Valproate reduces retinal ganglion cell apoptosis in rats after optic nerve crush
Feng Pan,Dan Hu ,Li-Juan Sun,Qian Bai,Yu-Sheng Wang,Xu Hou
Abstract The retinal ganglion cells of the optic nerve have a limited capacity for self-repair after injury.Valproate is a histone deacetylase inhibitor and multitarget drug,which has been demonstrated to protect retinal neurons.In this study,we established rat models of optic nerve-crush injury and injected valproate into the vitreous cavity immediately after modeling.We evaluated changes in the ultrastructure morphology of the endoplasmic reticulum of retinal ganglion cells over time via transmission electron microscope.Immunohistochemistry and western blot assay revealed that valproate upregulated the expression of the endoplasmic reticulum stress marker glucose-regulated protein 78 and downregulated the expression of transcription factor C/EBP homologous protein,phosphorylated eukaryotic translation initiation factor 2α,and caspase-12 in the endoplasmic reticulum of retinal ganglion cells.These findings suggest that valproate reduces apoptosis of retinal ganglion cells in the rat after optic nerve-crush injury by attenuating phosphorylated eukaryotic translation initiation factor 2α-C/EBP homologous protein signaling and caspase-12 activation during endoplasmic reticulum stress.These findings represent a newly discovered mechanism that regulates how valproate protects neurons.
Key Words:apoptosis;C/EBP homologous protein;caspase-12;endoplasmic reticulum;glucose-regulated protein 78;optic nerve crush;phosphorylated eukaryotic translation initiation factor 2α;retinal ganglion cells;unfolded protein response;valproate
Introduction
Traumatic optic neuropathy and glaucoma are both vision-threatening disorders often seen in the clinic.The pathological characteristics they have in common are retinal ganglion cell (RGC) death and subsequent optic nerve atrophy (Tham et al.,2014;Karimi et al.,2021).As a part of the central nervous system (CNS),RGCs (and thus the optic nerve) have a limited capacity for self-repair after acute trauma or chronic injury (Gokoffski et al.,2020).Although no effective therapy currently can enhance RGC regeneration (Laha et al.,2017),several strategies such as targeting intraocular inflammation,providing exogenous neurotrophic factors,reactivating the intrinsic growth capacity of mature RGCs,or modifying the extrinsic growth-inhibitory environment of the optic nerve have shown translational potenti al (Chun and Cestari,2017;Li et al.,2017;Williams et al.,2020).
Valproate (VPA) is a multitarget drug that was introduced in clinical practice over 50 years ago (Romoli et al.,2019).In addition to its use in the treatment of epilepsy and bipolar disorder,VPA is also a neuroprotective agent administered in acute CNS injuries (Chen et al.,2014).VPA-mediated neuroprotection in animal models of optic nerve crush (ONC) (Zhang et al.,2012b),optic neuritis (Liu et al.,2017),normal-tension glaucoma (Kimura et al.,2015b),ocular hypertension (Alsarraf et al.,2014a),and retinal ischemia injury (Zhang et al.,2012a) have been validated in succession during the last decade.Although the above findings support the clinical administration of VPA for ocular neurodegenerative diseases,the mechanisms through which it triggers neuroprotection are not yet clear.
As a histone deacetylase (HDAC) inhibitor,VPA induces the acetylation of histones,affects the methylation status of histones that support DNA,and thus ulti mately regulates epigenetic gene expression (de Campos Vidal and Mello,2020).The mechanism through which VPA mediates neuroprotection in cases of spinal cord and brain injury partially involves inhibition of HDAC (Chu et al.,2015;Chen et al.,2018;Silva et al.,2018).In addition,decreased retinal acetylation is a central event in ischemic retinal injury,and the hyperacetylation levels of histone 3 induced by VPA can provide neuroprotection (Alsarraf et al.,2014b).Interestingly,prolonged survival of RGCs treated with VPA in the animal model of ONC is not accompanied by altered acetylation of histones 3 or 4 (Biermann et al.,2010).Thus,the precise mechanism of action of VPA needs further investigation before it can be used in clinical trials for treatment of apoptotic diseases that affect RGCs.
The endoplasmic reticulum (ER) of cells has a protein-folding capacity that is regulated by a dynamic intracellular signaling pathway known as the unfolded protein response (UPR) (Hetz and Papa,2018).Injury-induced ER stress in retinal neurons triggers maladaptive UPR outputs and activation of apoptosis(Park et al.,2021).Key molecules in the UPR pathways have different effects on axon injury-induced apoptosis of RGCs (Hu et al.,2012).The ER chaperones glucose-regulated protein 78 (GRP78) and C/EBP homologous protein (CHOP)are marker molecules of ER stress that regulate RGC fate (Kumar et al.,2019).During ER stress,the balance between the pro-survival chaperone GRP78 and the pro-apoptotic factor CHOP drives cell destiny (Penas et al.,2011).To date,few data are available on whether VPA-induced reduction in apoptosis of injured RGCs involves the regulation of UPR signaling.In this study,we focused on the above questions and analyzed the mechanisms underlying VPA-mediated neuroprotection.
Methods
Animals
All animal experiments were approved by the Animal Care and Use Committee at the Fourth Military Medical University in April 2020 (approval No.KY20203131) and were performed according to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (National Institutes of Health,2011).Seven-to eight-week-old male Sprague-Dawley rats (250–300 g) bred in the specific-pathogen-free environment at the Animal Center of the Fourth Military Medical University (license No.SCXK(Shaan) 2019-001) were used for experiments.Housing conditions were as follows: room temperature,20–24°C;air humidity,50–60%,light/dark,alternating cycles of 12 hours.Each cage housed 2–4 rats.The rats were divided randomly into VPA and normal saline groups (n=6/group).
ONC and VPA treatment
The ONC model was performed as previously described (Siddiq et al.,2021).Briefly,rats were anesthetized with 1% pentobarbital sodium (50 mg/kg,Sinopharm,Shanghai,China) by intraperitoneal injection.The left optic nerve was exposed by blunt dissection with both the retinal blood supply and dura intact,and was crushed 2 mm away from the eyeball with forceps for 20 seconds (Martin Instruments,Tuttlingen,Germany).The incision in the skin was sutured.A sham procedure without the crush was performed in the right eye.After ONC injury,a 33-gauge needle (Hamilton Co.,Hamilton,KS,USA) was passed through the sclera at the ora serrata level immediately under the operating microscope.Then,VPA (500 mM,Sigma-Aldrich,St.Louis,MO,USA) dissolved in normal saline (5 µL) or normal saline as a vehicle control was injected into the vitreous cavity.A topical anti biotic eye drop (5 mg/mL,Levofloxacin,Santen,Japan) was applied to the wound at once.
Transmission electron microscopy
Transmission electron microscopy was used to observe changes in ultrastructure morphology of the retinal ER after ONC (6,12 hours,and 1,3,and 7 days).First,rats were transcardially perfused with 4% glutaraldehyde(PHYGENE,Shanghai,China) after anesthesia with 1% sodium pentobarbital(Sinopharm,Shanghai,China) administered intraperitoneally.The retina was dissected carefully and fixed with 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.4) for 1 hour at 25°C.Then,the retina was postfixed with 1% osmium tetroxide for 1 hour at 25°C,dehydrated through a graded alcohol series,and embedded in Epon812 epoxy resin.Ultrathin sections(70 nm) were cut by an ultramicrotome (Reichert-Nissei Ultracut S,Leica,Twinsburg,OH,USA) and stained with uranyl acetate and lead citrate.The stained sections were observed under a transmission electron microscope(JEM1440,JEOL,Tokyo,Japan).RGCs were identified as large cells in the RGC layer containing pale nuclei with one or two nucleoli,as reported previously(Saggu et al.,2010).The ER in the RCGs were identified as tubular sacs that were evenly distributed in the cytoplasm and showed a typical flat layer-like structure.
Hematoxylin and eosin staining and terminal deoxynucleoti dyl transferasemediated bioti nylated UTP nick end labeling staining
To investigate changes in RGC apoptosis after ONC injury,hematoxylin and eosin (HE) staining of RGCs was performed.Cryosections (10 µm) were made 3 and 7 days after injury from frozen rat eyes after perfusion with 4%glutaraldehyde (Beyotime,Shanghai,China).Briefly,the slides were washed and fixed in 4% paraformaldehyde for 10 minutes,stained in hematoxylin for 5 minutes,and then washed and dipped in 1% acid alcohol to enhance differentiation.Sections were re-washed and counterstained with eosin for 30 seconds before taking micrographs.The retinal thickness was measured two to three disc-diameters from the optic nerve.Samples having detached retinas or fragmentary tissues were discarded.
Apoptotic RGCs were evaluated with a terminal deoxynucleoti dyl transferasemediated biotinylated UTP nick end labeling (TUNEL)-staining kit (Roche,Nutley,NJ,USA) 3 and 7 days after ONC.Cells with brown or dark-brown nuclei were identified as TUNEL-positive cells.Cells with blue nuclei were identified as TUNEL-negative cells.The number of apoptotic RGCs (TUNELpositive) was counted per 200 µm within the ganglion cell layer in five randomly selected sections.All micrographs were taken two to three discdiameters from the optic nerve to minimize variability.
Immunofluorescent staining
Double-label immunofluorescence was performed on the sequential incubation of retina sections for ER stress markers.The sections were fixed with cold acetone for 5 minutes,then incubated with blocking buffer comprising 1% bovine serum albumin (Beyotime) and 0.3% Triton X-100 in phosphate-buffered saline for 1 hour at 25°C.The following anti bodies were all diluted in sterile phosphate-buffered saline.The slides were incubated first with GRP78 (rabbit,1:100,Cell Signaling,Danvers,MA,USA,Cat# 3177,RRID: AB_2119845) or CHOP (mouse,1:200,Cell Signaling,Cat# 2895,RRID:AB_2089254) overnight at 4°C followed by secondary anti body for 1 hour at 25°C.For double-labeling,the sections were washed with phosphate-buffered saline and incubated with β-tubulin-3 (mouse,1:1000,Sigma,St.Louis,MO,USA,Cat# WH0203068M4,RRID: AB_1844089) primary antibody followed by secondary anti body.The secondary anti bodies were anti -rabbit IgG (goat,1:400,Cat#A-11034,RRID: AB_2576217) and anti -mouse IgG (donkey,1:800,Cat# A-21203,RRID: AB_141633) (Invitrogen,Waltham,MA,USA).The retina sections were coverslipped with 50% glycerol and observed under a confocal laser scanning microscope (FluoView 300,Olympus,Tokyo,Japan).The quantification of the fluorescence intensity was performed using ImageJ soft ware 1.52a (National Institutes of Health,Bethesda,MD,USA) (Schneider et al.,2012).
Western blot assay
Western blot assay was performed for detecting the markers of ER stress and the related signaling pathway.All retina samples were lysed using radioimmunoprecipitation assay buffer with protease inhibitors and phosphatase inhibitors (Beyotime) to extract the total protein.Equally loaded proteins(20 µg) were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene difluoride membranes.A solution of 5% nonfat powdered milk was used to block the membranes for 1.5 hours at room temperature,then the membranes were incubated overnight with the primary antibodies (GRP78,rabbit,1:250,Abcam,Shanghai,China,Cat# 3158-1,RRID: AB_2 120005;CHOP,mouse,1:1000,Cell Signaling,Cat# 2895,RRID: AB_2 089254;phosphorylated eukaryotic translation initi ation factor 2α (p-eIF2α),rabbit,1:1000,Cell Signaling,Cat#9721,RRID: AB_ 330951;caspase-12,rabbit,1:2000,Cell Signaling,Cat#2202,RRID: AB_2 069200;β-acti n,rabbit,1:2000,Abcam,Cat# ab8227,RRID:AB_2305186),followed by incubation with the corresponding secondary antibody from Invitrogen (goat anti-rabbit IgG,1:400,Cat# A-11034,RRID:AB_2 576217;rat anti -mouse IgG,1:1000,Cat# 04-6020,RRID: AB_2532933)at room temperature for 1 hour.Western blot bands were detected by enhanced chemiluminescence reagents (Thermo Scientific,Shanghai,China)and quantified with ImageJ soft ware.
Stati stical analysis
No stati stical methods were used to predetermine sample sizes;however,our sample sizes are similar to those reported in a previous publication (Zhang et al.,2012b).No animals or data points were excluded from the analysis.Data were analyzed using Student’st-test or one-way analysis of variance followed by Dunnett’spost hoctest with SPSS soft ware (version 19.0;IBM,Armonk,NY,USA).Values are presented as the mean ± standard error of the mean (SEM).P-values <0.05 were considered stati stically significant.Each test was done in triplicate.
Results
Ultrastructure morphology of the ER in the RGCs after ONC
ER stress can be detected in axotomized RGCs,which might lead to the activation of apoptotic signaling pathways (Hu et al.,2012).To investigate the potential role of UPR signaling in this phenomenon,we used transmission electron microscopy to evaluate changes in the ultrastructure morphology of the RGC-ER over time after ONC.Normal ER was evenly distributed in the cytoplasm,showing a typical flat layer-like structure,with narrow and closed lumens,arranged in parallel and connected,with granular ribosomes attached to the inner membrane (Figure 1A).No obvious change was observed in the ER after 6 hours (Figure 1B).However,part of the ER cavity (Figure 1C) began to expand by 12 hours post-ONC.After 1 day,the number of expanded ER with degranulation (Figure 1D) increased and the original layered arrangement was disordered,indicating that the ER began to lose its original structural organization.After 3 days,the ER had expanded and swelled more obviously(Figure 1E).Some ER became vesicle-like and lost their normal shape,and endometrial degranulation was more common.After 7 days,the ER had lost the original regular layered arrangement,and some had atrophied into small vacuole-like structures (Figure 1F).These results suggest that the morphological changes in the ER is an important signal that indicates the time frame of RGC apoptosis.
Intravitreal VPA reduces RGC apoptosis after ONC
Twice daily subcutaneous VPA injection has been reported to promote the survival of RGCs after ONC (Zhang et al.,2012b).To investigate whether a single dose of VPA reduces RGC apoptosis in the current study,rats received an intravitreal injection immediately after ONC.TUNEL assay was subsequently performed on retina sections and TUNEL-positive cells were observed in the RGC layer (Figure 2AandB).RGC apoptosis was significantly lower in the VPA group than that in the control group (P<0.01;Figure 2C).These data demonstrate that intravitreal injection is a safe and reliable option for VPA delivery.
VPA attenuates retinal thinning and reduction in RGC number after ONC
We also examined retina sections to evaluate whether intravitreal VPA can prevent retinal thinning and reduce the number of RGCs that are lost after ONC.RGC dendrites synapse with axon terminals of bipolar cells in the inner plexiform layer (IPL) of the retina.RGC injury might change the function of bipolar cells (Zhang et al.,2022).Therefore,the thickness of the whole retina,IPL,and inner nuclear layers were evaluated.The average thickness of the normal whole retina was 158.2 ± 6.76 µm.ONC injury reduced the thickness of the whole retina in a time-dependent manner,with or without VPA treatment (Figure 3AandB).However,after 7 days,thinning of the whole retina and the IPL were attenuated after VPA treatment (P<0.01;Figure 3CandD).No significant difference in the thickness of the inner nuclear layer was observed between the VPA and normal saline groups at 3 and 7 days post-ONC (Figure 3E).The average number of RGCs in normal retina sections was 31.2 ± 4.12 per 350 µm.Although ONC injury resulted in a timedependent decrease in the number of RGCs regardless of group,the number of RGCs was significantly higher 7 days post-ONC in the VPA group than in the control group (P<0.05;Figure 3F).Thus,we confirmed the protective effect of VPA on the retinal neurons.
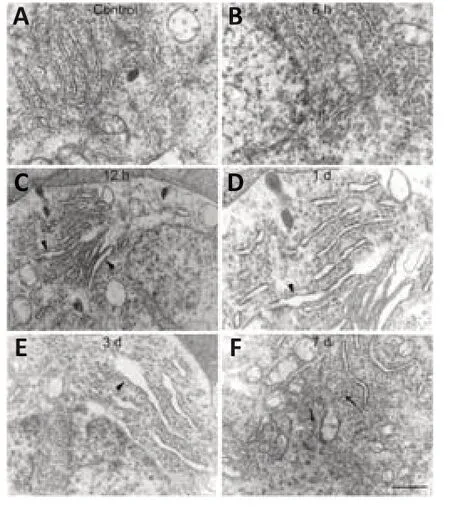
Figure 1|Ultrastructure morphological change in the endoplasmic reticulum (ER) of retinal ganglion cells (RGCs) in response to optic nerve crush (ONC).
GRP78 and CHOP are expressed in the retina and ONC enhances their expression in RGCs
GRP78 is a master chaperone during ER stress and regulates the signaling of UPR in neurodegenerative disorders (Gorbatyuk and Gorbatyuk,2013).We examined the location of GRP78 in the retinal sections and observed immunoreacti vity in the ganglion cell layer and inner nuclear layer of normal controls.The positive expression of GRP78 was co-localized primarily with β-tubulin-3 (RGCs marker;Jiang et al.,2015) and surrounded the RGC nuclei(Figure 4A).We found that GRP78 expression had increased by 6 hours after ONC,but levels decreased by day 7 (Figure 4B).
CHOP is another key mediator of the ER-stress response and an important pro-apoptotic signaling molecule after axonal injury (Syc-Mazurek et al.,2017).To investigate the location of CHOP in the retina sections,immunofluorescence staining was performed.We found slight acti vity in the ganglion cell layer of the control rats,which was co-localized with β-tubulin-3 in the RGCs (Figure 5).High expression of CHOP in the RGCs was induced by ONC and remained for 7 days.
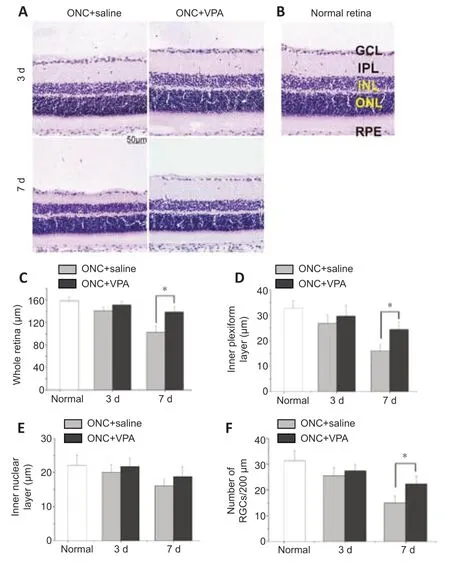
Figure 3|Valproate (VPA) decreases retinal thinning and the loss of retinal ganglion cells (RGCs) in the rat model of optic nerve crush (ONC).
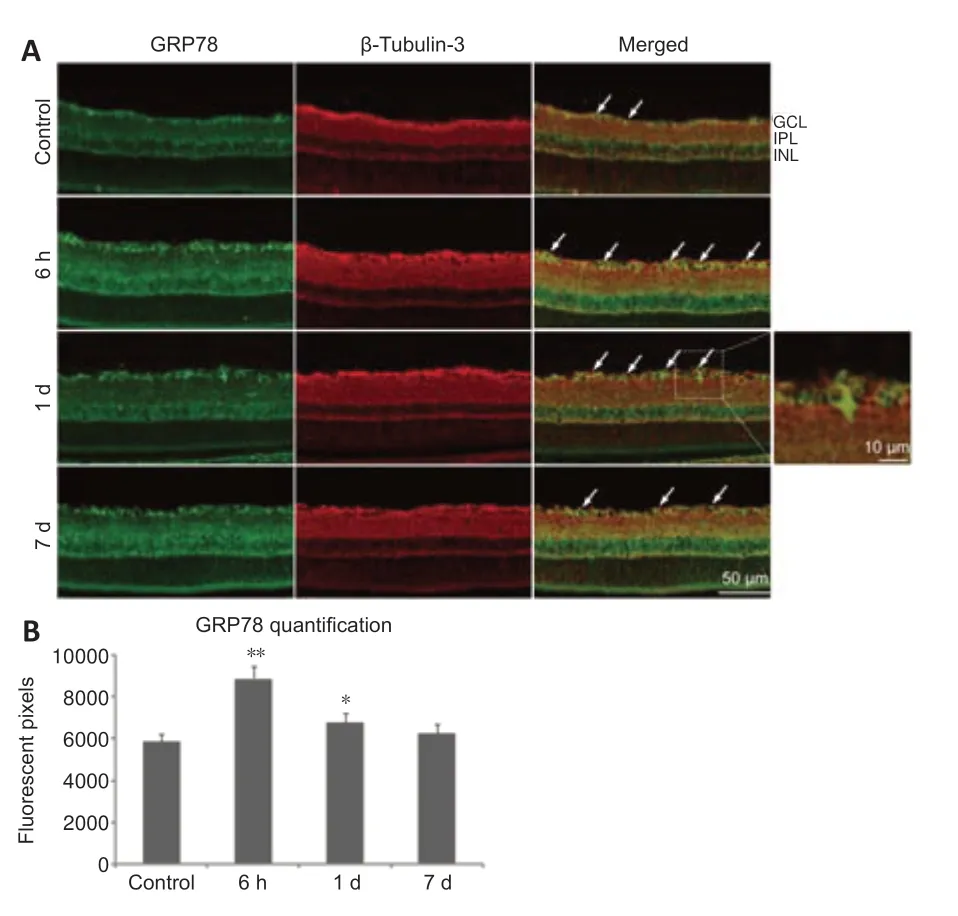
Figure 4|Glucose-regulated protein 78 (GRP78) is expressed in retinal ganglion cells(RGCs).
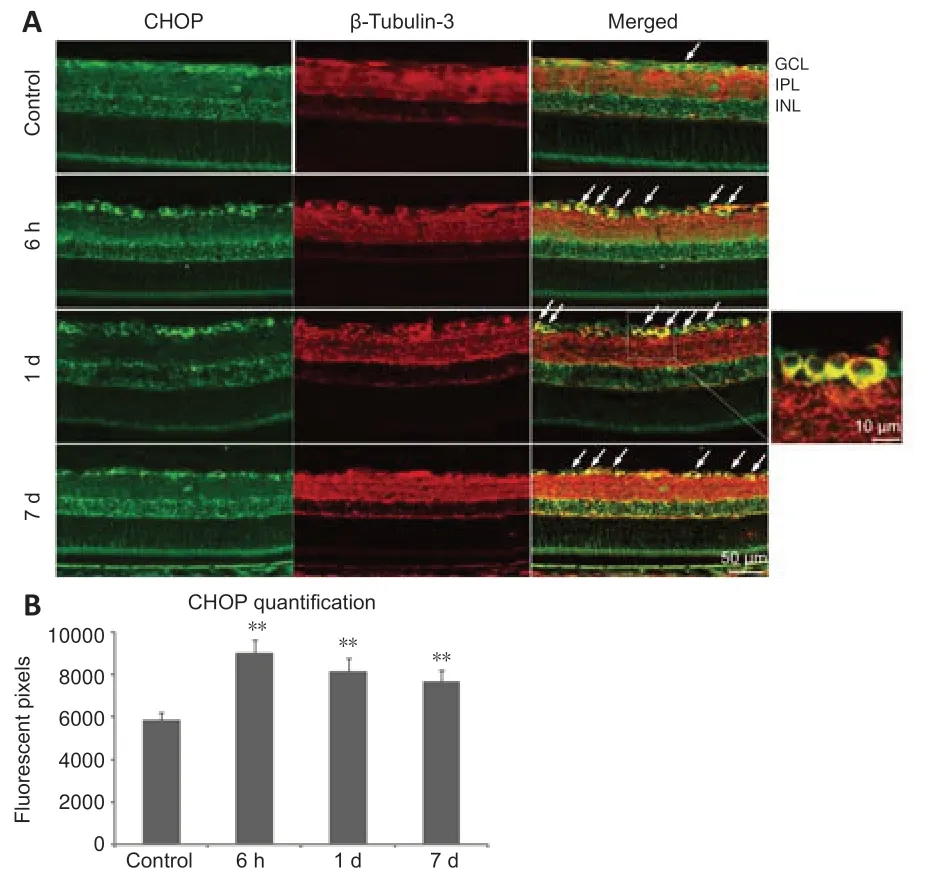
Figure 5|C/EBP homologous protein (CHOP) is expressed in retinal ganglion cells(RGCs).
To quantify the protein levels of GRP78 and CHOP,the whole neural retina was extracted and processed for immunoblotting (Figure 6A).Compared with controls,GRP78 expression was significantly enhanced 6 hours after VPA treatment (P<0.01;Figure 6B) and then decreased to normal levels by day 3.CHOP expression in the normal retina was low,but increased markedly after 6 hours and lasted at a high level for 7 days (P<0.01;Figure 6C).Expression of phosphorylated eIF2α in the retina was increased after 6 hours,but went down to normal levels by day 3 (P<0.01;Figure 6D).These findings indicate that both GRP78 and CHOP are up-regulated to help maintain ER homeostasis in the early period after injury.However,the balance between the neuroprotective effects of GRP78 and the neural lesions induced by CHOP was disturbed by the 3rdday post-injury,as evidenced by reduced GRP78 expression and high CHOP expression.The upstream regulator of CHOP,phosphorylated eIF2α,is activated during ER stress.
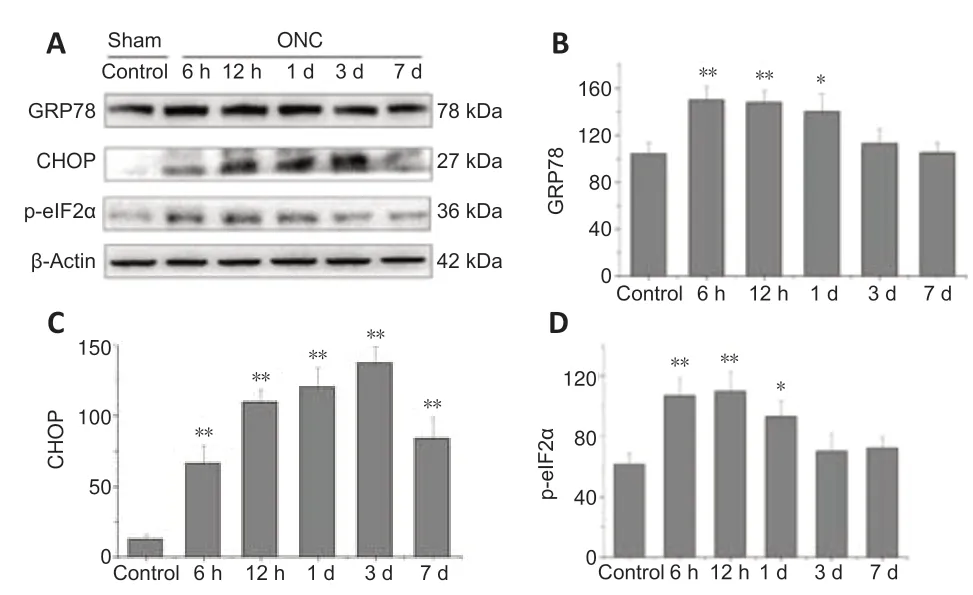
Figure 6|Optic nerve crush (ONC) enhances expression of glucose-regulated protein 78 (GRP78) and C/EBP homologous protein (CHOP) in the retina.
VPA-mediated protection of axotomized RGCs functions via the UPR pathway and caspase-12 inhibition
VPA has been reported to protect RGCs bothin vivoandin vitro,without any accompanying alterations in histone acetylation (Biermann et al.,2010,2011).To determine whether it protects the axotomized RGCs through regulation of UPR signaling,we measured GRP78 and CHOP protein levels via immunoblotting (Figure 7A).ONC injury gradually enhanced both GRP78 and CHOP expression over time in the VPA and normal saline groups.Importantly,VPA treatment led to higher GRP78 levels at each time point (Figure 7B).Interestingly,VPA treatment inhibited the expression of CHOP after 1 day (Figure 7C).These findings suggest that VPA plays a role in ER stress alleviation,resulting in the prolonged survival of injured RGCs.
Inhibition of the protein kinase RNA-like ER kinase (PERK)-eIF2α-CHOP pathway protects RGCs and preserves visual function in acute optic nerve trauma injury and chronic glaucomatous neuropathy (Hu,2016).We next determined that ONC injury enhanced expression levels of phosphorylated eIF2α in the retinas of both groups,but this up-regulation was significantly inhibited by VPA at day 1 (Figure 7D).These results confirm the hypothesis that the neuroprotective mechanism of VPA acts by targeting the PERK-eIF2α-CHOP pathway.
Caspase-12 is a key ER stress marker and pro-apoptotic factor for neuronal death (de la Cadena et al.,2014).When we tested protein extracts from the retinas of the model rats,we found increased levels of caspase-12.Compared with controls,the VPA group exhibited lower caspase-12 expression over time,and the differences was significant after 12 hours (Figure 7E).These data indicate that ER stress-related apoptosis induced by ONC can be attenuated by VPA through the inhibition of caspase-12 activation.
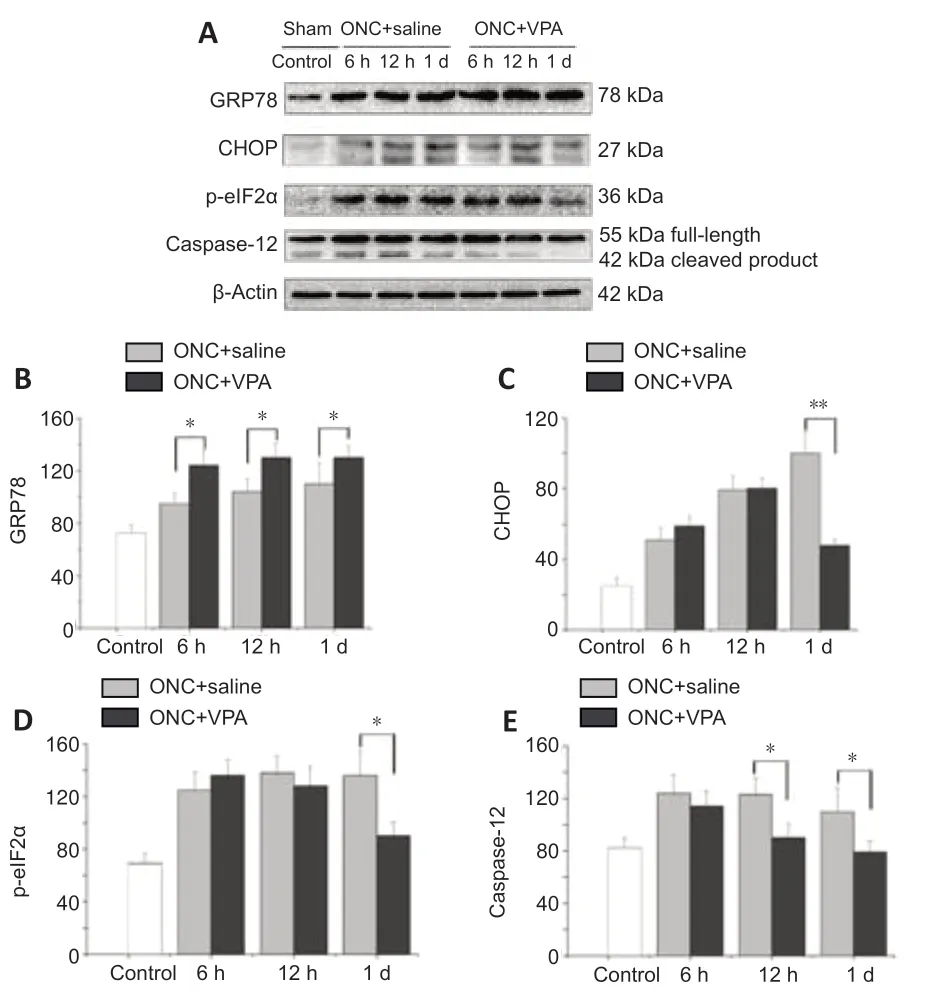
Figure 7|Valproate (VPA) inhibits pro-apoptotic signaling within the unfolded protein response (UPR) pathway after optic nerve crush (ONC).
Discussion
The optic nerve contains the axons of RGCs and is a crucial part of the visual pathway.Its damage caused by trauma,glaucoma,or ischemic neuropathy triggers apoptosis of neurons and jeopardizes visual function (Fudalej et al.,2021).Despite decades of research,the molecular mechanisms underlying RGC repair and survival remain poorly understood.The number of basic research and clinical studies of potential neuroprotective and neuroregenerative agents is still growing.Uncertain disease pathogenesis,drug mechanisms of action,and therapeutic targets has limited the number of clinical trials for new drugs that combat neurodegenerative diseases (Liu and Pang,2013).In this study,our findings indicated that valproate could inhibit optic nerve crush injury-induced activation of eIF2α-CHOP pathway and caspase-12 acti vity,which reduces apoptosis of RGCs during endoplasmic reticulum stress.
HDAC inhibitors have been shown to combat neurodegenerative conditions(Ziemka-Nalecz et al.,2018;Pickell et al.,2020).VPA is a broad-spectrum HDAC inhibitor,and numerous reports have shown that it is effective at preventing or delaying neuronal death and dysfunction (Monti et al.,2009;Chu et al.,2015;Romoli et al.,2019).In both N-methyl-D-aspartate-induced neurotoxicity and ONC models,VPA was able to stimulate the BDNF-TrkB pathway and increase RGC survival (Zhang et al.,2012b;Kimura et al.,2015a,2016).In vitro,VPA delayed spontaneous RGC death with only a slight effect on histone acetylation (Biermann et al.,2011).A few clinical trials have studied the therapeutic potenti al of VPA in ocular disorders.One randomized controlled trial showed that 3 months of oral VPA therapy improved visual acuity in patients with advanced glaucoma (Mahalingam et al.,2018).Another phase-2 multicenter clinical trial failed to provide support for oral VPA in the treatment of autosomal dominant retinitis pigmentosa (Birch et al.,2018).Thus,the molecular mechanisms through which VPA protects retinal neurons needs further study.
As an important part of the ER stress response,the UPR is activated to restore cellular homeostasis (Senft and Ronai,2015),alone or in coordination with other cell organelles (Costa et al.,2020).The cascade of signaling pathways triggered by the UPR regulates cell survival and cell death.Timely intervention that reduces ER stress is an essential option for rescuing neuron (Hetz and Papa,2018).In the present study,we first used transmission electron microscopy to observe how the RGC-ER ultrastructure morphology changed over time following ONC.We found that the changes began as early as 12 hours after injury.Afterward,the ER began to lose its original structural system (day 1) and began atrophying into small vacuoles structures (by day 7),indicating irreversible organelle injury and loss of ER function after a week.The ER functions to properly fold and process secreted transmembrane proteins (Oslowski and Urano,2011).The loss of the original ER structural system on the first day after injury suggests that treatment beginning at thistime will promote the most RGC survival.
To rescue the RGCs in the ONC animal model,one single dose of intravitreal VPA was administered immediately after crush injury.Compared with the control group,the number of apoptotic RGCs in VPA group were significantly less at 3 and 7 days after ONC.Meanwhile,the whole neural retina and the IPL were thicker in the VPA group than in the control group at 7 days postinjury.The retinal thickness two to three disc-diameters from the optic nerve was recorded by HE staining in all groups.The normal retinal thickness of the male Sprague-Dawley rat was similar to what has been previously reported (Li et al.,2021).These findings confirm that VPA has a neuroprotective effect in the ONC model (Biermann et al.,2010;Zhang et al.,2012b),and indicate that a single dose of intravitreal VPA results in RGC protection.Intravitreal,rather than subcutaneous,administration of VPA provides a valuable reference for future clinical trials.
GRP78 is found on the ER membrane and regulates protein quality control and degradation upon ER stress (Dores-Silva et al.,2020).GRP78 expression increases when the ER is stressed (Ibrahim et al.,2019) and its overexpression attenuates ER stress and protects neurons from cell death after ischemia or trauma to the nervous system (Weng et al.,2011;Casas,2017).CHOP is a member of CCAAT/enhancer-binding protein family and is involved in the regulation of genes that encode proteins that function in proliferation,differentiation and expression,and energy metabolism (Hu et al.,2018).CHOP-induced apoptosis in times of ER stress has been implicated in neurodegenerative diseases,diabetes,ischemic diseases,and tumor (Li et al.,2015).CHOP deficiency enhances cell function and reduces apoptosis(Noh et al.,2015;Kim et al.,2019;Shi et al.,2021).In our study,the normal retina sections showed positive immunoreacti vity for both GRP78 and CHOP anti bodies in the ganglion cell layer of the retina.CHOP expression is weak in a normal physiological state.After ONC,retinal expression of both CHOP and GRP78 were higher than normal at the 6-hour mark.Interestingly,the expression of GRP78 decreased to normal levels by the third day after injury,while CHOP levels remained high for 7 days.This imbalance between GRP78 and CHOP indicated inevitable death for RGCs.
The effect of intravitreal VPA on the expression of UPR-related factors may provide clues to the mechanisms underlying VPA-mediated neuroprotection.Immunoblotting showed that ONC injury enhanced the expression of GRP78 and CHOP gradually in a time-dependent manner within 1 day.VPA treatment boosted GRP78 expression at each time point and decreased CHOP expression at 1 day.Meanwhile,phosphorylated eIF2α,the upstream regulator of CHOP(Huang et al.,2017),was also inhibited.Phosphorylation of eIF2α attenuates protein translation at the beginning of the UPR,but promotes translation of activating transcription factor 4,selectively followed by CHOP up-regulation(Chen et al.,2022).VPA can promote transcription,which is opposite to the biological effect of p-eIF2α,suggesting that the inhibition of the eIF2α-CHOP pathway by VPA is neuroprotective.
In addition to the eIF2α-CHOP pathway,ER-induced apoptosis also occurs via the caspase-12 kinase pathway (Hu et al.,2018).Procaspase-12 is predominantly localized to the ER and is specifically activated by disturbances to ER homeostasis such as ER stress (Nakagawa and Yuan,2000).We observed that VPA reduced the upregulation caspase-12 expression induced by ONC in a time-dependent manner.This inhibition of caspase-12 might depend on the VPA-induced upregulation of GRP78 mentioned above.Over-expression of GRP78 might lead to attenuated activation of inositol requiring enzyme 1,thus limiting the amount of procaspase-12 that can be converted to caspase-12.The accumulation of procaspase-12 on the ER membrane would then be a sign of decrease of caspase-12 levels.The above hypothesis will be investigated in our next study.
Further investigations are already under way to clarify whether VPA also rescues the morphological changes to the ER,as well as the time course of GPR78 and CHOP expression in the neural retina layer after VPA treatment.The current study don’t examine the long-term effects (1 or 2 months) of intravitreal VPA on RGC protection.Thus,detailed information on the safety remains unknown and the value of clinical trials also need careful evaluation.In conclusion,the ER chaperones GRP78,CHOP,and p-eIF2α determine the fate of RGCs during ER stress.Our study clarifies the mechanism underlying VPA-mediated neuroprotection;VPA acts to inhibit ONC injury-induced activation of the eIF2α-CHOP pathway and caspase-12 acti vity.Targeting these key pro-survival and pro-apoptotic signaling molecules of the UPR should be included in neuroprotective strategies.
Acknowledgments:We thank Jing-Bo Su of Eye Institute of Chinese PLA,Xijing Hospital,Fourth Military Medical University for assistance in the animal experiments.
Author contributions:Study design: DH,YSW,XH;experimentimplementation: FP,LJS,QB;data analysis: DH,FP;manuscript draft : DH,XH.All authors approved the final version of the manuscript.
Conflicts of interest:The authors have no financial conflict of interest.
Open access statement:This is an open access journal,andarticles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- Bystanders or not? Microglia and lymphocytes in aging and stroke
- Alzheimer’s disease risk after COVID-19: a view from the perspective of the infecti ous hypothesis of neurodegeneration
- Serine and arginine rich splicing factor 1: a potenti al target for neuroprotection and other diseases
- Can glial cells save neurons in epilepsy?
- Lights for epilepsy: can photobiomodulation reduce seizures and offer neuroprotection?
- The landscape of cognitive impairment in superoxide dismutase 1-amyotrophic lateral sclerosis

