after SCI.GDNF is a well-known factor with neuroprotective properties.In the post-traumatic spinal cord,GDNF increases neuronal survival and promotes axon regeneration (Ibáñez and Andressoo,2017).The role of NCAM in this gene combination is proposed not only for stimulation of neuroregeneration (Sytnyk et al.,2017) but also to enhance the homing and survivability of the gene-modified WBC at the site of neurodegeneration in the spinal cord (Islamov et al.,2017).The BBB impaired after SCI (Whetstone et al.,2003;Nasser et al.,2016) provides permeability for recombinant molecules produced by gene-modified WBC in the bloodstream and increases the homing of WBC,including the gene-modified ones.It is important to mention that earlier we demonstrated the homing of WBC expressing reporter green fluorescent protein in the spinal cord at the site of a contusion a week after intravenous infusion of the autologous genetically-enriched leucoconcentrate in mini-pigs with SCI.Thus,the efficacy of the delivery of such a combination of recombinant genes by autologous WBC to stimulate neuroregeneration in the central nervous system was confirmed in the study,which demonstrated that the genetically-enriched leucoconcentrate induces positive changes in the post-traumatic spinal cord remodeling which were in line with the improvement in behavioral performance (successful attempts to stand and walk and normal pattern of urination).
Treatment of severe combined immunodeficiency,for example,of adenosine deaminase deficiency (ADA deficiency),based on the delivery of a functional gene into the patient’s leucocytes,is the first approach ofex vivogene therapy that was approved for use in practical medicine (Aiuti et al.,2017).Standing with the safety and efficacy of autologous WBC for cell-mediated gene therapy,we believe that leucoconcentrate prepared from the standard unit of a patient’s peripheral blood as a cellular carrier of therapeutic genes for the temporary production of biologically active molecules specific for correction of the certain disorders may be one of the breakthrough directions in gene therapy.
Limitations
Meanwhile,our results should be considered exploratory because of the limited number of animals,and the beneficial effects of GEL on posttraumatic spinal cord regeneration should also be confirmed on male minipigs.Additional experiments are also needed to optimize leukoconcentrate transduction with a combination of adenoviral vectors (Ad5/35-VEGF165,Ad5/35-GDNF,and Ad5/35-NCAM1) and the dose of the injected drug,also taking into account its possible re-administration.Further,a translational research will be necessary to analyze the pharmacokinetics of the recombinant VEGF,GDNF,and NCAM produced by GEL and their possible side effects.
Conclusion
Currently,there are no approved gene-cell constructs for the temporary production of therapeutic molecules for SCI treatment.In the present study,we employed an autologous genetically-enriched leucoconcentrate forex vivogene therapy of a moderate contusion SCI in a mini-pig model.Morphological and immunofluorescence analyses of the molecular and cellular changes revealed the positive effects of the intravenous infusion of the autologous leucoconcentrate producing the recombinant therapeutic molecules VEGF,GDNF,and NCAM on the remodeling of the damaged nerve tissue.In treated animals,we revealed a reduction of the inhibiting regeneration of cystic cavitations and glial scarring,increased survivability of the spinal cells,and regrowth of the axons accompanied by the increased number of myelinating cells which were consistent with the improvement in behavioral performance.The results of this work provide a solid platform for a new ex vivo gene therapy for SCI and will facilitate further translation of regenerative therapies in clinical neurology.
Acknowledgments:Some experiments were carried out using the equipment of the Collective Spectroanalytical Center of FRC Kazan Scientific Center of RAS.The authors are grateful to the students of Kazan State Medical University(Minyazeva Irina Salavatovna,Minekaev Tagir Farhatovich,and Kalistratova Julia Aleksandrovna) for their assistance in the study.
Author contributions:Conceptualization: RRI;data curation: FVB and MMS;formal analysis: LFN and IIS;investigation: AAI,MAD,RRG,DZG;methodology:MMS,DNS;project administration: RRI;validation: MMS,AAL,ILT;supervision:RRI;visualization: AAI,MAD,RRG;writing– original draft : RRI,MMS,FVB;writing– review &editing: RRI.All authors approved the final version of this paper.
Conflicts of interest:The authors declare no competing interests.
Availability of data and materials:All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:This is an open access journal,andarticles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Alexander Sosunov,Columbia University Vagelos College of Physicians and Surgeons,USA;Laura Calzà,University of Bologna,Italy.
Additional file:Open peer review reports 1 and 2.
- 中国神经再生研究(英文版)的其它文章
- Bystanders or not? Microglia and lymphocytes in aging and stroke
- Alzheimer’s disease risk after COVID-19: a view from the perspective of the infecti ous hypothesis of neurodegeneration
- Serine and arginine rich splicing factor 1: a potenti al target for neuroprotection and other diseases
- Can glial cells save neurons in epilepsy?
- Lights for epilepsy: can photobiomodulation reduce seizures and offer neuroprotection?
- The landscape of cognitive impairment in superoxide dismutase 1-amyotrophic lateral sclerosis

