The circ_0002538/miR-138-5p/plasmolipin axis regulates Schwann cell migration and myelination in diabetic peripheral neuropathy
Yu-Tian Liu ,Zhao Xu ,Wei Liu ,Sen Ren ,He-Wei XiongTao JiangJing ChenYu KangQian-Yun LiZi-Han WuHans-GüNther Machens,Xiao-Fan Yang,Zhen-Bing Chen
Abstract Circular RNAs (circRNAs) play a vital role in diabetic peripheral neuropathy.However,their expression and function in Schwann cells in individuals with diabetic peripheral neuropathy remain poorly understood.Here,we performed protein profiling and circRNA sequencing of sural nerves in patients with diabetic peripheral neuropathy and controls.Protein profiling revealed 265 differentially expressed proteins in the diabetic peripheral neuropathy group.Gene Ontology indicated that differentially expressed proteins were mainly enriched in myelination and mitochondrial oxidative phosphorylation.A real-time polymerase chain reaction assay performed to validate the circRNA sequencing results yielded 11 differentially expressed circRNAs.circ_0002538 was markedly downregulated in patients with diabetic peripheral neuropathy.Further in vitro experiments showed that overexpression of circ_0002538 promoted the migration of Schwann cells by upregulating plasmolipin (PLLP) expression.Moreover,overexpression of circ_0002538 in the sciatic nerve in a streptozotocin-induced mouse model of diabetic peripheral neuropathy alleviated demyelination and improved sciatic nerve function.The results of a mechanistic experiment showed that circ_0002538 promotes PLLP expression by sponging miR-138-5p,while a lack of circ_0002538 led to a PLLP deficiency that further suppressed Schwann cell migration.These findings suggest that the circ_0002538/miR-138-5p/PLLP axis can promote the migration of Schwann cells in diabetic peripheral neuropathy patients,improving myelin sheath structure and nerve function.Thus,this axis is a potential target for therapeutic treatment of diabetic peripheral neuropathy.
Key Words:circ_0 002538;circRNA sequencing;competing endogenous RNAs;demyelination;diabetic peripheral neuropathy;miR-138-5;myelination;plasmolipin;protein profiling;Schwann cells
Introduction
Diabetes mellitus is a major global health concern affecting more than 9% of the global population,and this is expected to increase over time (Feldman et al.,2019a).The most common complication of diabetes mellitus is diabetic peripheral neuropathy (DPN),which affects approximately 50% of people with diabetes during their lifetime (Pop-Busui et al.,2017).DPN is the key initiating factor of diabetic foot conditions that can lead to nontraumatic lower limb amputation,which can seriously reduce the quality of life and patient life expectancy (Feldman et al.,2019a;Selvarajah et al.,2019).DPN is characterized by pain,paresthesia,and loss of sensation,and is associated with axon atrophy,demyelination,weakened regenerative potential,and the loss of peripheral nerve fibers (Farmer et al.,2012).Although several therapeutic approaches have been introduced in clinical practice,the current DPN treatment has only been found to relieve some symptoms with limited effects (Singh et al.,2014).Current studies have found that the occurrence and development of DPN are largely caused by hyperglycemia,insulin deficiency,and dyslipidemia.However,the molecular mechanisms that lead to demyelination and neurological dysfunction remain unclear.Therefore,clarification of the molecular mechanism that promotes DPN initiation and development has important clinical significance and may lead to more effective treatments for DPN.
Circular RNAs (circRNAs) are a recently characterized type of noncoding RNA.They play a key role in the occurrence and development of many diseases and are highly evolutionarily conserved,stable,and tissue-specific (Zhang et al.,2019;Shi et al.,2020).circRNAs are involved in the modification of transcription or posttranscriptional gene expression,and their mode of action includes protein binding,translation,and microRNA (miRNA) sponges (Wang et al.,2020a).circRNA sequencing in spinal cord tissue and dorsal root ganglia of DPN mice revealed 135 and 15 differenti ally expressed circRNAs (Zhang et al.,2020;He et al.,2021),respectively,which were associated with the occurrence and development of neuronal abnormalities.However,the characteristics and functions of circRNAs in Schwann cells (SCs) in DPN remain unclear.
In the present study,we used circRNA sequencing and protein profiling analyses of nerve tissues from humans with or without DPN to explore the onset and developmental mechanisms of DPN.circ_0002538 is a circRNA derived from Kelch-like family member 8 (KLHL8) with downregulated expression in circRNA sequencing of nerves from patients with DPN,whose function has not previously been characterized.Moreover,we investigated the role of circ_0002538 in the development of DPNin vitroandin vivo.
Methods
Ethics statement
This study was approved by the Ethics Committee of Tongji Medical College,Huazhong University of Science and Technology (approval No.IEC 2021-S085,approved on March 31,2021),and informed consent was obtained from each patient.All animal study protocols were approved by the Animal Care Committee of Huazhong University of Science and Technology (No.2020-S2665,approved on December 1,2020).The timeline of the experiment was shown inFigure 1.
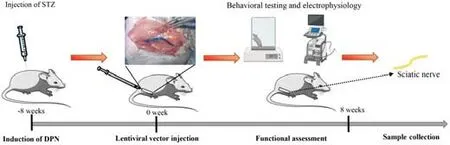
Figure 1|Schematic diagram illustrating the timeline of the experiment.
Patient tissue specimens
Sural nerve tissues and skin tissues were collected from 29 patients who underwent lower limb amputation at the Union Hospital and Liyuan Hospital of Huazhong University of Science and Technology from 2014 to 2020.The DPN diagnoses were based on a history of diabetes,typical symptoms,abnormal nerve conduction,and the exclusion of neuropathy with causes other than diabetes (Pop-Busui et al.,2017;Feldman et al.,2019b).For diabetic patients without nerve conduction data,we confirmed the diagnosis of DPN by performing a skin biopsy to assess intraepidermal nerve fiber density and utilizing transmission electron microscopy (HT7700,Hitachi,Hitachi,Japan) to confirm neuropathy in the peripheral nerves (Holland et al.,1997).Individuals diagnosed with the following diseases were excluded from the study: neuropathic deficits caused by other diseases,severe peripheral vascular disease,a history of major amputation,other serious chronic medical diseases,or alcohol and drug abuse.
Under a microscope,the epineurium of the sural nerve tissues in the distal calf was stripped,and the nerve bundles were drawn out and immediately snap-frozen in liquid nitrogen for further research.Skin tissues 10 cm above the lateral malleolus were collected for immunofluorescence staining of protein gene product 9.5.The intraepidermal nerve fiber density was calculated according to a previously described method (Vlcková-Moravcová et al.,2008).
Protein profiling analysis
Total proteins were extracted from three pairs of sural nerves from the patients with DPN and individuals without DPN using a protein lysis solution(4% sodium dodecyl sulfate,100 mM Tris HCl,pH 7.6).We then performed proteomic profiling using the tandem mass tag labeling system (Thermo Fisher Scientific,Waltham,MA,USA).We used a Q Exactive Plus high-resolution mass spectrometer (Thermo Fisher Scientific) to perform tandem mass tag quanti tative proteomic analysis,and the soft ware programs Mascot 2.6 (Matrix Science,Boston,MA,USA) and Proteome Discoverer 2.1 (Thermo Fisher Scientific) for library identification and quanti tative analysis,respectively (false discovery rate <0.01).
Gene Ontology and Kyoto Encyclopedia of Genes and Genomes enrichment analysis
The differentially expressed proteins or mRNAs were further analyzed via Gene Ontology (GO) enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis for functional prediction.We used GO analysis to annotate the cell components and biological processes based on the GO resource (http://www.geneontology.org),and pathway analysis to explore the enrichment of different pathways based on the KEGG database (http://www.genome.jp/kegg).The protein–protein interaction network analysis was based on the STRING database (https://string-db.org) and visualized using Cytoscape 3.7.2 (Shannon et al.,2003).
circRNA sequencing analysis
The sequencing libraries were constructed as described in a previous report(Lu et al.,2020).Briefly,the total RNA of the aforementioned three pairs of peripheral nerves was prepared using TRIzol reagent (Invitrogen,Carlsbad,CA,USA).The RNA integrity number was evaluated using the Agilent 2200 TapeStation (Agilent Technologies,Eugene,OR,USA),and all RNA samples with an RNA integrity number above 7.0 were subjected to further circRNA sequencing analysis.Before constructing the circRNA sequencing libraries,we used the Epicentre Ribo-Zero rRNA Removal Kit (Illumina,San Diego,CA,USA) to remove ribosomal RNA from the RNA samples,and incubated 40 U RNase R (Epicenter,Madison,WI,USA) with the total RNA at 37°C for 3 hours to remove linear RNA.The libraries were sequenced using the HiSeq-3000 sequencing platform,and we examined the differenti ally expressed circRNA between the sural nerves from patients with DPN and tissues from individuals without DPN using DESeq2 soft ware (v 2.11.40.2;Bioconductor,Inc.).
Cell culture and treatments
We isolated the primary SCs from human sural nerves (three donors were randomly selected from each group),as previously described,to examine the impaired function of SCs from DPN patients (Wang et al.,2020b).Briefly,the sural nerves were cut into 5-mm-long sections after the epineurium had been stripped and predegenerated in SC culture medium for 10 days.Next,the nerve segments were cut into 2-mm3pieces and transferred to a mixture containing Dulbecco’s modified Eagle’s medium (Thermo Fisher Scientific),10% fetal calf serum,0.125% type IV collagenase (Sigma-Aldrich,St.Louis,MO,USA),1.25 U/mL dispase II (Solaribo,Beijing,China),and 1% penicillinstreptomycin to digest for 18–20 hours.The cells were cultured in SC medium(ScienCell,Carlsbad,CA,USA).The SCs used in the other experiments were purchased from ScienCell Research Laboratories and cultured in SC medium containing 5% fetal calf serum.We added oxidized low-density lipoprotein(ox-LDL,BioVision,Exton,PA,USA) to the culture medium to mimic diabetic conditions.After growing to confluent or subconfluent cell layers,the SCs were cultured for another 6 days to examine plasmolipin (PLLP) expression as previously described (Gillen et al.,1996).SCs were identified via immunofluorescence staining with S100 calcium binding protein B and glial fibrillary acidic protein.HEK293 cells (ACC305,DMSZ,Braunschweig,Lower Saxony,Germany,RRID: CVCL_0045) were cultured in high glucose Dulbecco’s modified Eagle’s medium containing 10% fetal calf serum and 1% penicillin/streptomycin.The cells were cultured at 37°C in a humidified atmosphere containing 5% CO2.
Real-time polymerase chain reaction
We extracted the total RNA from the sural nerves and cells using TRIzol reagent (TaKaRa,Kyoto,Japan).The genomic DNA was isolated using a TIANamp Genomic DNA Kit (TianGen Biotech,Beijing,China) according to the manufacturer’s instructions.The RNA samples were then reverse transcribed into complementary DNA (cDNA) using the PrimeScriptTMRT Reagent (TaKaRa,RR036A).We performed real-time polymerase chain reactions (RT-PCRs) using a 7500 Real-time PCR System (Applied Biosystems,Carlsbad,CA,USA) with the Universal SYBR Green Master Mix (4913 914001;Roche,Shanghai,China).β-Actin was used as an internal control.The RT-PCR protocol was as follows:one cycle of 95°C for 10 minutes followed by 40 cycles of 95°C for 15 seconds and 60°C for 1 minute.Gene expression was quantified using the 2–∆∆Ctmethod (Livak and Schmittgen,2001).For circRNA,the total RNA was reverse transcribed to cDNA using the PrimeScriptTMRT reagent kit (TaKaRa,RR037A).We used convergent and divergent primers to detect the expression of linear RNA and circRNA transcripts.The primers are shown inAdditional Table 1.
Sanger sequencing
We conducted Sanger sequencing to verify the back-splicing position of circ_0002538.The total RNA was extracted from the SCs and reverse transcribed into cDNA.circ_0002538 was amplified with divergent primers and 2× Taq Master Mix (Vazyme,Nanjing,Jiangsu,China) using qPCR.The qPCR protocol was one cycle of 95°C for 5 minutes followed by 34 cycles of 95°C for 30 seconds,52°C for 30 seconds,and 72°C for 30 seconds.Then,the base sequences of the products were determined using Sanger sequencing and compared with the data in circBase (http://circrna.org/).
Nuclear and cytoplasmic separation assay
To detect the cellular localization of circRNAs,we extracted RNA from nuclear and cytoplasmic fractions using a cytoplasmic and nuclear RNA isolation kit(Norgen Biotek,Ontario,Canada) according to the manufacturer’s protocol.The relative expression levels of circ_0002538 in the nucleus and cytoplasm were detected via RT-PCR.We used GAPDH and U6 small nuclear RNA as internal controls.
Digestion with RNase R
For RNase R digestion,10 µg of total RNA was incubated with 2 U/µg RNase R (BioVision,Milpitas,CA,USA) at 37°C for 30 minutes.RNAs treated with the same process without RNase R were the mock group.The expression levels of KLHL8 and circ_0002538 were determined via RT-PCR.
Plasmid construction and stable transfection
circ_0002538 cDNA was synthesized by Tsingke Biological Technology (Wuhan,China) and cloned into the GV689 vector (Shanghai GeneChem Co.,Ltd.,Shanghai,China) to construct overexpression plasmids.Short hairpin RNA(shRNA) for circ_0002538 was designed using the CircInteractome tool and cloned into the GV493 vector (Shanghai GeneChem Co.,Ltd.) to construct silencing plasmids.The plasmids for the overexpression and knockdown of PLLP were designed and synthesized by Shanghai Gene Chemical Co.,Ltd.Then,the constructed plasmids were packaged into lentivirals (LVs)by Shanghai Gene Chemical Co.,Ltd.and cell transfection was performed according to the manufacturer’s instructions.The transfected cells were incubated with 2 µg/mL of puromycin (BIOFOX,Nantong,China) for 5 days,and the surviving cells were used as stable transfectants.
Oligonucleoti de transfection
miRNA mimics,miRNA inhibitors,and corresponding negative control oligonucleotides were synthesized by RiboBio (Guangzhou,China).The sequences used are listed inAdditional Table 2.Transfection was carried out using a PECTTM CP Transfection kit (RiboBio) with a final concentration of 50 nM for miRNA mimics and 100 nM for miRNA inhibitors,according to the manufacturer’s protocol.
Transwell assay
SC migration was determined using a Transwell chamber (8-µm pore size,Corning,Corning city,NY,USA) according to the manufacturer’s protocol.Approximately 2 × 104cells suspended in 200 µL of serum-free medium were added to the upper chamber,and a total of 650 µL of Schwann medium containing 5% fetal calf serum was added to the lower chamber as a chemical attractant.After a 24-hour incubation period,we evaluated cell migration by counting the number of migrated cells on the lower surface of the chamber in at least five random fields.
Western blot analysis
We tested the expression levels of PLLP protein in SCs and neural tissues via a western blot analysis.The protein was extracted using a radioimmunoprecipitation assay lysis buffer,supplemented with 1% protease inhibitor.Equal amounts of protein (30 µg) were separated in a 10% sodium dodecyl sulfate-polyacrylamide gel and then transferred to polyvinylidene fluoride membranes (Millipore,Darmstadt,Germany).The membranes were blocked in 5% (w/v) bovine serum albumin (Aladdin,Shanghai,China)before incubation with the primary antibodies at 4°C overnight.Then,the membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:5000,Aspen Biotechnology Co.,Ltd.,Wuhan,China,Cat# AS1107) for 1 hour at room temperature and visualized using a BioSpectrum Imaging System (UVP,Upland,CA,USA) with the Immobilon ECL substrate kit (Millipore,Darmstadt,Germany).We used primary anti bodies specific to PLLP (rabbit,1:700,Cusabio,Houston,TX,USA,Cat# CSB-PA896501LA01HU).All tests were repeated three times,and the typical images were provided.
RNA pulldown assay
To detect the combination of circRNAs and miRNAs,we performed RNA pulldown assays with biotinylated probes according to the manufacturer’s protocol (MCE,Shanghai,China,Cat# HY-K0208).In brief,the biotinylated probe or nonsense control probe (RiboBio) was incubated with M-280 streptavidin magnetic beads (MCE) at room temperature for 2 hours to generate probe-coated beads.Approximately 1 × 107SCs were crosslinked with 1% paraformaldehyde and then neutralized with 1.25 M glycine.Next,these cells were harvested,lysed,and incubated with probe-coated magnetic beads at 4°C overnight.After being washed,the RNA complexes bound to the beads were eluted and extracted using an Rneasy Mini Kit (Qiagen,Hilden,Germany).Then,the abundance of circRNA or miRNA was evaluated via RTPCR.
Dual-luciferase reporter assay
We predicted the binding sites of miR-138-5p targeting circ_0002538 and PLLP using RNAhybrid (Rehmsmeier et al.,2004) and TargetScan (McGeary et al.,2019),respectively.The wild-type or mut-circ_0002538 fragment was cloned into the downstream of the luciferase reporter gene of the pMIRreport vector (Promega,Madison,WI,USA),while wild-type or mut-PLLP fragment was inserted into the downstream of the hRluc (Renilla) reporter gene of the psi-check2 vector (Promega).The corresponding plasmid and miRNA mimic were cotransfected into HEK293T cells (5 × 104) seeded in a 12-well plate using Lipofectamine 2000 (Thermo Fisher Scientific).The firefly and Renilla luciferase acti vity of the cells was quantified using a Dual Luciferase Reporter System Kit (E1910,Promega) according to the manufacturer’s instructions.
Prediction of miRNAs targeting circ_0002538 or PLLP
We made predictions regarding the miRNAs that target circ_0002538 or PLLP to ascertain the connection between circ_0002538 and PLLP.The prediction process was conducted by RiboBio (Guangzhou,China).For PLLP,miRNAs predicted by at least three databases (miRDB,miRTarBase,miRWalk,and TargetScan) were considered candidates (Dweep et al.,2011;McGeary et al.,2019;Chen and Wang,2020;Huang et al.,2020).For circ_0002538,miRNAs predicted by at least two databases (RNAhybrid,miRanda,and TargetScan)were considered candidates (Rehmsmeier et al.,2004;McGeary et al.,2019).We used a Venn diagram to find the common miRNAs (Hulsen et al.,2008).
Induction of diabetes
Due to the high similarity to human and the stability in genes,mice were used to explore circ_0002538 functionin vivo(Perlman,2016).Sex is one factor influencing variations in diabetes induction.As estrogen interferes with streptozotocin (STZ) action,female animals are less sensitive to the diabetogenic action of STZ than male animals.Further,male mice are more commonly used in neuroscience research (Beery and Zucker,2011).As a result,we chose to use male animals for our study.Compared with other age groups,rodents aged 8–9 weeks show maximal induction of diabetes(Goyal et al.,2016).Thus,we used rodents in this age group.The induction of diabetes was conducted as previously described (Wang et al.,2020b).Briefly,a total of 60 male (8-week-old) C57BL/6J mice (specific-pathogen-free level,SiPeiFu,Beijing,China,SCXK2019-0010) were intraperitoneally injected with STZ (Sigma-Aldrich) at a dose of 50 mg/kg for 5 consecutive days.Subsequently,45 mice had fasting blood glucose levels of 16.7 mM or higher and were thus diagnosed with diabetes (Wang et al.,2020b).Forty mice with significantly increased mechanical and thermal thresholds were diagnosed with DPN (Fan et al.,2020).We randomly selected one side of the sciatic nerve to be injected with circ_0002538 (circ_0002538 group) and injected the other side with LV-vector (vehicle group,n=40).
Surgery and lenti viral vector injection
We injected a LV-vector into the sciatic nerve of the mice with DPN,as previously described (Tannemaat et al.,2008).Briefly,after exposure and isolation of the sciatic nerve,2.5 µL of lentiviral solution (6 × 106TU LVcirc_0002538 or LV-GFP vector) was injected into the distal peroneal and ti bial branches of the sciatic nerve through the epineurium using a 10-µL Hamilton syringe (Hamilton Co.,Reno,NV,USA).Fast Green (Sigma-Aldrich) at a final concentration of 0.1% was added to the lentiviral solution to monitor the injection process and ensure that there was no obvious leakage.A 2.5-µL lenti viral solution containing 6 × 106TU LV-sh-PLLP or LV-vector was injected into the sciatic nerve of normal mice to determine the role of PLLP.The epineurium at the injection site was repaired with 10-0 nylon sutures under an operating microscope (Xinti an Medical Instrument Co.,LTD,Zhenjiang,China).
Behavioral testing and electrophysiology
Eight weeks after diabetic induction,we assessed thermal and mechanical nociceptive thresholds via double-blind trials.Before the nociceptive behavior test,the mice were acclimated to the environment for at least half an hour.Mechanical allodynia was assessed using von Frey filaments (Danmic Aesthesio,Campbell,CA,USA),as described previously (Xu et al.,2015;Pan et al.,2019).A brisk withdrawal or flinching of the paw was considered a positive response.The inter-test interval between the two sides of the plantar hind paw was more than 15 minutes,and the 50% force withdrawal threshold was determined for the plantar hind paws using the “up-and-down” method(Chaplan et al.,1994).The thermal nociceptive threshold was assessed using the hot plate test (Masocha et al.,2016).A mouse was placed in a Plexiglas cylinder on a hot plate (Model 7280,Ugo Basile,Gemonio,Italy),and the time required for the stimulus to elicit behavioral changes (such as paw licking,stomping,and withdrawal of the hindpaw) was recorded.
At 8 weeks post-surgery,we evaluated the nerve conduction velocity of the sciatic nerve as a sign of DPN.The sciatic nerve conduction velocity was measured via orthodromic recording techniques,as described previously(Ii et al.,2005;Baum et al.,2016;Wang et al.,2020b).The sensory nerve conduction velocity and motor nerve conduction velocity were calculated using an electromyograph (Nicolet,Madison,WI,USA) according to a previous method (Ii et al.,2005).
Hematoxylin and eosin staining,immunofluorescence analysis
We conducted hematoxylin and eosin (HE) staining to evaluate the intraepidermal nerve fiber density of skin samples from diabetic and nondiabetic individuals.The samples were collected and fixed in paraformaldehyde(4%) within 2 hours of amputation,then dehydrated and embedded in paraffin.Four-micron-thick slices of skin were prepared and subjected to HE (Bioyear,Wuhan,China) to examine subcutaneous nerves in the skin.
We used protein gene product 9.5 to evaluate the number of subcutaneous nerves in the skin samples.Glial fibrillary acidic protein and S100 calcium binding protein B were used to characterize primary SCs extracted from the sural nerves.We used myelin protein zero (MPZ) to locate SCs in the sciatic nerves of the DPN mice.The mice were sacrificed 8 weeks after the operation,and the bioluminescence of green fluorescent protein (GFP)-expressing cells was detected via fluorescence microscopy (Olympus,Tokyo,Japan).Then,the sciatic nerve tissues were collected for morphological analysis.For immunofluorescence analyses,we incubated primary anti bodies against protein gene product 9.5 (rabbit,1:300,Proteintech,Wuhan,China,Cat#14730-1-AP,RRID: AB_2210497),glial fibrillary acidic protein (rabbit,1:400,Abcam,Carlsbad,CA,USA,Cat# ab68428,RRID: AB_1209224),S100 calcium binding protein B (rabbit,1:200,Abcam,Cat# ab52642,RRID: AB_882426),and MPZ (rabbit,1:200,Abcam,Cat# ab183868,RRID: AB_2895675)overnight at 4°C.On the second day,we incubated goat anti -rabbit secondary anti body (Fluor® 488,1:400,Abcam,Cat# ab150077) at 37°C for 1 hour.We used 2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride (Biosharp,Wuhan,China,Cat# BL105A) to stain the cell nuclei.Fifteen-micrometer-thick frozen sections of nerve tissues were stained with MPZ.Images were obtained using a fluorescence microscope (Olympus,Tokyo,Japan),with at least three visual fields for each sample.
Transmission electron microscopy
The collected nerves were cut into 5-mm long sections,prefixed in 2.5%glutaraldehyde for 30 minutes,and then postfixed in 1% osmium tettroxide for 1 hour.After dehydration and embedding in epoxy resin,ultrathin sections(60 µm) were prepared and stained with uranyl acetate and lead citrate.Images were captured under a transmission electron microscope (HT7700,Hitachi),and 15 random images were captured for each sample.
Stati stical analysis
According to previous methods (Charan and Kantharia,2013),we determined a minimum sample size of 35 mice.Considering the potenti al for unexpected death in the experiment and the failure of the STZ-induced diabetes model,we used a sample size of 60.
The data are expressed as the mean ± standard deviation (SD),median(interquartile range (IQR)),or number (%).Pvalues were obtained using the pairedt-test,independent-samplest-test,or Fisher’s exact test(normal distribution) combined with the Mann-WhitneyUtest (nonnormal distribution) or one-way analysis of variance with Tukey’spost hoctest (more than two groups).P<0.05 was considered significant,and all statistical analyses were performed using Graphpad Prism 8.0 (GraphPad Soft ware,San Diego,CA,USA,www.graphpad.com).
Results
Characteristics of patients and confirmation of DPN
Twenty-nine patients from two terti ary teaching hospitals were recruited for the study.The median age of the DPN group was 60.0 years (IQR: 56.0–67.0 years) and that of the non-DPN group was 63.5 years (IQR: 55.75–65.0 years).The calf skin and sural nerve were intact in all patients when undergoing amputation.Detailed patient information is provided inAdditional Table 3.Because some of the patients had not undergone nerve conduction studies,which is the gold standard for diagnosing DPN,we attempted to verify the diagnosis using other indicators.HE staining revealed a decreased number of subcutaneous nerves in the skin of the lateral malleolus in the DPN group(Additional Figure 1AandB),which was confirmed by protein gene product 9.5 staining of axons (Additional Figure 1CandD).Furthermore,the numbers of axons and intact myelin sheaths were decreased in the nerves of the DPN group,as shown by transmission electron microscopy (Additional Figure 1EandF).We thus confirmed DPN in the collected diabetic peripheral nerves.
Impaired myelination and SC migration in the peripheral nerves of the DPN group
Protein profiling analyses were performed on three pairs of peripheral nerves in the DPN and non-DPN groups.A total of 5353 proteins were identified,and 265 proteins were significantly [P<0.05,|fold change (FC)| ≥ 1.3]differenti ally expressed in the DPN group (Additional Table 4),as shown by the hierarchical cluster analysis (Figure 2A).GO cellular component analysis indicated that the differentially expressed proteins were mainly found in the mitochondrion and myelin sheath (Figure 2BandAdditional Table 5).GO biological process analysis showed that 390 terms were significantly enriched,among which myelination was potentially related to DPN (Figure 2CandAdditional Table 6).The proteins related to myelination were serine incorporator 5,PLLP,gap junction protein gamma 3,proteolipid protein 1,periaxin,and MPZ.GO molecular function analysis showed significant enrichment in G protein-coupled serotonin receptor binding and protein binding (Figure 2DandAdditional Table 7).KEGG pathway analysis revealed that 77 pathways were significantly enriched,among which oxidative phosphorylation and the glucagon signaling pathways were potenti ally related to DPN (Figure 2EandAdditional Table 8).Figure 2Fshows a protein–protein interaction network constructed according to the differentially expressed proteins and showing the interactions among these proteins.These results indicate that abnormal myelination might play an important role in the pathogenesis of DPN.
Myelin is composed of SCs,which are indispensable for the physiological functions of peripheral nerves (Salzer,2015).Previously,impaired SC migration was reported to contribute to the abnormal myelination and demyelination of peripheral nerves (Anliker et al.,2013;Yi et al.,2019).Thus,we compared the function of SCs from nerves in the DPN and control groups.The primary SCs isolated from the peripheral nerves exhibited a long spindle shape under an optical microscope (Additional Figure 2A).These were confirmed via positive immunofluorescence staining of S100 calcium binding protein B and glial fibrillary acidic protein (Additional Figure 2B).Cell migration assays showed significantly impaired migration of SCs derived from patients with DPN (Figure 2GandH).
Characterization of circ_0002538 and its function in SCs
We performed circRNA sequencing for the three pairs of peripheral nerves to uncover their characteristics in the development of DPN.In diabetic peripheral nerves,we identified a total of 15637 circRNAs.A total of 169 circRNAs showed significantly (P<0.01,q<0.05,readings ≥ 50,FC ≥ 2)dysregulated expression in the DPN group: 116 circRNAs had significantly downregulated expression and 53 circRNAs had significantly upregulated expression (Additional Table 9).The differentially expressed circRNAs(DEcircRNAs) were directly displayed by hierarchical cluster analysis (Figure 3A).The DEcircRNAs were verified using RT-PCR,and the results showed that six circRNAs with downregulated expression and five with upregulated expression were confirmed in the DPN group (Figure 3BandC).These DEcircRNAs may play an important role in the pathogenesis of DPN.
To further investigate the function of DEcircRNAs in DPN,we focused on circRNA circ_0002538,which showed a 2.14-FC decrease in expression in the DPN group compared with the non-DPN group.circ_0002538 is formed by head-to-tail splicing of exon 2 of the KLHL8 gene,which is located on chromosome 4 (q22.1) (Figure 3D).Sanger sequencing verified the headto-tail splicing,which was consistent with the data in circBase (Figure 3D).circ_0002538 could be amplified by RT-PCR using divergent primers in cDNA but not in genomic DNA (Figure 3E).circ_0002538 was barely altered after incubation with RNase R comparing to the mock group (Figure 3F),which further confirmed that circ_0002538 has a loop structure.
We confirmed that circ_0002538 expression was decreased in DPN tissues(Figure 3C).Then,we transfected LV-circ_0002538-shRNA into SCs to simulate the pathological process of SCs during DPN.shRNA significantly reduced circ_0002538 expression without affecting the KLHL8 mRNA expression(Figure3G).We chose sh-circ_0002538 #2 in the following experiments because it had a high inhibitory efficiency compared with the other shRNAs.Migration assays revealed that the knockdown of circ_0002538 impeded the migration of SCs (Figure 3HandI).We further validated the effects of circ_0002538 in the circ_0002538-overexpressing SCs.The expression level of circ_0002538 in these stable overexpression cells was substanti ally increased,while there was no change in the KLHL8 mRNA level (Additional Figure 3A).Migration assays revealed that the overexpression of circ_0002538 increased the number of SCs that migrated to the lower chamber (Additional Figure 3BandC).These findings indicate that circ_0002538 was involved in regulating SC migrationin vitro.
Overexpression of circ_0002538 improves the neuropathic phenotype and symptoms of DPN
To further assess the role of circ_0002538 in DPNin vivo,we injected circ_0002538 LV into mice with DPN (Figure 4A).We used a fluorescence microscope to examine GFP-positive cells in the sciatic nerve at the 8th week after surgery,and found that injection of the LV-vector led to long-term transgene expression in the sciatic nerve (Figure 4B).RT-PCR revealed that circ_0002538 expression in the circ_0002538 group was higher than that in the vector group (Figure 4C).Immunofluorescence showed that GFP-positive cells also expressed MPZ protein in the circ_0002538 overexpression group,indicating that circ_0002538 was stably expressed in SCs (Figure 4D).
To further examine the effect of circ_0002538 on the signs and symptoms of DPNin vivo,we conducted behavioral tests and neurophysiological measurements.Compared with the control vector group,the circ_0002538 group showed improved thermal and mechanical thresholds (Figure 4EandF).Electrophysiological records showed that compared with those of the control group,the sensory and motor nerve conduction velocities of the circ_0002538 group were significantly increased (Figure 4GandH).These results demonstrated that the upregulation of circ_0002538 expression improved the function of the sciatic nerve in diabetic mice with DPN.Transmission electron microscopy revealed that the percentage of abnormal myelin sheaths,which manifested as myelin infoldings,vacuolization,and uneven thickness,increased in the DPN group but significantly decreased in the circ_0002538 group (Figure 4IandJ).These results suggest that the overexpression of circ_0002538 ameliorated the symptoms of DPN by improving myelination.
Overexpression of circ_0002538 increases PLLP expression
To examine the effect of circ_0002538 on myelination-related proteins,we detected the expression of serine incorporator 5,PLLP,gap junction protein gamma 3,proteolipid protein 1,periaxin,and MPZ in the circ_0002538-overexpressing SCs because protein profiling indicated that these molecules are dysregulated in DPN.RT-PCR showed that circ_0002538 regulated the expression of PLLP,gap junction protein gamma 3,and proteolipid protein 1,and PLLP showed the greatest FC (Figure 5A).Western blotting further revealed that knocking down circ_0002538 led to the downregulation of PLLP expression (Figure 5Bleft).Accordingly,the overexpression of circ_0002538 increased PLLP protein expression in SCs (Figure 5Bright).These results confirmed that circ_0002538 could regulate the expression of PLLP.
To simulate diabetic conditions,we added ox-LDL to the culture medium.RTPCR revealed decreased PLLP expression in the ox-LDL-cultured SCs.We used 100-µg/mL ox-LDL in the following experiments because it produced a more significant effect (Figure 5CandD).RT-PCR showed that the overexpression of circ_0002538 increased PLLP expression in the SCs cultured with ox-LDL.This was further confirmed by western blotting (Figure 5EandF).We also investigated PLLP expression in the nerve tissues from the patients with DPN via western blots.PLLP expression was significantly downregulated in the nerve tissues of the patients with DPN compared with those without DPN (Figure 5GandH).In addition,the administration of circ_0002538 LV significantly increased the expression of PLLP in the sciatic nerve of the mice with DPN compared with the administration of control LV (Figure 5IandJ).These results indicated that circ_0002538 could regulate the expression of PLLPin vitroandin vivo.
PLLP regulates SC migration and myelination
To further verify the role of PLLP in SCs,we transfected a lentiviral vector containing the PLLP gene into SCs.RT-PCR showed that the PLLP overexpression cells had significantly increased PLLP expression,which was further confirmed by the western blotting results (Figure 6AandB).We performed a mRNA-sequencing analysis of the SCs transduced with the LV carrying either PLLP or the control vector.A total of 23448 mRNAs were identified,and 1671 mRNAs met the filtering criteria (P<0.05,FC ≥ 2)(Additional Table 10).The filtered mRNAs were further analyzed using GO analysis for functional prediction (Additional Tables 11–13).GO biological process analysis showed that the filtered mRNAs were significantly enriched in neutrophil migration,regulation of neutrophil migration,positive regulation of neutrophil migration,and positive regulation of leukocyte migration,indicating that PLLP might be related to cell migration (Additional Figure 4A).Transwell assays confirmed that the overexpression of PLLP significantly increased SC migration (Figure 6CandD).We further validated the role of PLLP by knocking it down.RT-PCR revealed that PLLP expression was decreased in PLLP knockdown SCs (Figure 6E).Transwell assays showed that the knockdown of PLLP effectively inhibited SC migration (Figure 6FandG).These results indicate that PLLP affects SC migration.
To verify the effect of PLLP on peripheral nerve myelinationin vivo,we injected sh-PLLP LV into the mouse sciatic nerve.Western blotting revealed that PLLP was decreased in the PLLP knockdown group compared with the control vector group (Figure 6HandI).The ratio of myelin abnormalities was strongly increased in the PLLP knockdown group,as shown by transmission electron microscopy (Figure 6JandK).These results indicate that PLLP might regulate myelination in peripheral nerves.
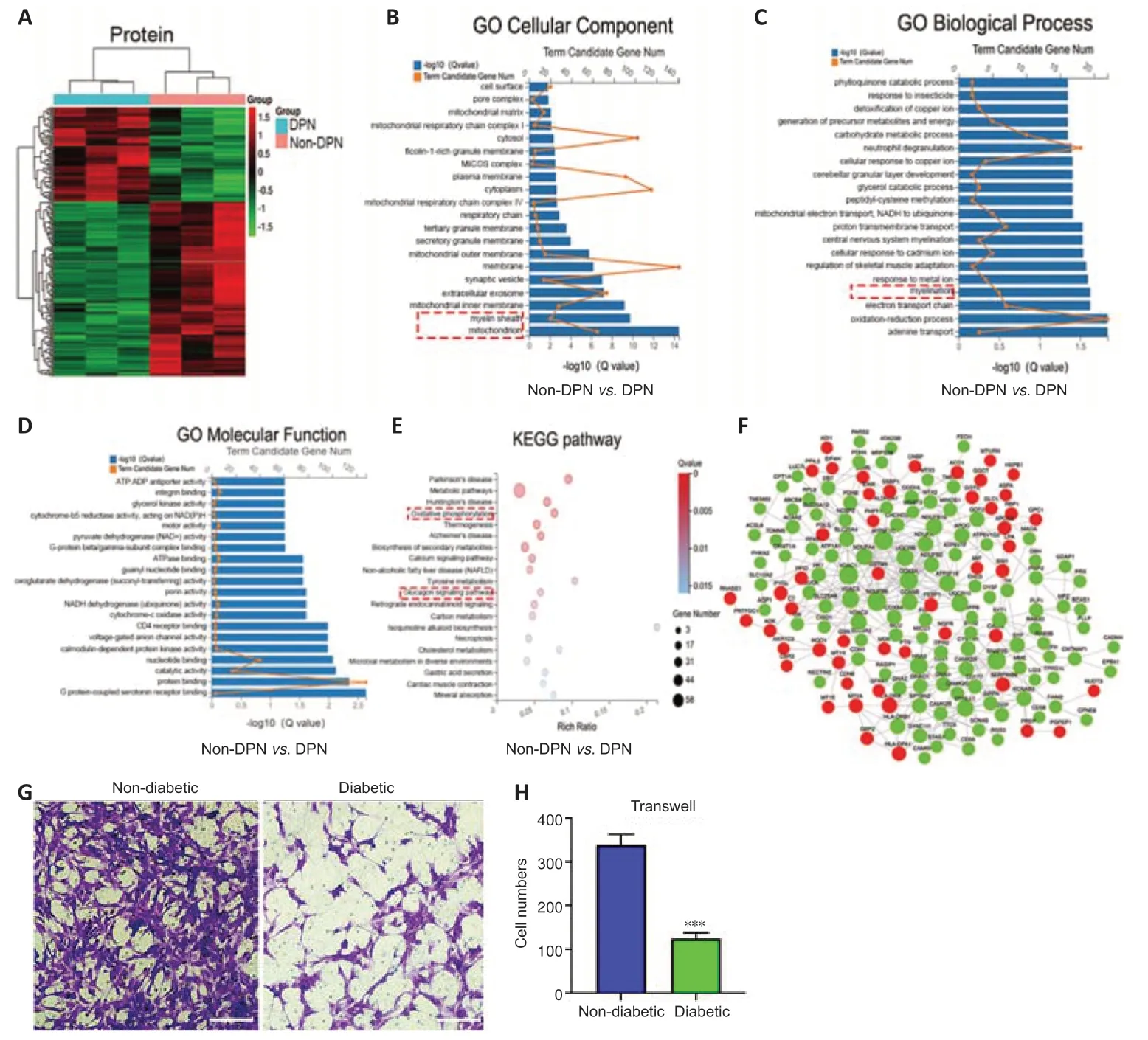
Figure 2|Protein profiling analysis and the detection of SC function in DPN.
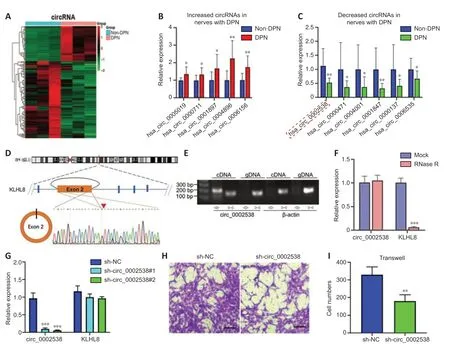
Figure 3|Characterization of circ_0002538 and its function in SCs.
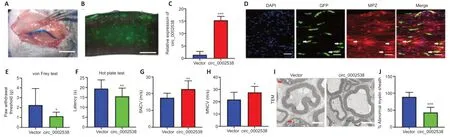
Figure 4|Overexpression of circ_0002538 improves demyelination and symptoms of DPN.
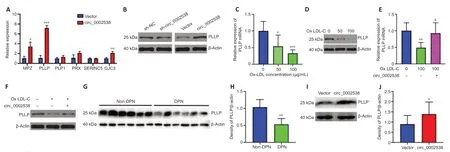
Figure 5|circ_0002538 regulates the expression of PLLP in vitro and in vivo.
circ_0002538 serves as a sponge for miR-138-5p in SCs
The most common function of circRNAs is to act as sponges for miRNAs,thus regulating downstream target genes.We located circ_0002538 in cellular components via nuclear and cytoplasmic separation experiments.RT-PCR analysis showed that circ_0002538 was predominantly localized in the cytoplasm (Figure 7A),indicating that it might target specific miRNAs to regulate PLLP expression.Forty-eight candidate miRNAs were predicted to bind to PLLP and 130 candidate miRNAs were predicted to bind to circ_0002538 (Additional Tables 14and15).After overlapping the candidate miRNAs of PLLP and the candidate miRNAs of circ_0002538,only two miRNAs(miR-138-5p and miR-3714) were found (Figure 7B).We conducted pulldown assays using the biotinylated circ_0002538 probe to verify the interaction between circ_0002538 and the two candidate miRNAs.The circ_0002538 probe effectively pulled down circ_0002538 (Figure 7C),and miR-138-5p was significantly enriched in the circ_0002538 probe sponge complex,while miR-3714 was not significantly enriched (Figure 7D).RT-PCR and agarose gel electrophoresis confirmed that the miR-138-5p probe could prominently pull down circ_0002538 (Figure 7EandF).We further verified this interaction using a dual-luciferase reporter assay.A schematic model showed the putative binding site of circ_0002538 and miR-138-5p (Figure 7G).Luciferase reporter assays demonstrated that miR-138-5p decreased the luciferase activity of HEK293T cells in the wild-type circ_0002538 group but had no effect in the mutant group (Figure 7H),demonstrating the direct binding between circ_0002538 and miR-138-5p in SCs.Taken together,these data demonstrate that circ_0002538 acts as a miRNA sponge for miR-138-5p in SCs.
miR-138-5p inhibits the migration of SCs by targeting PLLP
To investigate the function of miR-138-5p,we transfected miR-138-5p mimic or inhibitor into SCs.In the migration assays,the number of SCs that migrated to the lower chamber was significantly reduced after transfection with the miR-138-5p mimics.In contrast,the miR-138-5p inhibitor enhanced SC migration (Figure 8AandB).Then,we used a dual-luciferase reporter assay to determine whether miR-138-5p could bind to PLLP to regulate its expression.Figure 8Cshows the predicted binding sites and mutated sites of miR-138-5p on the 3′UTR of PLLP.The overexpression of miR-138-5p significantly weakened the relative Rluc activity of the wild-type plasmids but not the mutant plasmids (Figure 8D),suggesting that miR-138-5p could directly bind to the PLLP 3′UTR and block its activity.Western blot analysis further demonstrated that the miR-138-5p mimics significantly reduced PLLP protein expression,while the miR-138-5p inhibitors increased PLLP protein expression(Figure 8E).These results revealed that miR-138-5p could strongly suppress SC migration by targeting PLLP.
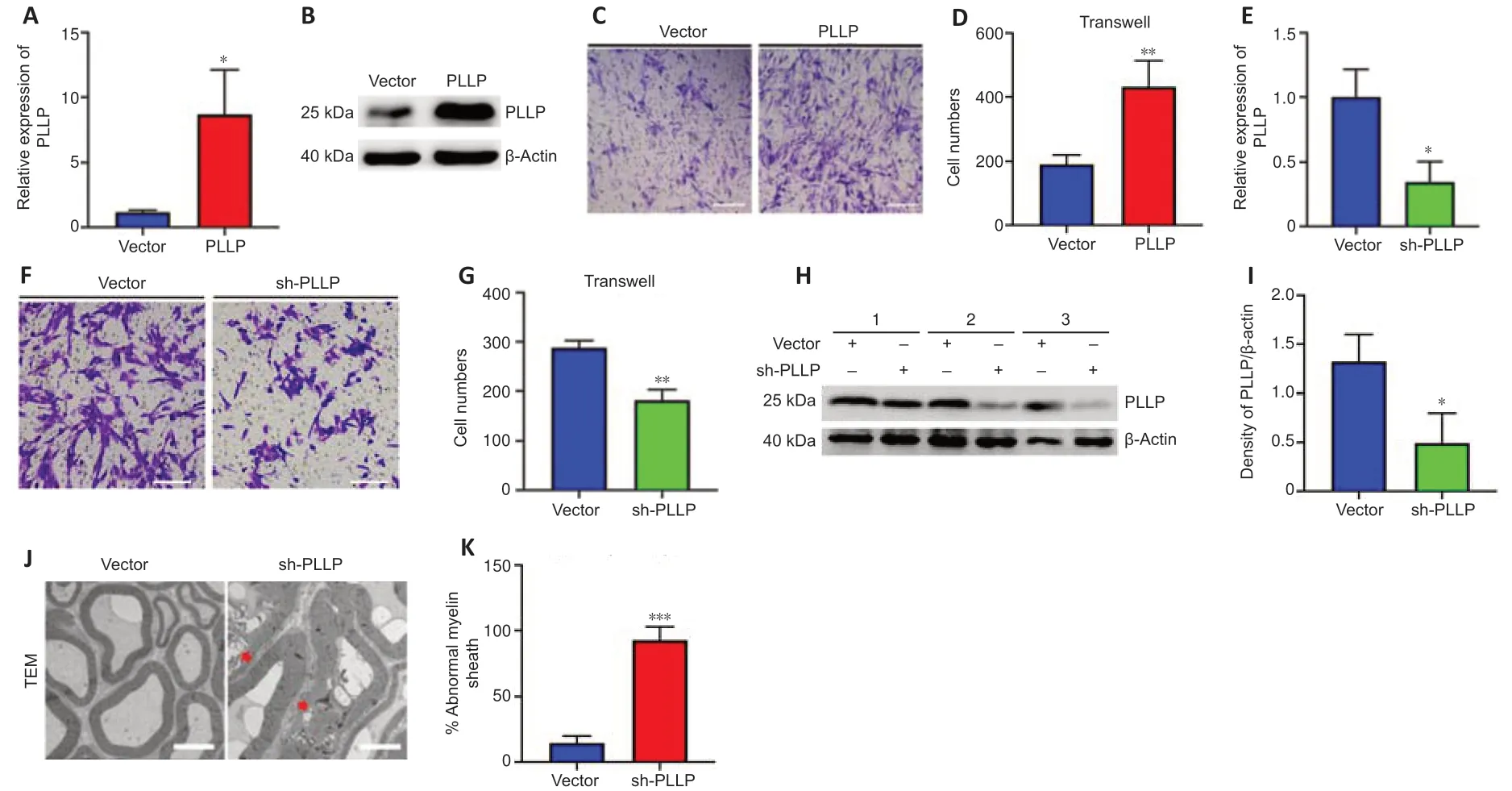
Figure 6|PLLP regulates SC migration and myelination.
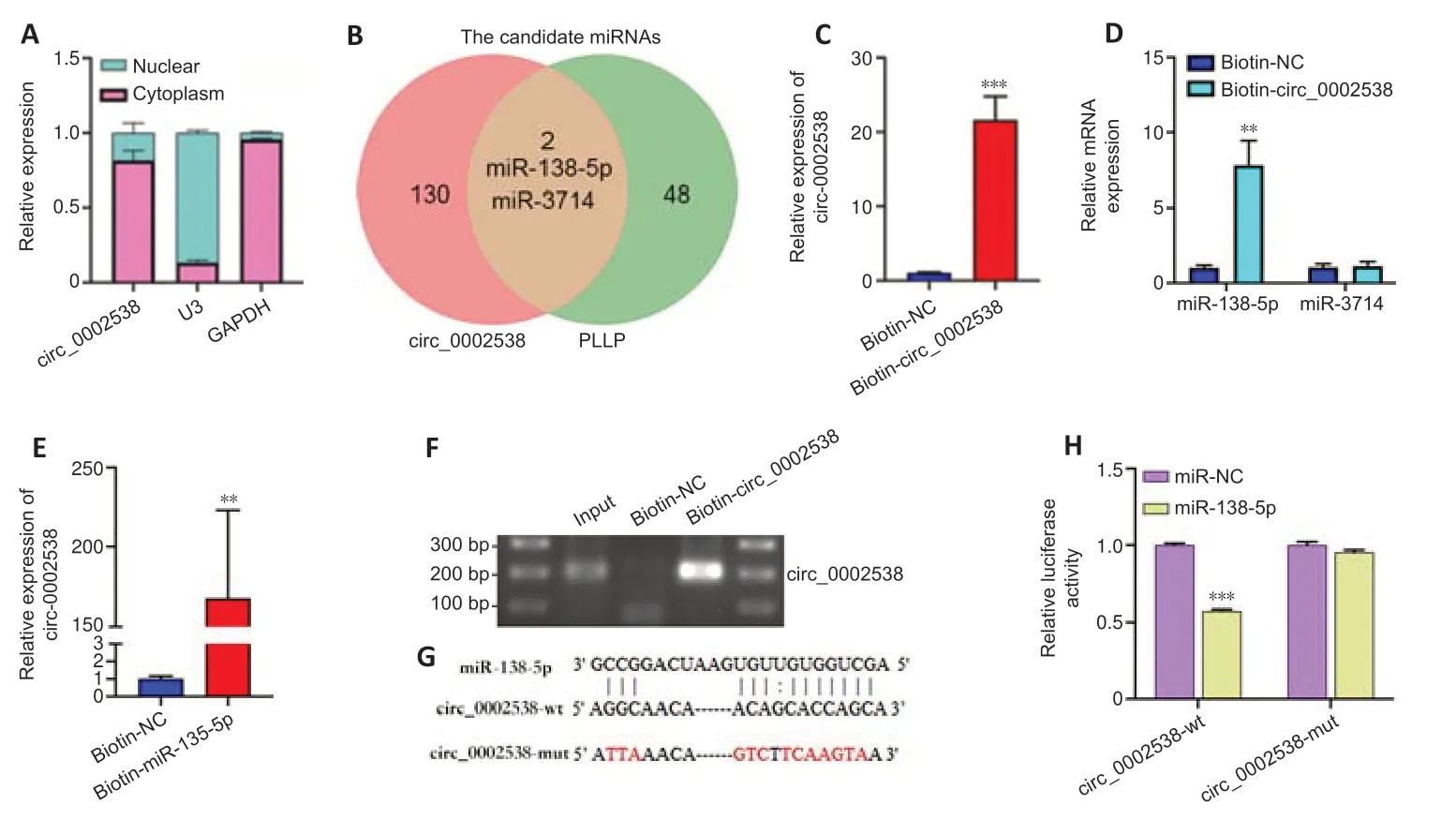
Figure 7|circ_0002538 acts as a sponge for miR-138-5p in SCs.
miR-138-5p reverses the effect of circ_0002538 on SCs
We demonstrated that circ_0002538 could sponge miR-138-5p and that miR-138-5p could inhibit SC migration by targeting PLLP.Subsequently,we explored whether circ_0002538 could regulate PLLP through miR-138-5p.The SCs cotransfected with the miR-138-5p mimics and circ_0002538 exhibited decreased migration compared with the SCs transfected with circ_0002538 only (Figure 9AandB),which indicated that ectopic expression of miR-138-5p could parti ally eliminate the promoting effect of circ_0002538.Western blot analysis showed that the SCs cotransfected with the miR-138-5p mimic and circ_0002538 exhibited reduced PLLP expression compared with the SCs transfected with circ_0002538 only (Figure 9CandD).The above results demonstrated that circ_0002538 regulated SC migration in part by sponging miR-138-5p and subsequently influencing PLLP expression.
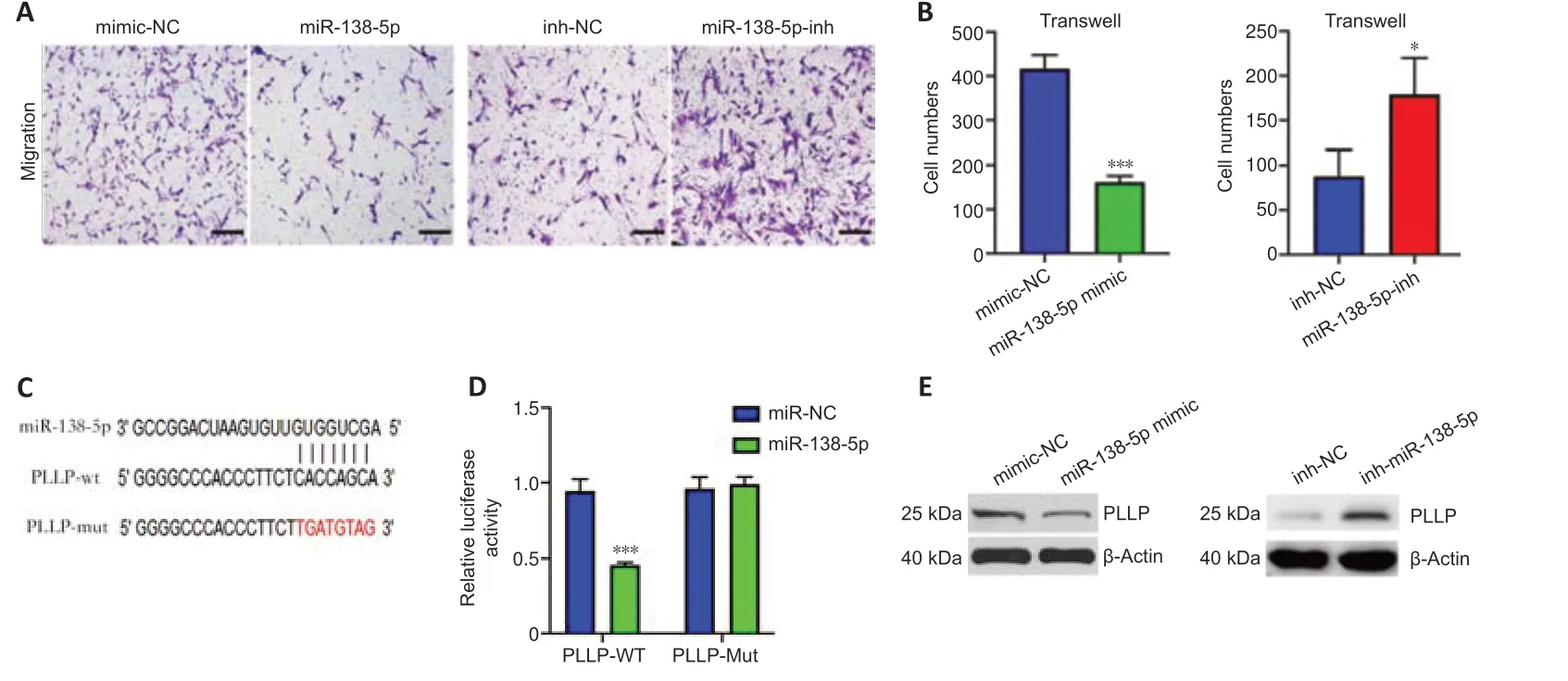
Figure 8|miR-138-5p inhibits SC migration by targeting PLLP.
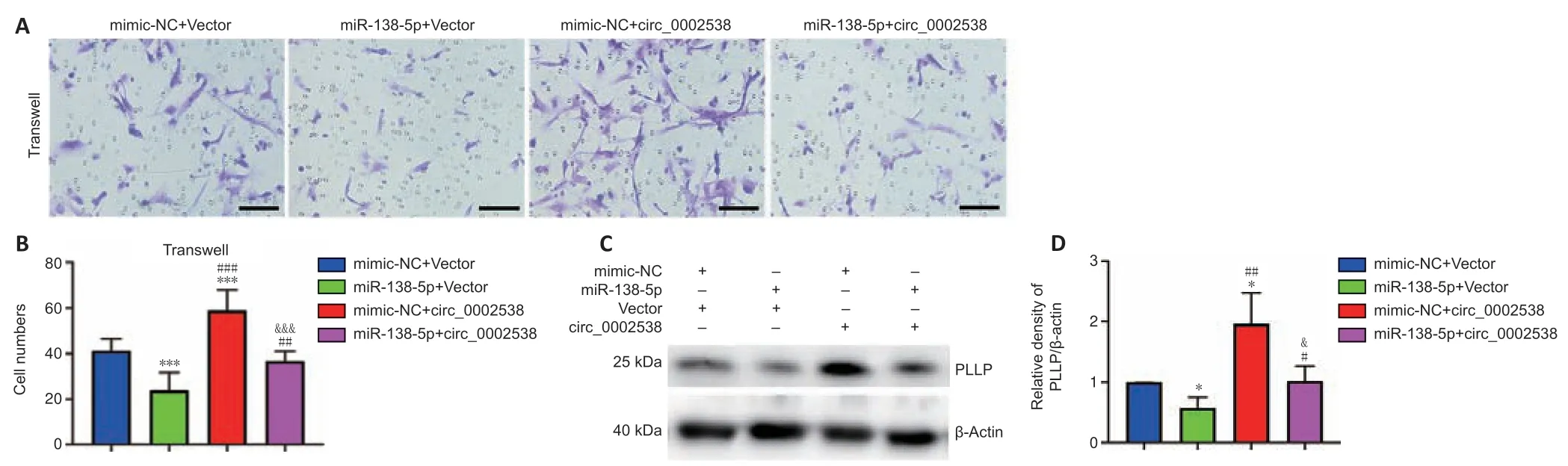
Figure 9|miR-138-5p reverses the circ_0002538-mediated promotion of SCs.
Discussion
DPN is the most common complication of diabetes,and thus represents a major burden to healthcare systems and society worldwide (Selvarajah et al.,2019).Few studies have been used circRNA sequencing to study the etiology of human DPN.Although nontraumatic amputations are mainly caused by DPN,the actual number of calf amputations each year is not high,limiting the availability of sural nerve samples from individuals with DPN.We collected peripheral nerve tissues from individuals with or without DPN and performed circRNA sequencing and protein profiling.We verified the results of circRNA sequencing and further showed that circ_0002538 could ameliorate symptoms in diabetic mice with DPN by promoting the migration and myelination of SCs.Therefore,our data indicate that the overexpression of circ_0002538 may be a promising treatment for patients with DPN.
Transcriptomic alterations often occur during the pathogenesis and progression of diseases.Previous studies have identified hundreds of differenti ally expressed genes in patients with static or progressive diabetic neuropathy that are functionally enriched in pathways,including the regulation of axonogenesis and lipid metabolism (Hur et al.,2011).A microarray analysis of the dorsal root ganglia of diabetic rats found that DE mRNAs with downregulated expression were significantly enriched in various biological processes,including myelination,peripheral nervous system myelination,axon guidance,and the regulation of axon production (Guo et al.,2018).Further,aberrantly expressed mRNAs in SCs isolated from the sciatic nerves of diabetic rats were enriched in downregulated biological processes related to myelination,axonogenesis,and axon development (Wang et al.,2020b).In this study,we identified 265 proteins with dysregulated expression in peripheral nerves from DPN patients that were enriched in myelination.SCs provide protection and nutritional support to enable myelinated axons to maintain normal physiological functions,and impaired SC function eventually leads to axonal loss (Dey et al.,2013).Therefore,we focused on the influence of SCs on DPN.We evaluated the function of SCs from patients with diabetes and found that these SCs had reduced migration,consistent with the results of previous studies (Gumy et al.,2008;Jia et al.,2018).
Although circRNAs were originally thought to be byproducts of abnormal splicing events (Cocquerelle et al.,1993),recent studies have shown that certain circRNAs are involved in some important physiological processes.However,the role of circRNAs in the SCs of DPN has rarely been examined,especially in human DPN.Zhang et al.(2020) reported 15 DEcircRNAs in the dorsal root ganglia between wild-type mice and mice with diabetes mellitus.Liu et al.(2019) reported that mmu_circRNA_006636 could relieve high glucose-induced apoptosis and autophagy in RSC96 cells.In our study,116 circRNAs had downregulated expression and 53 circRNAs had upregulated expression in DPN.Among them,11 circRNAs were verified,of which circ_0000711 and circ_0006156 were previously reported to play important roles in tumors (Li et al.,2018;Hong et al.,2019;Chen et al.,2020;He et al.,2020),but none were found to be involved in neuropathy.The functions of most verified DEcircRNAs are sti ll unclear.Therefore,more research is needed to explore the potenti al roles of noncoding RNAs in human DPN.
One of the most common and important functions of circRNAs is to act as competing endogenous RNAs that sequester miRNAs through their binding sites and then modulate the acti vity of miRNAs on their target genes (Salmena et al.,2011).Although the function of circ_0002538 has not previously been characterized,we found decreased circ_0002538 expression in the nerves of patients with DPN.Further,we found that the overexpression of circ_0002538 improved the symptoms of DPN in diabetic mice.Transmission electron microscopy demonstrated that the administration of circ_0002538 decreased the number of damaged myelin sheaths in DPN,indicating that circ_0002538 might help repair damaged myelin sheaths by improving myelination.According to the GO biological process analysis,the proteins with dysregulated expression identified using protein profiling were significantly enriched in the myelination process,indicating that circ_0002538 improved DPN by regulating myelination-related proteins.The expression of myelination-related proteins was detected in the circ_0002538-overexpressing SCs,which demonstrated that circ_0002538 could regulate the expression of PLLP.Based on the computational predictions and experimental validation of candidate miRNAs binding circ_0002538 and PLLP,we selected miR-138-5p for the construction of competing endogenous RNAs.The circ_0002538-miR-138-5p-PLLP axis was demonstrated using RNA pulldown assays,dual luciferase assays,and a mouse model of DPN.We further verified that circ_0002538 could competitively adsorb miR-138-5p to antagonize its suppression of PLLP.
DPN is involved in deleterious changes in peripheral nerves,such as myelin damage (Cermenati et al.,2012).The myelin sheath is a multi layer membrane produced by SCs that allows efficient transmission of nerve impulses.PLLP has been found to assemble myelin membrane precursor domains via its ability to attract liquid-ordered lipids between the Golgicomplex and plasma membrane (Yaffe et al.,2015),and PLLP expression was found to be elevated in nerve stumps following axotomy (Bosse et al.,2003).However,the characteristics and functions of PLLP have not been examined in DPN.In our research,we found that PLLP regulated the migration of SCs,which is an important step preceding myelination and remyelination of the peripheral nervous system (Anliker et al.,2013).Impaired or delayed SC migration contributes to abnormal myelination and demyelination of peripheral nerves(Anliker et al.,2013;Yi et al.,2019).These data are consistent with our finding that silencing PLLP can lead to impaired SC migration and peripheral nerve demyelination in mice.PLLP expression was decreased in diabetic mice with DPN.The increased expression of PLLP,mediated by the overexpression of circ_0002538,improved demyelination.Therefore,we concluded that circ_0002538 and PLLP might play important roles in DPN,and thus might be useful in the development of treatments for demyelinating diseases.
This study had several limitations.First,the number of nerve samples used for sequencing and verification was relatively small.Second,to minimize the influence of other cells,we only used nerve bundles for sequencing and subsequent verification.However,we still cannot completely exclude the influence of other components in peripheral nerves,such as axons,fibroblasts,endothelial cells,and inflammatory cells.Their effects on DPN are the subjects of further research.Third,as circRNAs can interact with different proteins or be translated in a way that mediates their biological roles,further research is needed to identi fy more circRNAs related to the pathogenesis of DPN.Finally,although we validated the protective effects of circ_0002538 in mice and found an improvement in the neuropathic phenotype and symptoms of DPN,the therapeutic effects on humans need to be verified.
In conclusion,this study reported the results of circRNA sequencing and protein profiling of peripheral nerves from individuals with DPN.As a result,we verified 11 DEcircRNAs in the DPN and control groups.Furthermore,our study demonstrated that circ_0002538 expression was downregulated in patients with DPN and that increased expression of circ_0002538 improved the symptoms of diabetic mice with DPN.Mechanistically,circ_0002538 regulated SC migration and myelination,at least in part,through the miR-138-5p/PLLP axis.Collectively,our study illuminated the key role of the circ_0002538/miR-138-5p/PLLP axis in DPN.Our results provide new insight into the mechanisms and potenti al treatments for DPN.
Author contributions:Study design: YTL,ZX,HGM,ZBC;sample collection:YTL,ZX,SR,HWX,WL,TJ,JC,XFY,YK,QYL,ZHW;data verify the sequencing:YTL,ZX,SR,HWX,WL;cell experiments: YTL,ZX,WL,TJ,JC;animal data collection and analysis: YTL,ZX,XFY,YK,ZHW,QYL;manuscript draft and review: YTL,ZX,XFY,HGM,ZBC.All authors approved the final version of the manuscript.
Conflicts of interest:The authors declare no competing interests.
Availability of data and materials:All data generated or analyzed during this study are included in this published article and its supplementary information files.
Open access statement:This is an open access journal,andarticles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional files:
Additional Table 1:Primers used in this study.
Additional Table 2:Nucleic acid sequences used in this study.
Additional Table 3:Basic characteristics of patients included in the study.
Additional Table 4:The differenti ally expressed proteins analyzed in this study were selected from the results of protein profiling analysis with fold change (FC)>1.3 and P <0.05.
Additional Table 5:GO cellular component analysis of differenti ally expressed proteins.
Additional Table 6:GO biological process analysis of differenti ally expressed proteins.
Additional Table 7:GO molecular function analysis of differenti ally expressed proteins.
Additional Table 8:KEGG pathway analysis of differenti ally expressed proteins.
Additional Table 9:The DEcircRNAs analyzed in this study were selected from the results of circRNA sequencing analysis with FC >2.0,P<0.01,q <0.05 and readings ≥ 50.
Additional Table 10:The differenti ally expressed mRNAs analyzed in this study were selected from the results of mRNA sequencing analysis.
Additional Table 11:GO biological process analysis of filtered mRNAs.
Additional Table 12:GO cellular component analysis of filtered mRNAs.
Additional Table 13:GO molecular function analysis of filtered mRNAs.
Additional Table 14:Candidate miRNAs binding to circ_0002538 predicted by RNAhybrid,miRanda and TargetScan.
Additional Table 15:The candidate miRNAs binding to PLLP predicted by miRDB,miRTarBase,miRWalk and TargetScan.
Additional Figure 1:Confirmation of DPN in the collected peripheral nervetissues.
Additional Figure 2:Identification of SCs isolated from sural nerves of patients.
Additional Figure 3:Overexpression of circ_0002538 promoted SC migration.
Additional Figure 4:The filtered mRNAs in the mRNA sequencing results of the PLLP-overexpressing SCs and the control SCs were further analyzed with GO enrichment analysis.
- 中国神经再生研究(英文版)的其它文章
- Bystanders or not? Microglia and lymphocytes in aging and stroke
- Alzheimer’s disease risk after COVID-19: a view from the perspective of the infecti ous hypothesis of neurodegeneration
- Serine and arginine rich splicing factor 1: a potenti al target for neuroprotection and other diseases
- Can glial cells save neurons in epilepsy?
- Lights for epilepsy: can photobiomodulation reduce seizures and offer neuroprotection?
- The landscape of cognitive impairment in superoxide dismutase 1-amyotrophic lateral sclerosis

