The default mode network is affected in the early stage of simian immunodeficiency virus infection:a longitudinal study
Zhen-Chao Tang ,Jiao-Jiao Liu ,Xue-Tong Ding,Dan Liu,Hong-Wei Qiao,Xiao-Jie HuangHui Zhang,Jie Tian,Hong-Jun Li
Abstract Acquired immune deficiency syndrome infection can lead to cognitive dysfunction represented by changes in the default mode network.Most recent studies have been cross-sectional and thus have not revealed dynamic changes in the default mode network following acquired immune deficiency syndrome infection and antiretroviral therapy.Specifically,when brain imaging data at only one time point are analyzed,determining the duration at which the default mode network is the most effective following antiretroviral therapy after the occurrence of acquired immune deficiency syndrome.However,because infection times and other factors are often uncertain,longitudinal studies cannot be conducted directly in the clinic.Therefore,in this study,we performed a longitudinal study on the dynamic changes in the default mode network over time in a rhesus monkey model of simian immunodeficiency virus infection.We found marked changes in default mode network connectivity in 11 pairs of regions of interest at baseline and 10 days and 4 weeks after virus inoculation.Significant interactions between treatment and time were observed in the default mode network connectivity of regions of interest pairs area 31/V6.R and area 8/frontal eye field (FEF).L,area 8/FEF.L and caudal temporal parietal occipital area (TPOC).R,and area 31/V6.R and TPOC.L.ART administered 4 weeks after infection not only interrupted the progress of simian immunodeficiency virus infection but also preserved brain function to a large extent.These findings suggest that the default mode network is affected in the early stage of simian immunodeficiency virus infection and that it may serve as a potential biomarker for early changes in brain function and an objective indicator for making early clinical intervention decisions.
Key Words:acquired immune deficiency syndrome;analysis of variance;antiretroviral therapy;default mode network;functional magnetic resonance imaging;human immunodeficiency virus;longitudinal study;rhesus monkeys;simian immunodeficiency virus;SIV-mac239
Introduction
Human immunodeficiency virus (HIV) infection can lead to brain impairments that worsen with the duration of the disease,which greatly affects the quality of life of HIV patients,even in the current era of highly active anti retroviral therapy (ART) (Nightingale et al.,2014;Watkins and Treisman,2015;Tang et al.,2017a,b).The prolongation of HIV infection leads to the dysfunction of multiple brain networks,especially the default mode network (DMN),which is the most active network at rest and is closely related to cognitive function(Mak et al.,2017;Smith et al.,2018).Several previous cross-sectional studies have reported that the dysfunction of the DMN caused by HIV infection is associated with cognitive deficits (Ortega et al.,2015;Cohen et al.,2018).In earlier studies,HIV infection was shown to result in decreased functional connecti vity in the DMN in HIV-infected (Thomas et al.,2013) and perinatallyacquired HIV patients (Herting et al.,2015).Furthermore,it has been shown that decreased functional connecti vity of the DMN is correlated with poorer neurocognitive performance (du Plessis et al.,2017).In addition,another study found that abnormal activation of the posterior cingulate cortex in the DMN of HIV patients is related to deficits in Hopkins Verbal Learning Test-2 learning performance (Cohen et al.,2018).Neurocognitive performance and functional connectivity of the DMN can be improved by providing patients with ART (Zhuang et al.,2017),and more recently,studies have demonstrated that changes in the DMN are also closely related to various factors,such as aging (Egbert et al.,2019) and HIV self-management interventions (Webel et al.,2019).
However,most previous studies used a cross-sectional experimental design,and magnetic resonance imaging (MRI) data were acquired at one time point only (Cohen et al.,2018;Egbert et al.,2019;Webel et al.,2019).Although cross-sectional studies are effective in investigating DMN changes,they are unable to reveal dynamic changes in the DMN following acquired immune deficiency syndrome (AIDS) infection or ART over the disease or treatment course.Specifically,cross-sectional studies are unable to determine how early the DMN is affected by AIDS infection because MRI data are acquired at only one time.Therefore,more longitudinal studies are needed to specifically examine dynamic DMN changes.Furthermore,longitudinal studies are crucial for determining whether early ART is sufficient to prevent the dysfunction of the DMN in HIV patients (Samboju et al.,2018).However,longitudinal studies are challenging to conduct in HIV patients because of the numerous uncontrollable factors.Instead of directly investigating brain changes in HIV patients,simian immunodeficiency virus (SIV)-infected rhesus monkeys can be used as an analogous primate model to analogize brain damage caused by AIDS,with a particular focus on longitudinal brain changes during the progression of HIV infection (Tang et al.,2016).Indeed,unified functions associated with the DMN have been observed in both rhesus monkeys and human beings (Li and Zhang,2017,2018;Arsenault et al.,2018),which demonstrates that the SIV-infected rhesus monkey model is an appropriate model for the longitudinal investigation of DMN changes caused by AIDS infection.
In this study,we conducted a longitudinal study investigating DMN changes in SIV-infected rhesus monkeys.We aimed to investigate changes in the DMN during the early stage of SIV infection and whether these changes are related to the immunological and biochemical functions as proxies for the effects of HIV infection.
Methods
Design
Ten male rhesus monkeys were randomly inoculated with SIV and allocated into therapy and control groups.Clinical characteristics and MRI scans were acquired at six time points at an interval of 7 to 12 weeks.ART was administered to the therapy group from the fourth time point.The DMN was extracted from the resting-state functional MRI (fMRI) data of the monkeys.Analysis of variance (ANOVA) was performed for the DMN connectivity of the first three time points to investigate longitudinal connectivity changes.We also investigated the interaction effect between the factors of time and treatment on DMN connecti vity to explore changes in DMN connecti vity due to ART.The relationships among changes in DMN connecti vity and laboratory biochemical characteristics/immunological characteristics were also explored.
Animal screening before the main experiments
The current study was approved by the Institutional Animal Care and Use Committee of Beihang University (IACUC approval No.LHJ18001) on December 11,2018 and conducted according to the Guide for the Care and Use of Laboratory Animals of the Institute of Laboratory Animal Science and the Weatherall report for the Use of Non-Human Primates in Research(Weatherall,2006) to ensure individual safety and macaque welfare.Before performing the longitudinal procedure,health screenings and indirect immunofluorescence assays were performed in all the rhesus monkeys to confirm their health conditions and exclude possible SIV,simian type-D retrovirus,or simian T-cell lymphotropic virus-I infection.The housing surroundings were maintained at 16–26°C,humidity 40–70%,and 12/12-hour light/dark cycle.
Rhesus monkey model of SIV infection and data collection
The SIV-infected rhesus macaque model is used as an analog for HIV infection.Clinically,the population of HIV patients is predominantly male.In recent years,experiments using rhesus macaques have become increasingly expensive and difficult to conduct.Moreover,female monkeys are usually retained for breeding and are often not for sale,which limits experimentation with female monkeys.Thus,most previous SIV studies have used only male monkeys (Zhao et al.,2017;Mintzopoulos et al.,2019;Hsu et al.,2021).Ten male Chinese-origin rhesus macaques (weight 4.7 ± 0.6 kg;age 3.8 ± 0.3 years;provided by Beijing Gelsin Institute of Biological Resources (license No.SCXK [Jing] 2017-0027) were inoculated intravenously with SIVmac239 and observed longitudinally.SIV-mac239 was provided by the Virology Group,Microbiology Room,Institute of Medical Laboratory Animals,Chinese Academy of Medical Sciences.Ten macaques were intravenously inoculated with a 50-fold half-tissue culture-infective dose of SIVmac239 via the brachial vein.The sample size was calculated according to a matched case-control power analysis.To meet biosafety conditions,all macaques were housed and fed in an Association for Assessment and Accreditation of Laboratory Animal Care-accredited bio-safety level 3 laboratory.We defined baseline as 2 weeks before SIVmac239 inoculation.Immunological characteristics,including peripheral blood CD4+and CD8+T-cell counts,immunoglobulin M (IgM),and immunoglobulin G (IgG),were collected at baseline and 1,5,12,24,and 36 weeks post-virus inoculation.CD4+:CD8+ratios were calculated using the T-cell counts.In addition,laboratory biochemical characteristics,including plasma SIV RNA viral load,cerebrospinal fluid (CSF) viral load,glucose (Glu),total bilirubin (T-Bil),alanine aminotransferase (ALT),aspartate aminotransferase(AST),alkaline phosphatase (ALP),γ-glutamyl transpeptidase (γ-GT),total protein (TP),albumin (ALB),triglyceride (TG),total cholesterol (TC),creati nine(CREA),uric acid (UA),urea,creatine kinase (CK),and lactate dehydrogenase(LDH) were also measured at each time point.Glu,T-Bil,ALT,AST,ALP,γ-GT,TP,ALB,TG,TC,CREA,UA,Urea,CK,LDH,IgM,and IgG were not measured in two monkeys at the fourth time point.CSF viral load was only measured at 5,12,24,and 36 weeks post-virus inoculation.MRI scans were acquired at baseline and 10 days,4,12,24 weeks,and 36 weeks post-virus inoculation.The monkeys were randomly assigned to a therapy (five monkeys)or control group (five monkeys).All monkeys were anesthetized by intramuscular injection of ketamine hydrochloride (5–10 mg/kg;Taizhou Kangpu Pharmaceutical Co.,Ltd,Taizhou,China) before they underwent immunological characteristic data,laboratory biochemical characteristic data,and MRI data collection.The five monkeys in the therapy group received ART of emtricitabine (FTC) (50 mg/kg/day),tenofovir disoproxil fumarate (TDF) (5.1 mg/kg/day),and dolutegravir (DTG) (2.5 mg/kg/day) between the third and fourth time points (40 days post-virus inoculation).FTC and TDF were mixed and injected simultaneously,and DTG was administered separately 1 hour before FTC and TDF were administered.Details on the housing environment and parameters of the MRI scan acquisition are provided inAdditional file 1.An illustration of the longitudinal experimental design is provided inFigure 1.
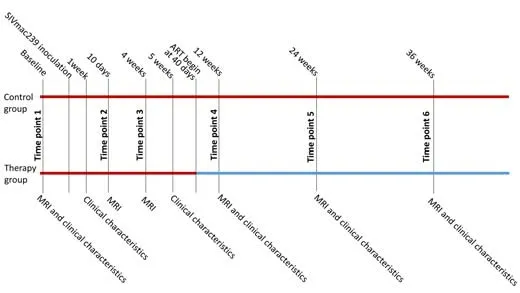
Figure 1|The longitudinal experimental design.
Biochemical/immunological characteristics analysis Longitudinal changes in laboratory biochemical/immunological characteristics
Laboratory biochemical characteristics included plasma SIV RNA viral load,CSF viral load,Glu,T-Bil,ALT,AST,ALP,γ-GT,TP,ALB,TG,TC,CREA,UA,CK,and LDH.Immunological characteristics included blood CD4+cell counts,peripheral blood CD8+cell counts,CD4+: CD8+ratios,IgM,and IgG.Serum biochemical indexes (e.g.,Glu,T-Bil,ALT,AST,ALP,γ-GT,TP,ALB,TG,TC,CREA,UA,CK,and LDH) were detected using an automatic biochemical analyzer(Mindray BS-360S,Shenzhen,China).Peripheral blood T lymphocyte subsets CD4+,CD8+,and CD4+:CD8+ratio were obtained by ethylenediaminetetraacetic acid anticoagulation (Xue et al.,2016) using flow cytometry (Accuri C6,BD,Franklin Lakes,NJ,USA).Viral loads were measured using the SYBR Green I real-time polymerase chain reaction (PCR) method with the ABI 7500 Real-time PCR Instrument (Thermo Fisher Scientific,Waltham,MA,USA).
Previous studies have shown that SIV infection leads to changes in laboratory biochemical and immunological characteristics (de Medeiros et al.,2021;de Almeida et al.,2022).To quantify longitudinal changes in these characteristics,we performed one-way ANOVA of the laboratory biochemical and immunological characteristic values from the first three time points,using time as the main factor.To investigate the interaction between ART and infection time,two-way ANOVA,followed by least significant difference multiple comparisons,was conducted for the laboratory biochemical and immunological characteristic values from the final three time points,using treatment (level: control and therapy groups) and time (level: three time points) as the two main factors.A threshold ofP<0.05 with false discovery rate (FDR) correction was considered significant.
Longitudinal changes in the DMN network
The DMN network was constructed from fMRI data after preprocessing.Details of the preprocessing and construction of the DMN network are provided inAdditional file 1.To assess longitudinal changes in DMN connectivity,we performed a series of ANOVAs for DMN connectivity and average of DMN connecti vity (DMNave) (definitions of DMN connecti vity and DMNave are described in the Construction of the DMN section).Specifically,to investigate early changes due to SIV infection,one-way ANOVAs of DMN connecti vity and DMNave from the first three time points were conducted,using infection time as the main factor.To investigate the impact of therapy and infection time,two-way ANOVAs were performed for DMN connecti vity and DMNave from the last three time points,with treatment (level: control and therapy groups) and time (level: three time points) as the two main factors (P<0.05,FDR corrected).
The relationship between the DMN and laboratory biochemical/immunological characteristics
Pearson’s correlation analysis was conducted between DMN connectivity and laboratory biochemical characteristics for the first and last three time points.The biochemical characteristics analyzed included plasma SIV RNA viral load,CSF viral load,Glu,T-Bil,ALT,AST,ALP,γ-GT,TP,ALB,TG,TC,CREA,UA,CK,and LDH,and the immunological characteristics analyzed included blood CD4+cell counts,peripheral blood CD8+cell counts,CD4+: CD8+ratios,IgM,and IgG.CSF viral load was not measured for the first three time points in any of the monkeys,and Glu,T-Bil,ALT,AST,ALP,γ-GT,TP,ALB,TG,TC,CREA,UA,Urea,CK,LDH,IgM,and IgG were not measured for the fourth time point in two monkeys.Therefore,these characteristics were not included in the correlation analysis.The number of laboratory biochemical and immunological characteristics was 20 for the first three time points and 21 for the last three time points.The significance of the correlation was tested using bootstrap resampling analysis (1000 replications).After Bonferronicorrection for the number of laboratory biochemical and immunological characteristics,aP<0.0025 was considered significant for the first three time points,and aP<0.0024 was considered significant for the last three time points.SPSS 22 (IBM Corp.,Armonk,NY,USA) was used for all stati stical analyses.The raters were blinded to group assignment.
Results
Biochemical/immunological characteristics
The biochemical/immunological characteristics used for the longitudinal analysis were body weight,peripheral blood CD4+cell counts,CD8+T-cell counts,CD4+:CD8+ratio,plasma SIV RNA,Glu,T-Bil,ALT,AST,ALP,γ-GT,TP,ALB,TG,TC,CREA,UA,Urea,CK,LDH,IgM,and IgG,which are summarized inTables 1and2.Biochemical/immunological characteristics changes due to SIV infection were observed in the early stage of SIV infection.For immunological characteristics,significant changes were observed in peripheral blood CD4+cell counts (F(2,18)=5.335,P=0.015),peripheral blood CD8+T-cell counts (F(2,18)=8.282,P=0.003),CD4+:CD8+ratio (F(2,18)=19.438,P<0.001),IgM (F(2,16)=14.391,P=0.005),and IgG (F(2,16)=37.631,P<0.001).For laboratory biochemical characteristics,significant changes were observed in plasma SIV RNA (F(2,18)=736.695,P<0.001),Glu (F(2,16)=53.104,P<0.001),AST (F(2,16)=14.583,P<0.001),ALP (F(2,16)=21.745,P<0.001),γGT (F(2,16)=5.963,P=0.012),TP (F(2,16)=13.117,P<0.001),TG (F(2,16)=7.074,P=0.006),TC (F(2,16)=5.575,P=0.015),urea (F(2,16)=3.698,P=0.048),CK (F(2,16)=7.124,P=0.006),and LDH (F(2,16)=15.279,P<0.001).No other biochemical/immunological characteristics showed significant changes during the first three time points following SIV infection.
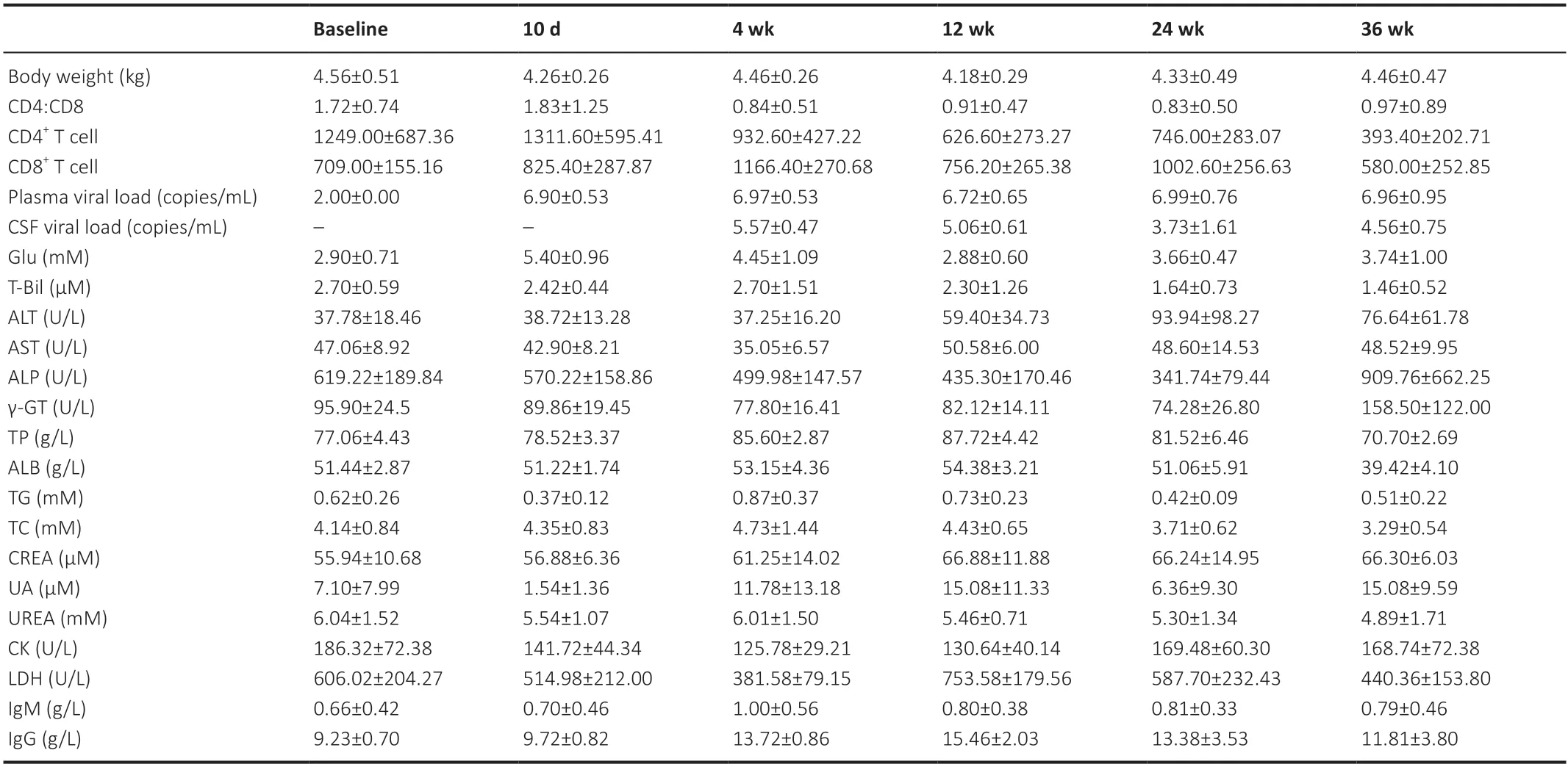
Table 1|Clinical characteristics of the animals in the control group
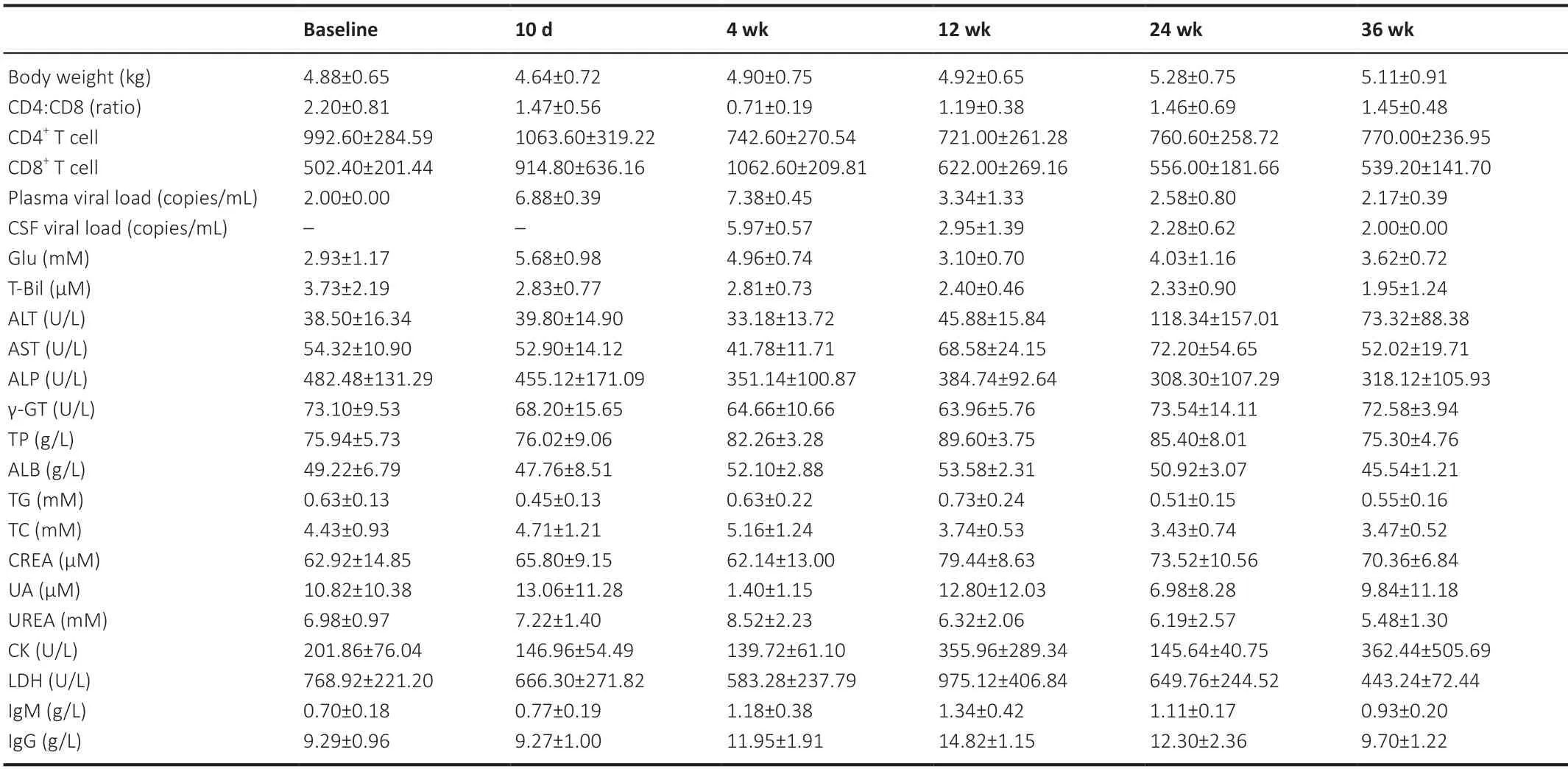
Table 2|Clinical characteristics of the animals in the therapy group
For biochemical/immunological characteristics changes following SIV infection and ART treatment,we found significant interactions between treatment and infection time for the immunological characteristics of peripheral blood CD4+cell counts (infection time:F(2,16)=6.654,P=0.008;treatment:F(1,8)=1.146,P=0.316;interaction:F(2,16)=8.160,P=0.004) and peripheral blood CD8+cell counts (infection time:F(2,16)=5.439,P=0.016;treatment:F(1,8)=2.728,P=0.137;interaction:F(2,16)=5.036,P=0.020),the biochemical characteristics of ALB (infection time:F(2,16)=38.317,P<0.001;treatment:F(1,8)=1.088,P=0.327;interaction:F(2,16)=3.924,P=0.041) and TC (infection time:F(2,16)=14.245,P<0.001;treatment:F(1,8)=0.563,P=0.474;interaction:F(2,16)=5.003,P=0.021).No other biochemical/immunological characteristics showed a significant treatment by infection time interaction.
DMN
Post hocanalysis further revealed that DMNave 4 weeks post-virus inoculation was significantly different from that at baseline (P=0.036,FDR corrected).
In addition,we found significant changes in DMN connecti vity in 11 pairs of ROIs during the first three time points,which comprised area 8/FEF.R and area 31/V6.L,area 46.L and area 8/FEF.L,area 46.L and area 8/FEF.R,area 46.L and precuneate gyrus (PGM).R,area 46.L and parieto-occipital area (PO)/V6.R,area 46.R and area 8/FEF.L,area 46.R and area 8/FEF.R,area 46.R and PO/V6.R,area 8/FEF.L and area 8/FEF.R,area 8/FEF.L and PO/V6.R,and PO/V6.R and area 8/FEF.R.We also visualized the DMN connecti vity of these 11 pairs of ROIs on a normalized T1 monkey brain map inFigure 2A.As the disease progressed,we observed further significant changes in DMN connecti vity in 24 pairs of ROIs during the first three time points,which comprised area 31/V6A.L and area 31/V6A.R,area 31/V6A.L and PGM.R,area 31/V6A.L and PO/V6.R,area 31/V6A.L and TPOC.L,area 31/V6A.L and TPOC.R,area 31/V6A.R and PGM.L,area 31/V6A.R and PGM.R,area 31/V6A.R and PO/V6.R,area 31/V6A.R and TPOC.R,area 46.R and area 8/FEF.L,area 46.R and TPOC.L,area 46.R and TPOC.R,area 8/FEF.R and TPOC.R,PGM.L and TPOC.R,PGM.R and PO/V6.L,PGM.R and PO/V6.R,PGM.R and TPOC.L,PGM.R and TPOC.R,PO/V6.L and PO/V6.R,PO/V6.L and TPOC.L,PO/V6.L and TPOC.R,PO/V6.R and TPOC.L,PO/V6.R and TPOC.R,and TPOC.L and TPOC.R.These are visualized on a normalized T1 monkey brain map inFigure 2B.
The two-way ANOVA of DMN changes following SIV infection and ART treatment revealed significant interactions between treatment and time for the DMN connecti vity of the following ROI pairs: area 31/V6.R and area 8/FEF.L (infection time:F(2,16)=0.632,P=0.544;treatment:F(1,8)=2.751,P=0.136;interaction:F(2,16)=3.993,P=0.039),area 8/FEF.L and TPOC.R (infection time:F(2,16)=9.543,P=0.002;treatment:F(1,8)=13.475,P=0.006;interaction:F(2,16)=3.718,P=0.047),and area 31/V6.R and TPOC.L (infection time:F(2,16)=2.383,P=0.124;treatment:F(1,8)=0.223,P=0.649;interaction:F(2,16)=3.695,P=0.048).These three pairs of ROIs are visualized inFigure 2C.The results of the post hoc analysis are provided inAdditional file 1.
Relationship between DMN connecti vity and laboratory biochemical/immunological characteristics
The relationships between DMN and laboratory biochemical/immunological characteristics for the first and last three time points were analyzed separately.For immunological characteristics,we observed that the CD4+:CD8+ratio was positively correlated with connecti vity between area 46.L and area 8/FEF.R (r=0.563,P=0.001).IgG was negatively correlated with the DMN connecti vity of three ROI pairs,area 46.L and area 8/FEF.R (r=−0.594,P=0.001),area 46.R and area 8/FEF.R (r=−0.578,P=0.001),and area 8/FEF.R and PO/V6.R (r=−0.574,P=0.001;Figure 3).
For laboratory biochemical characteristics,we showed that TP was positively correlated with DMN connectivity between area 46.R and area 8/FEF.R (r=−0.54,P=0.002) and between area 8/FEF.R and PO/V6.R (r=−0.587,P=0.001).UA was positively correlated with DMN connectivity between area 46.L and area 8/FEF.L (r=0.548,P=0.002),between area 46.L and PGM.R (r=0.545,P=0.002),and between area 46.R and area 8/FEF.L (r=0.581,P=0.001).We also found that CK was positively correlated with DMN connecti vity between area 8/FEF.L and PO/V6.R (r=0.557,P=0.002).Plasma SIV RNA was negatively correlated with DMN connecti vity of three ROI pairs: area 46.L and PO/V6.R (r=−0.585,P=0.001),area 46.R and PO/V6.R (r=−0.553,P=0.002),and area 8/FEF.L and PO/V6.R (r=−0.542,P=0.002;Figure 3).For the last three time points,we found no significant correlation between changes in DMN connecti vity and laboratory biochemical/immunological characteristics.
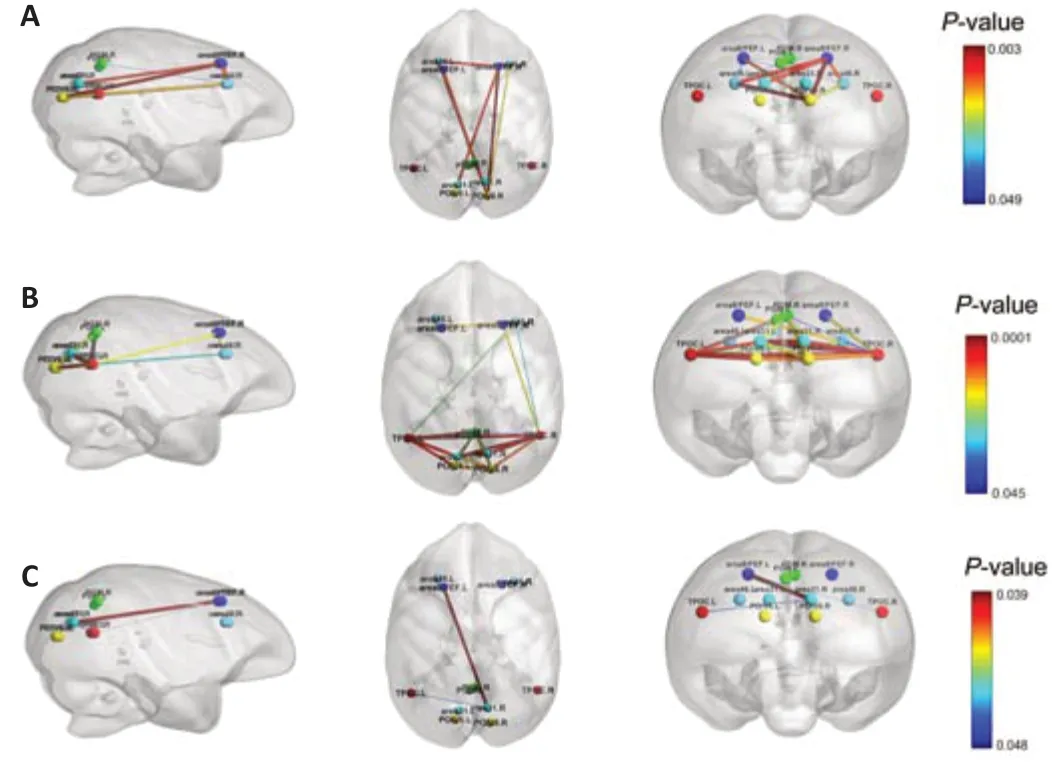
Figure 2|Significant changes in default mode network (DMN) functional connecti vity.
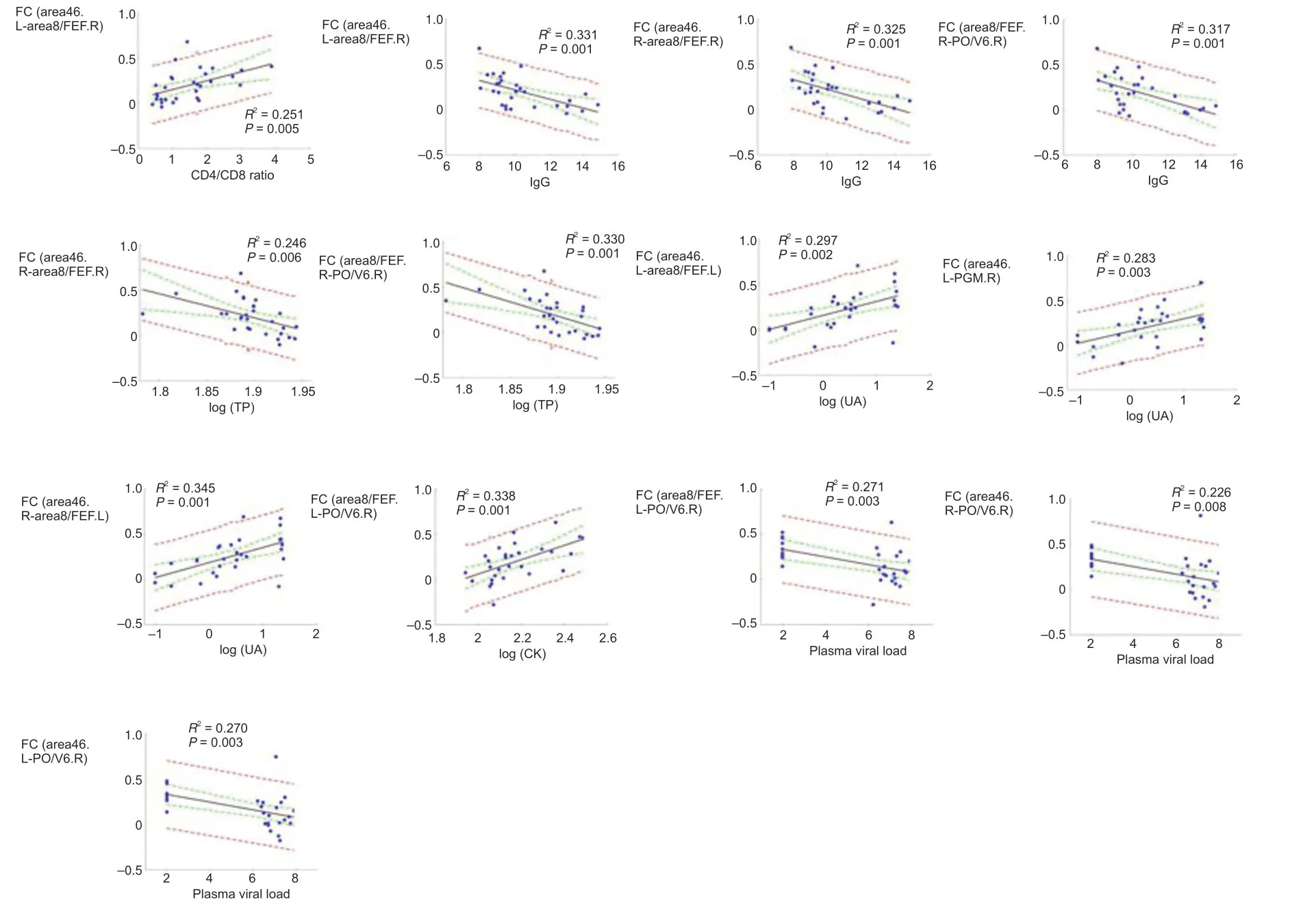
Figure 3|Correlation between clinical characteristics and DMN connecti vity (Pearson’s correlation analysis).
Discussion
We investigated longitudinal changes in DMN connecti vity and biochemical/immunological characteristics due to SIV infection and ART in an SIV-infected rhesus monkey model.We found that SIV infection caused significant changes in DMN connecti vity in area 8/FEF,area 46,and PO/V6,and these changes could be detected as early as 4 weeks post-viral inoculation.Moreover,we found ART had a significant effect on the recovery of DMN connectivity among area 8/FEF,TPOC,and area 31/V6.We also found significant changes in peripheral blood CD4+cell counts,peripheral blood CD8+T-cell counts,CD4+:CD8+ratio,and IgG following SIV infection and a significant interaction between ART and time for peripheral blood CD4+cell count changes only,which indicated that only peripheral blood CD4+cell counts were modulated by ART,whereas CD4+:CD8+ratio and peripheral blood CD8+T-cell counts were not improved by ART.We found significant correlations between the changes in DMN connectivity and various laboratory biochemical/immunological characteristics.Our results provide strong evidence for the modulation of the DMN and biochemical/immunological characteristics following SIV infection and ART.
SIV infection leads to early changes in DMN connecti vity
Previous studies have reported that HIV infection leads to cognitive dysfunction (Casagrande et al.,2021;Maki et al.,2021;Rubin and Maki,2021);thus,it is likely that SIV infection in the macaque would also result in similar dysfunctions.However,how early such dysfunctions can be detected is currently unclear.Because the DMN is involved in numerous cognitive functions (Fan et al.,2021;Yeshurun et al.,2021),it can serve as an efficient model to explore this issue longitudinally.We showed that SIV infection led to a reduction in DMN connectivity among area 8/FEF,area 46,and PO/V6.As reported in previous studies,area 8/FEF and area 46 in the macaque are functionally similar to frontal cortical regions in the human (Petrides and Pandya,1999;Koyama et al.,2004),and these regions are vulnerable to damage caused by AIDS (Rubin et al.,2016;Hassanzadeh-Behbahani et al.,2020;Zhang et al.,2020).We also found that DMN connecti vity of PO/V6 decreased during the early stages of infection.PO/V6 is located in the monkey visual cortex,which is homologous to the human visual area (Pitzalis et al.,2006).Our results are largely consistent with previous studies on AIDS in humans that demonstrated cross-species homology of SIV infection in macaques and dysfunctions in DMN regions in humans.Notably,the frontal cortex was affected as early as 4 weeks following infection.Thus,we speculate that DMN connectivity in macaque monkeys could serve as a potential biomarker for early brain damage caused by AIDS infection.In addition,DMN changes have been observed in patients with HIV infection (Ortega et al.,2015;Cohen et al.,2018).Therefore,although verification in a longitudinal study of HIV infection is needed,similar DMN changes following SIV and HIV infection also support the use of DMN connecti vity as a potenti al biomarker for early brain damage following HIV infection.
Early detection of brain damage is crucial for providing early clinical intervention to HIV-infected patients.A previous study in patients with HIV showed that sustained infection leads to cognitive impairments,such as HIVassociated neurocognitive disorders (Clifford and Ances,2013;Saylor et al.,2016) and HIV-associated dementia (Watkins and Treisman,2015;Clifford,2017),which are primary factors that significantly impact the quality of life of HIV patients.Clinically,cognitive impairments are more commonly observed during later infection stages than during the very early stage (Holt et al.,2012;Guha et al.,2016).In addition,clinical interventions are mostly administered after the onset of cognitive decline,at which point,reversing cognitive impairment becomes difficult.According to our results,brain damage caused by AIDS infection can be detected by evaluating DMN connecti vity as early as 4 weeks after infection;thus,DMN connecti vity change may be used as a marker for providing early clinical intervention to protect the integrity of the brain from HIV infection.
ART restores DMN connecti vity
Our results showed that ART restored DMN connectivity among area 8/FEF,TPOC,and area 31/V6.In line with our findings,previous studies have reported that ART improves the cognitive performance of HIV-infected patients (DʼAntoni et al.,2018;Kanmogne et al.,2020).Our longitudinal findings may explain that previous reports of improved cognitive function are attributed to the recovery of DMN functional connectivity.Notably,we observed the restoration of connecti vity among area 8/FEF,TPOC,and area 31/V6 following ART,which suggested that an integrated subnetwork within the DMN is responsible for the modulation of both visual and motor function.Our results indicated that prolonged ART restores the functions of the DMN.Therefore,timely ART could protect the cognitive function of HIV-infected patients.
ART is unanimously considered an efficient HIV treatment approach for preventing the replication of the virus,reducing viral load,and reconstructing immune function (Arendt and Nolting,2012;Bauer et al.,2014).However,there is debate regarding the exacerbation of damage to the central nervous system due to the drug toxicity of ART (Schouten et al.,2011).Therefore,it remains uncertain whether administering ART as early as possible benefits or harms the central nervous system.Our findings provided direct evidence thattimely ART could help protect cognitive function.
Relationship between DMN connecti vity changes and laboratory biochemical/immunological characteristics
We found significant correlations among DMN changes and immunological characteristics during the first three time points.Specifically,eight regions of decreased functional connecti vity were significantly correlated with increased plasma SIV RNA,which indicated that both brain and immunological functions exhibit similar changes in the early stages of SIV infection.However,because no significant correlations were observed among DMN changes and immunological characteristics during the last three time points,these similar changes may be interrupted by ART.
Limitations
The current study has several limitations.First,the sample size of the current study was relatively small because of the difficulti es and costs associated with conducting animal experiments.Second,we did not perform any cognitive tests because of difficulties in training animals.In the future,studies using large sample sizes and cognitive tests will be needed to validate our findings.
Conclusion
In the current study,we demonstrated that DMN functional connecti vity is affected shortly after SIV infection and that the administration of ART not only interrupts the progression of SIV infection but also preserves brain function.These findings indicated that the DMN could serve as a potent biomarker for early brain damage.It is of significant clinical value to detect early brain damage caused by AIDS to provide early intervention to patients with HIV-associated neurocognitive disorders or HIV-associated dementi a.In addition,our study provides a reference for other investigations of treatment for AIDS based on animal models.We also revealed that DMN connecti vity changes were significantly correlated with various laboratory biochemical/immunological characteristics,which suggested that DMN changes,as a potenti al biomarker,correspond to immunological and biochemical changes caused by AIDS infection.
Author contributions:Study design and administration: HZ,JT,HJL;experiment implementation: HWQ,XJH;data acquisition: DL,JJL;dataprocessing: ZCT,XTD;manuscript draft : ZCT,JJL,XTD,DL;manuscript revision:ZCT,JJL,HZ,JT,HJL.All authors approved the final version of the manuscript.
Conflicts of interest:The authors declare that they have no competing interests.
Availability of data and materials:All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:This is an open access journal,andarticles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows othersto remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional file:
Additional file 1:Additional information for data acquisition,processing,and results.
- 中国神经再生研究(英文版)的其它文章
- Bystanders or not? Microglia and lymphocytes in aging and stroke
- Alzheimer’s disease risk after COVID-19: a view from the perspective of the infecti ous hypothesis of neurodegeneration
- Serine and arginine rich splicing factor 1: a potenti al target for neuroprotection and other diseases
- Can glial cells save neurons in epilepsy?
- Lights for epilepsy: can photobiomodulation reduce seizures and offer neuroprotection?
- The landscape of cognitive impairment in superoxide dismutase 1-amyotrophic lateral sclerosis

