Recent advances on oxazolidinones synthesize from carbon dioxide
WANG Bian-ling ,GUO Zhi-qiang ,WEI Xue-hong
(1. Shanxi Key Laboratory of Functional Molecules, School of Chemistry and Chemical Engineering, Shanxi University,Taiyuan 030006, China;2. Shanxi Key Laboratory of Functional Molecules, Scientific Instrument Center, Shanxi University, Taiyuan 030006, China)
Abstract: Carbon dioxide capture and utilization (CCU) is the best way to solve the problem of reducing concentration of carbon dioxide in the atmosphere, and it has a good prospect for development. On this basis, chemical researchers have explored the methods of synthesizing valuable organic compounds with CO2 as carbon source. The oxazolidinones are commonly used to synthesize drugs, and they are significant in organic synthesis as chiral molecules and intermediates. The synthetic methods of oxazolidinones have emerged in recent years. Furthermore, the methods of using carbon dioxide as a carbon source have attracted many researchers. In earlier years, people explored cycloaddition reactions of carbon dioxide and aziridines to synthesize oxazolidinones, and they took alkali metals, Cr, Al or other metals as catalysts to improve the efficiency of the reactions. Because of the cost and the principle of green synthesis, it is more suitable for large-scale reaction to select cheap and easily available ionic liquids or no catalysts. In addition, carbon dioxide and compounds such as β-amino alcohols, unsaturated amines and 1, 2-dihalohydrogenated compounds can obtain moderate or even excellent yields under different reaction conditions. In this paper, we summarized the synthetic methods of oxazolidinones using CO2 with different raw materials in recent years.
Key words: carbon dioxide;oxazolidinones;aziridines;epoxides;β-amino alcohols
Carbon dioxide (CO2) as the mainly greenhouse gas has led to climate change[1,2]. Carbon capture and storage (CCS)[3,4], carbon capture and utilization(CCU)[5,6]are two most promising approaches to reduce energy conservation and emission reduction. However,CCS required abundant energy in CO2desorption. In order to overcome this disadvantage of CCS and achieve the purpose of directly fixing carbon dioxide,CCU is more extensive in reducing carbon dioxide concentration[7]. Carbon dioxide is low-toxic, available,abundant and renewable[8]. Compared to CO and methyl iodide, it is more stable and more likely to store. It has been reported that carbon dioxide as a C1 resource can synthesize a series of high-value chemical products including methanol[9], carboxylic acids[10],cyclic carbonates[11], oxazolidinones[12]and other important organic compounds[13]. Significantly, in these transformations, this paper focused on the synthesis of oxazolidinones.
Oxazolidinones are heterocyclic compounds which contain five-membered ring including nitrogen and oxygen[14]. Its moiety was discovered in few biological nature structures, such as streptazolin[15].Oxazolidinones and their derivatives contributed to the biological activity of various artificial medicines,including antimicrobial drugs[16,17], antidepressant[18]and analgesic drugs[19,20].
Oxazolidinones played an important role in chemical synthesis as organic synthesis intermediates[21]and effective chiral auxiliaries[22]. Therefore, a large number of expedient and efficient synthetic routes for generation of oxazolidinones and their derivatives have been developed[23−25]. Traditionally, there were abundant means to synthesize oxazolidinones using carbon oxide[26−28], isocyanate[29−33], phosgene[34]and their derivatives as C1 sources. Phosgene named ‘Choky Gas’ is a highly toxic reagent[35], which may cause damage to human and environment. And the strong base atmosphere limited the use of some substrates containing special functional groups. In recent years,toxic carbon sources such as phosgene and CO have been replaced by CO2[35,36], and many methods for the synthesis of oxazolidinones and their derivatives have been developed. And organic chemists put forward

1 Synthesis of oxazolidinones from CO2 and aziridines
Aziridine is also called cycloethylamine or ethanolamine, and it is an important ternary compound[39]. It has a similar structure to ethylene oxide and it is wildly used in making reactive dyes and anticancer agents[40]. The nature of the aziridine has been studied in the past few years[41], and the synthesis of oxazolidinones is one of its applications[29,42](Figure 1).
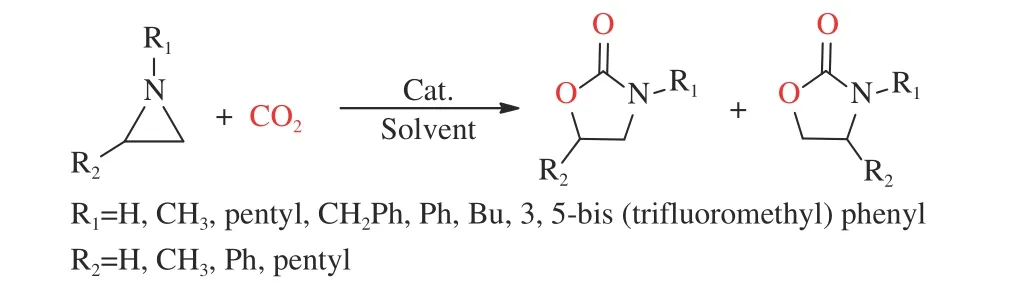
Figure 1 A general route to synthesize oxazolidinones from aziridines and CO2
1.1 Metal-complexes catalyzed synthesis of oxazolidinones using CO2 and aziridines
Up to date, some procedures have been reported for the cycloaddition reaction of aziridines and carbon dioxide using various metal-base catalysts, for example,organoantimony (Sb) halides[43], nickel (Ni)complexes[44], zirconyl (Zr)-base complexes[45], aluminum(Al) compounds[46,47]and so on (Figure 2).
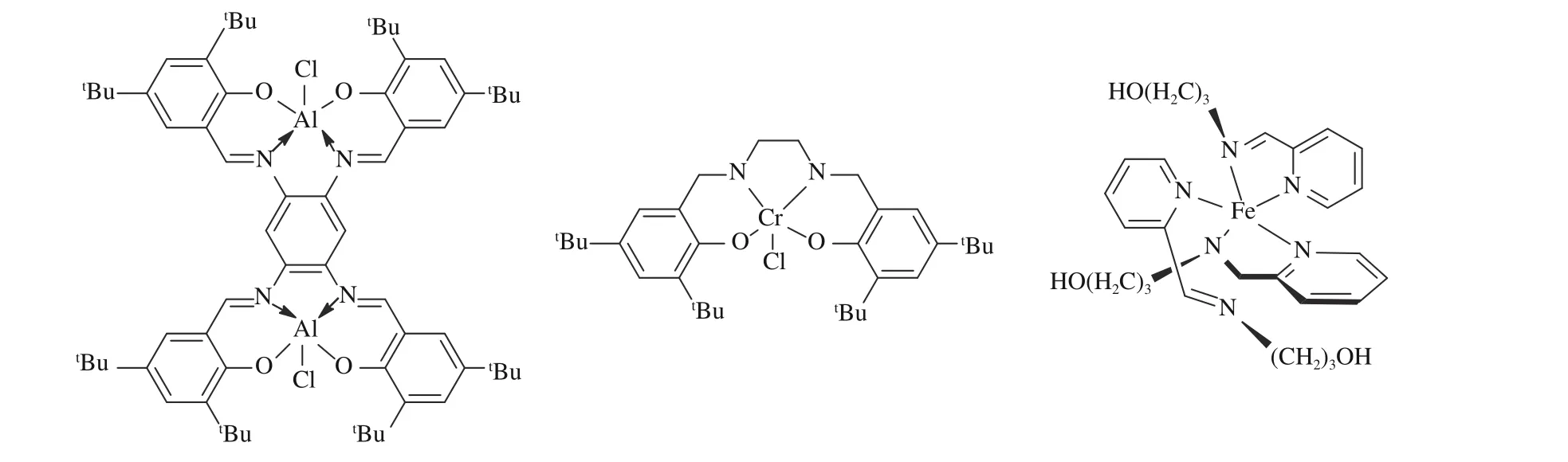
Figure 2 Structure of metal catalysts for catalytic reaction of CO2 and aziridines[47,51,53](with permission from Royal Society of Chemistry, Wiley-VCH and Elsevier)
The catalytic system composed of alkali metal halides and ammonium salts was reported to effectively catalyze the conversion of aziridine to the corresponding oxazolidinone[48,49]. In 2003, Sudo et al.[48]reported a route to synthetize 2-oxazolidinones employing lithium bromide as catalyst. It was noteworthy that the reaction could convert to the corresponding oxazolidinone with a good yield inNmethylpyrrolidone (NMP) at room temperature under atmospheric pressure. Also, they studied another substance, CS2, which is similar to CO2. The results indicated that it can also undergo cycloaddition reactions getting sulfur analogue of oxazolidinones in the same catalytic system.
In a closely related study, the lithium iodide (LiI)catalyzed reaction of aziridine and CO2was reported by Hancock and his partner[49]. In this way, they could obtain two regional isomers in THF. Importantly,adding HMPA (hexamthylphosphoramide) as cosolvent and improving pressure of CO2can greatly improve the isomeric ratio and get mostly 4-substituent oxazolidinones.
After that, Chromium (III)/DMAP (4-dimethylaminopyridine) as catalytic system has been proved that it can catalyze synthesis of oxazolidinones[46,47]. It was the first method to get 5-substitute as the major products instead of 4-substitute isomers, as shown in Figure 3. But high steric hindrance of N-substitute required harder conditions to obtain higher yields. This group further researched(salen) Cr (III) Cl/DMAP catalyst system in 2015[47]. It attached importance to theoretical calculation by density functional theory (DFT). The combination of theory and practice proved the feasibility of this catalyst system.
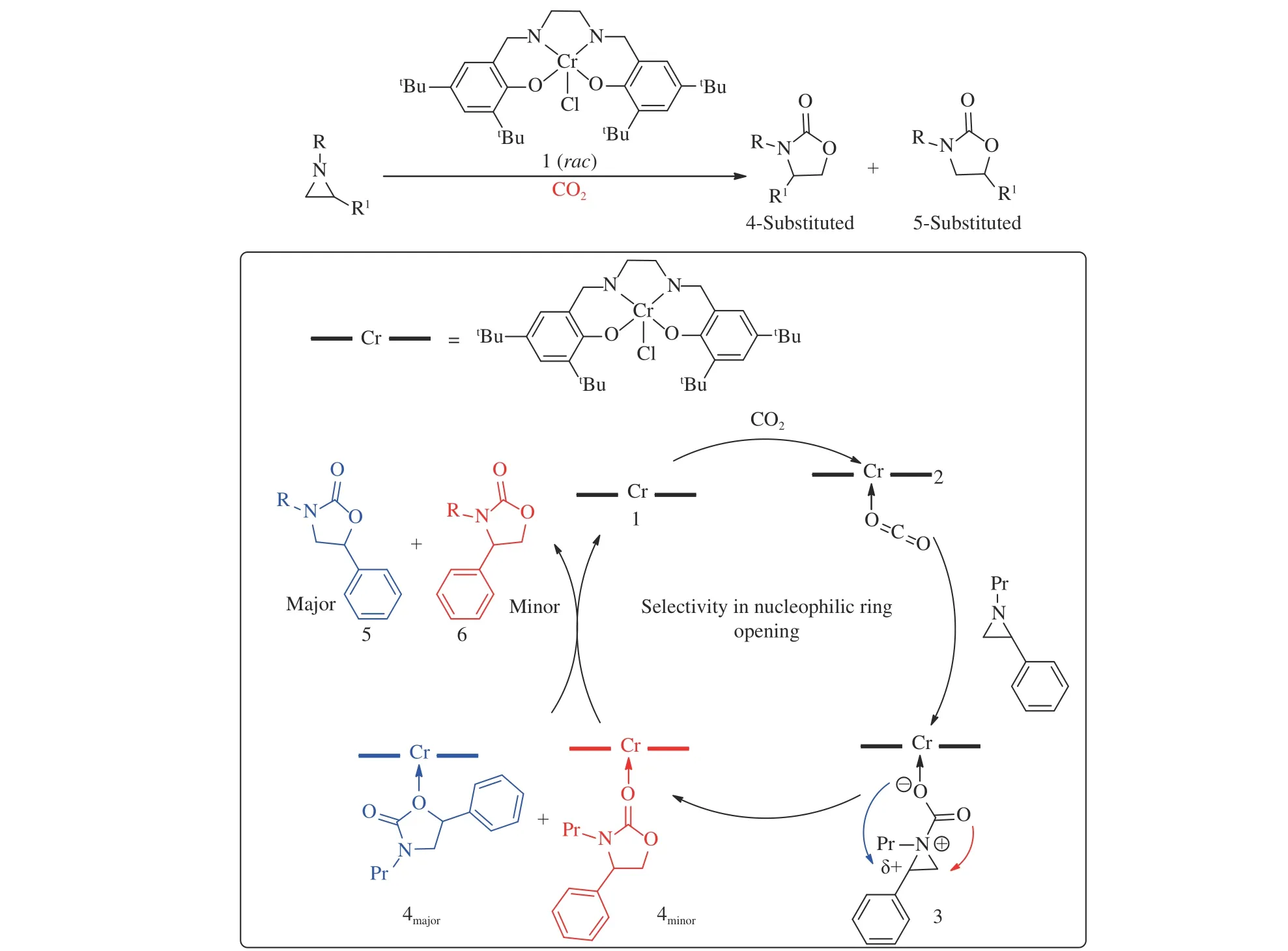
Figure 3 A proposed catalytic cycle for the formation of 5- and 4- substituted oxazolidinones from the coupling of aziridine and CO2 in the sole presence of the Cr catalyst[46,47](with permission from American Chemical Society and Royal Society of Chemistry)
In this respect, Wu et al. reported an efficient and renewable catalyst for synthesis of 5-aryl-2-oxazolidinones from aziridines and CO2by using zirconyl chloride (ZrCl4) as a solid catalyst without solvent in 2009[45]. The cationic cluster[Zr4(OH)8(H2O)16]8+in crystal of zirconyl chloride activated and catalyzed reaction. This protocol not only afforded moderate conditions and excellent yields but also got good regional selectivity under optimize conditions. Because of high steric hindrance of Nsubstituted group, 1-(2,2-dimethylpropyl)-2-phenylaziridine need longer reaction time. And the results indicated that benzene ring with electron-donating group was more active than that with electronwithdrawing. In addition, catalytic agent of this reaction could be separated by filtration and reused several times.
Furthermore, 2-oxazolidinones and their derivatives were also achieved via cycloaddition of CO2and aziridines using ruthenium porphyrin as a catalyst[50]. Under the optimized conditions, the aziridine, 1% mol [Ru(TPP)(NAr)2] (TPP=dianion of mesotetrakis-(phenyl) porphyin, Ar=3, 5(CF3C6H3)),and 10% mol TBACl (tetrabutyl ammonium chloride)at 100 °C under 0.6 MPa of CO2was able to obtain a yield of 60%−95% with up to almost 100%regioselectivities.
In addition, the Al complexes also could catalyze the synthesis of oxazolidinone using 1-benzyl-2-phenyl-aziridine as a model substrate. Sengoden and his group[51]explored the methods to form oxazolidinones by coupling CO2and aziridines. In this dispatch, they synthesized three readily available aluminum complexes. There are several advantages of this project including relatively low temperature (50 °C),low atmospheric pressure (1 bar), good regional selectivity, giving the desired oxazolidinones in moderate to excellent yields and recovering catalytic agent without loss of catalytic activity. Meanwhile, the study in scope of substrates with different groups found that no matter which group it contained, a good yield can be obtained by this method. In order to understand the chemical reaction, they proposed a possible mechanism, as shown in Figure 4. Aziridines and catalyst combined to form chelate and aziridine ringopening due to intramolecular nucleophilic substitution, then carbon dioxide inserted into complex A getting complex B. Finally, complex B went to the product by cyclizing.
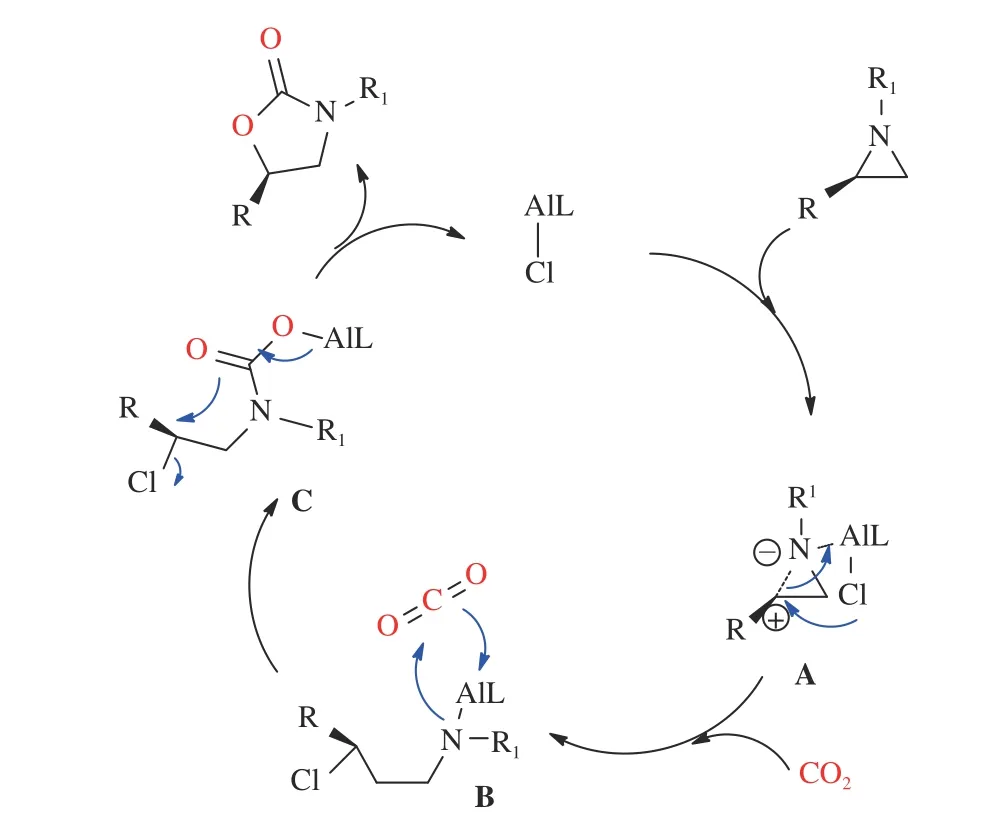
Figure 4 Plausible catalytic cycle using aluminum complexes[51](with permission from Wiley-VCH)
Recently, non-precious metal carbamates catalyst which synthesized by niobium (Nb) molecule combined with chloride-carbamate complex and tetrabutylammonium bromide (TBAB) was used to catalyze the coupling reaction of the aziridines and CO2under mild conditions[52]. At the same time, Kim et al.[53]reported Fe-iminopyridine (IP) as catalyst and TPPI(tetraphenylphosphonoum iodide) as co-catalyst to form cyclic carbonates. Meanwhile, they studied the synthetic application for five-membered heterocycles.In optimal reactions (10 bar CO2, 0.1% catalyst,1% additive, 50 °C), they discovered that oxazolidinones from cycloaddition of carbon dioxide and aziridines can also get excellent yields in this catalytic system. The position of substitutes in aziridines determined what kind of oxazolidines you get.
1.2 Ionic liquid catalyzed synthesis of oxazolidinones using CO2 and aziridines
Metal including V[43], Ni[44], Zr[45], Al[46,47]and so on as catalyst can promote synthesis of oxazolidinones.However, metal catalysts are expensive and not available. Besides, residual metals will be harmful to the environment. It is a better choice to choose a greener catalyst in the reaction. Ionic liquid (IL)materials are promising eletrolytes with striking physicochemical properties for energy and organic synthesis[54]. ILs are available and environmentally friendly catalysts. They are widely used in the synthesis of oxazolidinones. Tertbutyl ammonium bromide and tertbutyl ammonium iodide have been developed as quaternary ammonium catalysts[48]. In this reaction, halogen ions acted as available to accelerate the opening of epoxide compounds and the reaction temperature had an effect on regional selectivity[42].
Further investigations were made by Kawanami et al. in 2005[55]. They reported the carboxylation of aziridines with supercritical carbon dioxide (scCO2).Symmetric tetraalkyl ammonium salts catalyzed this reaction could provide higher turnover frequency(TOF) value and excellent yield. Under this catalyst system, the reaction can rapidly be carried out in 5 min and it can get excellent yields (95%).
Due to the condition of supercritical CO2is difficult to achieve in the laboratory, it is not suitable for extensive applications. In 2010, He ’s group[56]studied a series of Lewis basic ionic liquids in synthetizing of oxazolidinones from aziridines and CO2without any organic solvent or additives. It was an essay where reported aziridines reacted with CO2using[C4DABCO] Br (1-butyl-4-aza-1-azaniabicyclo [2.2.2]octane bromide) as catalyst. The overall cycloaddition conditions achieving in this message were more attractive than that using metal as catalyst. There was no loss of catalytic properties after four times.
Poly (ionic liquids) is a kind of catalysts for the catalyzed synthesis of five-membered heterocyclic compounds[57]. In this catalytic system, the significant advantages of this reaction are the absence of metal,high regional selectivity (almost for 5-substituted),high yield (>95%) and the formation of no by-products.Sonzini et al.[58]took advantage of TPPH2/TBACl(TPPH2=dianion of tetraphenyl porphyrin; TBACl=tetrabutylammonium chloride) to synthetize oxazolidinones. Using 1-(3,5-bis(trifluoromethyl)phenyl)-2-phenylaziridine as a model substance, they discussed the influence of catalyst/co-catalyst, CO2pressure, reaction temperature and reaction time on the reaction. By controlling the experiment, the optimize reaction conditions are 1.2 MPa of CO2pressure, 125 °C and the ratio of TPPH2/BACl to aziridine is 1∶5∶100.
Importantly, the reaction in the absence of solvent with ILs catalyst was hardly realized. Recently,Bresciani et al.[59]successfully prepared [NH2Et2] as an efficient catalyst in the absence of solvent for the reaction via aziridine and CO2under atmospheric CO2pressure at room temperature. Oxazolidinones and its derivatives were obtained from related aziridine. And this group proved the feasibility of the reaction by density functional theory.
1.3 Synthesis oxazolidinones by capturing CO2 on supported catalyst
There were milder conditions with supported catalyst in the synthesis of oxazolidinones. Liu et al.[60]reported a catalyst which is quaternary ammonium bromide functionalized polyethylene glycol(PEG6000(NBu3Br)2) for the synthesis of 5-substituted-2-oxazolidinones from CO2and various aziridines without other organic solvent or cocatalyst in 2008.The mass spectrometry (MS) and1H nuclear magnetic resonance spectroscopy (1H NMR) indicated the trace amounts of side products. This reaction not only achieved good catalytic yield, but also had extremely high regional selectivity. Most importantly, the catalyst was able to be recovered via centrifugation, without significant loss in catalytic activity and selectivity.A plausible reaction mechanism was presented, as shown in Figure 5. There were two possible pathways depending on the nature of R group with alkyl substitution at the N-position. Initially, CO2coordinated with aziridine, then a nucleophilic attacked by anionic Br−, the cyclization was facilitated by the intramolecular nucleophilic attack, regenerated the catalyst and further offered the final 5-substituted-2-oxazolidinones. The similar catalyst is polyethylene glycol (PEG400) embedded in potassium bromide was reported by Kumar and Jain[61]. They took [K+(PEG)Br−] as a highly efficient medium to get the desired production for the reaction of aziridines and CO2. Under the optimization conditions (1 atm CO2,60 °C), an army of aziridines as reactant were used to synthesize the corresponding oxazolidinone with fairly good yields.

Figure 5 A possible mechanism for the PEG6000(NBu3Br)2-catalyzed cycloaddition of CO2 with aziridine[60](with permission from ACS Publications)
In 2013, another supported catalyst named MCM-41 which is a kind of ordered mesoporous materials were reported by Nale et al.[62]. They demonstrated a protocol using MCM-41 (a kind of ordered mesoporous materials) as catalyst to the regioselective synthesis of 2-oxazolidinones and their derivatives. The reaction was carried out at room temperature that the benchmark cycloaddition aziridine and CO2(5 MPa)in 8 h using 14.6% amine functionalized MCM-4(25%) gave the quality results.
Recently, metal-organic frameworks (MOF) were merging in many aspects. Kathalikkattil et al.[63]first reported the synthesis of 2-oxazolidinones using MOF as catalyst in 2015. A water stable zinc-MOF (ZnGlu)catalyst was active and selective for the rapid reaction of CO2with multiple aziridines under mild conditions(1 MPa of CO2pressure, at room temperature).
Covalent organic framework (COF) is another kind of polyporous material. In 2016, Saptal et al.[64]reported a method to get oxazolidinones by reacting aziridines with carbon dioxide using 2,3-dihydroxyterephalaldehyde as catalyst. They studied the effects of co-catalyst, temperature and time on the reaction with 2-phenyl aziridine as a model substrate.The results indicated that the optimization conditions as follows: 5 mmol aziridine, 0.01 mmol 2, 3-Dha Tph COF (Dha= 2, 3-dihydroxyterephthalaldehyde; Tph=5,10, 15, 20-tetrakis (4-amino phenyl)-21H, 23Hporphin), 0.05 mmol TBAI, 2 MPa of CO2pressure,50 °C. Under the optimization conditions, a variety of substrates were tested. The results indicated that it can get moderate yields for desired products with corresponding different substituents of aziridines.Aziridines with a steric hindrance substituent could get better yield at higher temperature and more-time.Besides, COF catalyst can recycle 5 times without any loss.
In addition, Fibrous nanosilica (KCC-1) was investigated as a catalyst because of their high surface areas, robust stabilities and excellent lipophilicities.Sadeghzadeh et al.[65]studied the application of KCC-1,sodium tripoluphosphate (STPP) and 3-aminopropyltriethoxysilane in the synthesis of 2-oxazolidinones. They acted good catalyst activity and got satisfactory yield at room temperature under 0.5 MPa of CO2pressure in 30 min without solvent.Recently, Liu et al.[66]also described imidazolium functionalized ionic liquids onto mesoporous SBA-15.Ensuing SBA-15@DMIL-HOCH2COO was utilized as an efficient catalyst for the cycloaddition of aziridines and carbon dioxide to provide their products in an excellent yields at 80 °C under lower pressure (0.4 MPa).
1.4 Catalyst-free synthesis of oxazolidinones using CO2 and aziridines
In 2010, He’s group[67]reported a catalyst-free and solvent-free project to the formation of 2-oxazolidinone derivatives through the cycloaddition of CO2and aziridines. They studied the reaction mechanism in situ FT-IR technique. Later, Phung et al.[68]demonstrated that a library of 5-aryl-2-oxazolidinones in high yield through CO2(4 atm) at room temperature with corresponding aziridines in the absence of catalyst.And they made use of high speed ball milling (HSBH)to decrease the reaction time. The results showed that each reaction can get convenient yield within 17 h.
2 Synthesis of oxazolidinones from CO2 and β-amino alcohols
β-amino alcohol is widely found in bioactive,natural and unnatural amino acids[69]. It can be used as an intermediate in the synthesis of many heterocyclic organic compounds[70]including indoles[71]and benzoxazines[72]. Also, it exists in many medicines[73],such as anti-HBV drugs[74], antihypertensives[75], and other drugs[76−78]. Amino alcohols, which are derivatives of various amino acids[79], were widely used in carbon dioxide capture and as important raw materials in synthetizing oxazolidinones[27,80](Figure 6).
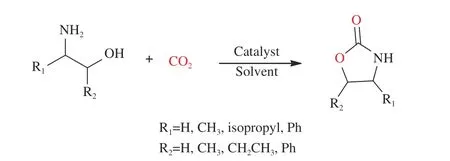
Figure 6 A route to synthesize oxazolidinones from CO2 and β-amino alcohol
2.1 Metal-complexes catalyzed synthesis of oxazolidinones using CO2 and β-amino alcohols
Ogawa’s group[81]first reported the synthesis of oxazolidinones using metal-catalyst fromβ-amino alcohol and CO2in 1985. In this catalytic system composing of triphenylstibine oxide and Molecular Sieves 3A, the corresponding oxazolidinones can get 80%−94% yields. Recently, Ph3SbO as catalyst catalyzed the reaction of amino alcohols with CO2was reported[82]. In this approach, dehydrating was the most important segment. The reactions took place at 20−175 °C in benzene which getting 85%−98% yields without other side-products. It provided a new method to synthetize oxazolidinones for us.
Molecular sieve is easy to be inactivate and it also need frequent dry. It is necessary to seek new catalysts.In previous reports, Ce was often used as a catalyst to promote the reaction. Inspired by previous studies,Juárez et al.[83]attempted to use Cesium oxide as a catalyst to the reaction of β-amino alcohol and carbon dioxide proceed fully. The results indicated that the synthesis of oxazolidinones could be carried out by amino alcohol and CO2in ethanol at 160 °C with ceria nanoparticles (nano-CeO2) catalyst. In this paper, other particles were tested including γ-Al2O3, P-25 TiO2,ZrO2, MgO and Y2O3as well as Au/np-CeO2(5 nm) at gold loadings from 0.79% to 2.46%, but it didn't get the expected results . This method can also be used to synthesize large heterocyclic compounds.
Nanoparticle processing is difficult and the process is complex. Meanwhile the cost is high, and it is difficult to be popularized in a large area. In 2012,1,3-dichloro-1,1,3,3-tetraalkyldistannoxanes catalyzed the reaction of 2-aminoalcohols and carbon dioxide was reported by Pulla et al.[84]. In this catalytic system,Sn as a center combined with other different substituent catalyzed the reaction. Just as guessed, different substituents had different catalytic effects on the reaction. To our delight, it showed the best catalytic performance in the reaction what is using chlorostannoxane with both butyl substituents on metal center under higher pressure (1.72 MPa) at 150 °C.Taking different substituent of the substrate, it still can get high yield with optimal conditions.
CeO2has acid-base and redox properties, and is widely used in organic synthesis as heterogeneous catalysts, but most of the reaction catalyzed by CeO2usually at high temperature. Therefore, the study of reaction at lower temperature is particularly important.In 2013, Tamura et al.[85]attempted to catalyze the selectively synthesis of oxazolidinones with CeO2at lower temperature (423 K) in acetonitrile. In this way,amino alcohols with various substituent were able to obtain satisfactory yield (>99%) and selectivity(>99%).
At the same year, Foo et al.[86]explored a new route in order to assess the possibility in dehydration reaction of amino alcohol and CO2. Taking L-valinol as a reaction substrate, the effect of different bases on the reaction was explored. It was worth noting that alkali metal salts were capable to promote the reaction. To our delight, the best result was obtained with Cs2CO3among other salts including Li2CO3, Na2CO3, K2CO3,Rb2CO3, KHCO3, CsHCO3and so on in 1 mL dimethyl sulfoxide (DMSO) under atmospheric carbon dioxide pressure at 150 °C for one day.
2.2 Non-metal catalyzed synthesis of oxazolidinones using CO2 and β-amino alcohols
Although these metals can get excellent yield, but they are expensive and poor stability. Importantly, their damage to the human and environment greatly limits the application of these methods. The appearance of ionic liquids compensates for these defects of metal catalysis.
Kawanami et al.[87]synthetized 2-oxazolidinone through coupling β-amino alcohol with supercritical carbon dioxide usingN,N'- dicyclohexylcarbodiimide(DCC) in 2002. The product can get the maximum yield of 96.7% without solvent in 12 h under 8.6 MPa of CO2pressure. In 2004, Dinsmore et al.[88]published a paper about Mitsunobu reagents applied in dehydration strategy. It was demonstrated that both primary and secondary amines could react with CO2and 2-amino alcohol, the corresponding oxazolidinones as sole products with great yields in the presence of DBU (1,8-diazabicyclo [5.4.0] undec-7-ene)/n-Bu3P/DBAD(ditert-butyl azodicarboxylate)/acetonitrile (CH3CN).
Ionic liquids composed of 100% anion and cation,and no neutral molecules. They were super acidic and for certain reaction can act as a catalyst what is relatively cheap without metal residue. They can catalyze the carbonylation of CO2and β-amino alcohol.Carbonylation of CO2and β-amino alcohol by 1-butyl-3-methylimidazolium bromide (BMIM-Br) has also been reported by Fu et al.[89]. They firstly showed an attractive route to produce oxazolidinones starting from 2-amino alcohols with CO2used BMIM-Br as catalyst and K2CO3as an additive at 150 °C. In addition, the synthetic methods have been explored in the electrochemical aspects[90,91], and the corresponding oxazolidinones can also be obtained.
3 Synthesis of oxazolidinones from CO2,epoxides and amines
Propylene oxide (PO) is one of the important synthetic intermediates[92], which can be used to synthesize polyurethane plastics, polyglycol esters,unsaturated resins and surfactants[93−95]because of more actively and easier to open ring. Meanwhile, it could be used to capture CO2and form a variety of useful compounds, for example, oxazolidinone is one of them(Figure 7).
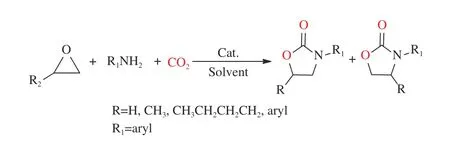
Figure 7 A route to synthesize oxazolidinones from epoxides, amines and CO2
3.1 Ionic liquid catalyzed synthesis of oxazolidinones using CO2 with epoxides and amines
Ionic liquids have been extensively investigated with a wide range of interesting applications[96,97]due to their high thermal stability, negligible vapor pressure,high loading capacity, easy recyclability and diversiform structure/property modulation.
Recently, Wang et al.[98]proved that 1-butyl-3-methimidazole ammonium bromide (BmimBr) and 1-butyl-3-imidazole methyl acetate (BmimOAc) ionic liquid as catalyst at 140 °C in 9 h, avoiding prefunctionalizing of the substrate via the direct insertion of CO2into poorly nucleophilic anilines and epoxides.Shortly afterwards, the equal lookup team[99]found that 1,8-diazabicyclo [5.4.0] undec-7-ene (DBU)/DBUderived bromide ionic liquid (HDBUBr) could also as catalyst system to effectively catalyze the conversion of CO2, anilines, and epoxides. However, higher reaction temperature (130 °C) and higher CO2pressure (2.5 MPa)were required for the catalytic activity. Unfortunately,this system had poor reactivity for the epoxides bearing substituents.
Higher temperature and higher CO2pressure conditions are not only difficult to achieve in the laboratory, but also dangerous. In 2017, Yao’s group[100]developed the use of quaternary ammonium salts and additive for the oxazolidinones synthesis from cycloaddition of amines, epoxides and CO2. In this method, only a balloon of carbon dioxide was needed,and the temperature used was much lower than previous reports. The catalytic activity study of these quaternary ammonium was found that DBU was the best one than the others. And DBU performed better activity than other organic salts and inorganic salt. As a result, TBAI/DBU was selected as optimized catalytic system. A great deal of anilines and epoxide reacted with CO2(ballon) gave corresponding 5-substitutent-2-oxazolidines using 0.5% TBAI and 5% DBU in 115 °C.It’s interesting that this method is suitable for both aliphatic and aromatic anilines. However, disubstituted epoxides only can get lower yield (11%) because of steric effect.
3.2 Base catalyzed synthesis of oxazolidinones using CO2 with epoxides and amines
In a study led by Seo et al.[101], K3PO4was utilized in the synthesis of oxazolidinones in 2017. This onepot reaction was applicable to a variety of terminal epoxides and amines. Various amines bearing either electron-donating or electron-withdrawing were all tolerated in the reaction. The advantages of this method were efficient, simple operation, inexpensive catalyst.It can also get good yields when the reaction conducted on a 10 g-scale. On the basis of previous researches and findings of investigations, based on the formation of phenyl isocyanate and based on 1, 2-aminoalcoho,two reaction mechanisms were proposed, as shown in Figure 8.
At the same year, the group of Sadeghzadeh[102]designed Spirulina (Arthrospira) platensis biomasses supported ionic liquid to synthesize 2-oxazolidinone derivatives via the cycloaddition of ethylene oxide with aromatic amines and carbon dioxide under 1 MPa pressure at 100 °C. In order to demonstrate the applicability of this method, the research group selected materials with representative substituents for the reaction exploration. The result made out that all reaction can go on wheels in this catalytic system and provide a considerable yield of the required products.
In 2019, Liu ’s group[103]developed a route to synthesize 3-aryl-2-oxazolidinones with multifunction organocatalytic system combined with 1, 8-diazabicyclo[5.4.0]undec-7-ene (DBU) . Amines of various functional groups can be tolerated at 90 °C in 4 h under 5 bar pressure with imidazole salts and DBU as catalyst. On the basis of the control experimental findings, a plausible pathway is proposed. Furthermore,An atom-economic reaction through cycloaddition of CO2to epoxides and amines in 12 h at 100 °C under a balloon carbon dioxide was developed by Cui ’s group[104]. In this reaction, the catalytic system is consisting of rhodamine B and DBU.
3.3 Metal-complex catalyzed synthesis of oxazolidinones using CO2 with epoxides and amines
Ionic liquid catalyzed reactions require higher pressure or higher temperature to be readily available in the laboratory and unsuitable for mass production in factories. In 2016, Xu et al.[105]reported the synthesis of rare-earth-metal complexes combined with aminebridge tri(phenolato) ligands and used them to participate in the cycloaddition reaction of CO2and epoxide. The reactions were carried out under 10 bar pressure of CO2at 95 °C in Nd combined with aminebridge tri(phenolato)/NBu4Br/DBU catalyst system in 9 h. The anilines bearing ortho-substituents with either electron withdrawing or electron donating was no product formed. However, others can get moderate to excellent products. What’s more, disubstituent epoxide(1, 2-epoxy-2-methylpropane) only get <10% yield due to steric hindrance.
In addition to Nd, the highly-active Fe(II)complexes have also been found that they were competitive alternatives to organic-catalysts for the preparation of oxazolidinones from epoxide, amine and CO2[106]. Taking representative epoxide compounds as reactant, all epoxides bearing aliphatic substituents reacted smoothly with aniline and CO2to give the desired products in good to excellent yield with Fecomplex catalyst. The observation from control experiments, they supposed a possible mechanism. The experiment reacted in a relatively mild atmosphere with an atomic efficiency of 100%. It is environment friendly and in line with the current green chemistry theory.
Metal catalysts are able to active carbon dioxide and promote reaction process. However, most metals are harmful and catalyst systems are complex (Figure 9).Heterogeneous catalysts are popular in organic synthesis because they are green and available.
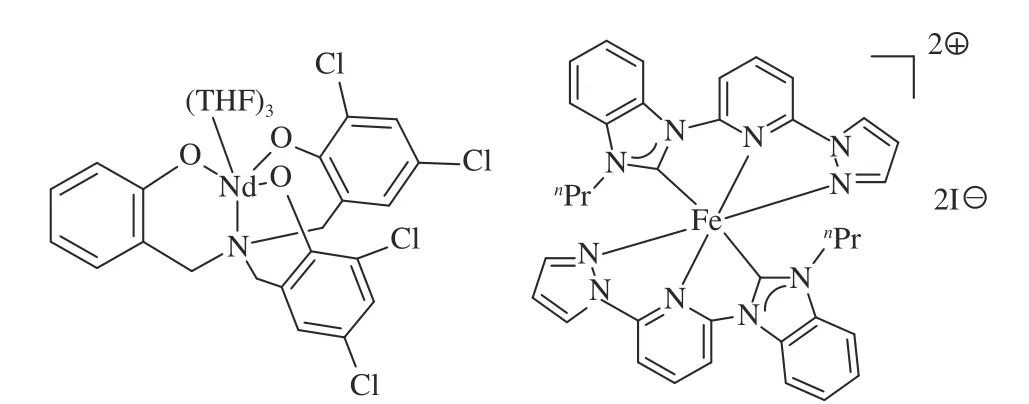
Figure 9 Structure of catalysts for catalytic reaction by CO2 with epoxides and amines[105,106](with permission from Wiley-VCH and Elsevier)
3.4 Heterogeneous catalyzed synthesis of oxazolidinones using CO2 with epoxides and amines
In 2020, Helal et al.[107]explored defectengineering metal-organic framework (UIO-66-40) as a catalyst for the reaction of amine, epoxide and carbon dioxide with solvent free at 85 °C in 12 h. It was the first successful report that MOF incorporated dual active sites catalyzed CO2-fixation to form oxazolidinones. At the same year, Feng et al.[108]prepared PVA(polyvinyl alcohol)-DFNT (dendritic fbrous nano-TiO2)/Ni NFs (nanofbres) as a catalyst to catalyze the cycloaddition of CO2, epoxides and amines.
In addition, a Nickel based Metal-Organic Framework (Ni-MOF) were synthesized and characterized as catalysts in the formation of 5-substituted oxazolidinones with amines, epoxides and CO2by Helal et al.[109]in 2021. The reaction can take place under milder conditions such as ambient pressure without any organic solvent. Simultaneously, either different para substituted aromatic amines or different epoxides as reagent can get good to excellent yields in this reaction using Ni-MOF as catalyst. The catalyst was found in a nine-run cycling reaction without any loss. Nevertheless, this method only worked for aromatic amines. Aliphatic amines were not doing anything in this condition. At last, the reaction process was studied and further study was carried out to deepen the understanding of the reaction.
4 Synthesis of oxazolidinones from CO2 and unsaturated amines
Unsaturated amines are terminal alkynes which can get by the chemoselective hydrogenation reaction from unsaturated nitro compounds[110]. They are considered as substrates to synthesize of alkaloids[111].Besides, they are also important reactants with carbon dioxide for the synthesis of oxazolidinones (Figure 10).
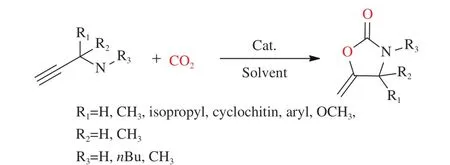
Figure 10 A route to synthesize oxazolidinones from unsaturated amines and CO2
4.1 Metal-complex catalyzed synthesis of oxazolidinones using CO2 with unsaturated amines
There is a common method to promote the reaction by using metal-complexes as catalysts. At first, the important role of transition metals--Ru[112]in the synthesis of oxazolidinones was studied. Ru with tricyclohexyphosphine in toluene can catalyze the reaction of N-substituted propargyl amines and carbon dioxide. Meanwhile, Mitsudo et al.[112]proposed a possible mechanism. Also, they explored the scope of amines.
Later, inspired by the previous studies, Shi et al.[113]developed the catalysis of another transition metals palladium in the process of oxazolidinones.They examined the catalytic performance of the catalyst formed by Pd or Ru compounds. The results indicated that when toluene as solvent, 5% Pd(OAc)2as a catalyst can obtain better yield than the others at 20 °C in the whole day.
In the above methods, there are higher pressure and longer time. Therefore, it is very necessary to reduce the reaction pressure and shorten the reaction time. Recently, García-domínguez et al.[114]still took Pd as a catalyst and CuI/DABCO as co-catalyst to promote the reaction process. In this catalytic system,propargylamine with higher steric hindrance were able to get moderate yields at 40 °C in 22 h under atmosphere. At last, they also explored the process of the reaction by controlling variables. Recently, the same catalytic system was reported by Ghosh et al.[115].It was different from the previous articles that they made use of Pd nanoparticles to activate carbon dioxide.
Besides, Ag transition metal and its derivatives usually as catalyst in organic chemistry[116]. Yoshide et al.[117]attempted to use this metal as a catalyst in the reaction of CO2with different unsaturated amines in 2009. It was the first report focused on silver salts and super base as a catalyst system for the reaction of unsaturated amines incorporation of carbon dioxide.Under the conditions of 0.1 MPa of CO2pressure and 25 °C in 1.5 mL of DMSO with 2% silver acetate, the yields up to almost quantitative. N-unsubstituted or Nheteroaryl-substituted, N-alkyl-substituted, and N-arylsubstituted all can get yields of >90%.
Following the above work, the important role of silver in the synthesis of oxazolidinones from unsaturated amines with carbon dioxide was further studied by the Shishido and his students[118]. They selectedN-benzyl-propargylamine as a model substrate in the initial conditions that is at 60 °C for 240 h. Later,they selected different catalyst and co-catalyst in DMSO or DMSO/H2O (10:1). Taking representative propargylic amines, they can get the best yield in DMSO with different amounts of AgNO3catalyst and DBU cocatalyst at 60 °C under atmosphere.
To sum up, silver was very important in the synthesis of oxazolidinone in activating unsaturated bonds in propargylic amines and it was the effective catalyst in the cyclization of propargylic amines and carbon dioxide. Other metals including Cu[119], Zn[120,121],Au[122,123]were all able to catalyze the cyclization of propargylic amines and carbon dioxide.
4.2 Base catalyzed synthesis of oxazolidinones using CO2 with unsaturated amines
Metal catalyst can promote the reaction, but it also brings a series of problems. In order to solve those problems, Costa et al.[124]reported that the reaction ofN-benzyl-N-(1, l -dimethylprop-2-ynyl)amine and 1 bar carbon dioxide took place smoothly in the conditions that is 7-methyl-1,5,7-triazabicycl [4.4.0] dec-5-ene(MTBD) as a catalyst in 20 °C for 24 h.
In 2008, another base DBU was reported as a catalyst to increase the reaction rate and improve the reaction yields by Yoshida et al.[125]. In this report, only spent 0.5−12 h they can get desired yields with DBU in acetonitrile under atmosphere at 80 °C.
Ionic liquid, as the most popular catalyst, is also indispensable in the synthesis of oxazolidinones from unsaturated amines and carbon dioxide because of its activation[126]. Han ’s group[127]found that 1-butyl-3-methylimidazolium acetate ([Bmim][OAc]) effectively catalyzed the cycloaddition of propargylamine and carbon dioxide. It can work with 1-butyl-3-methylimidazolium bis((trifluoromethyl)sulfonyl)imide([Bmim][Tf2N]) and get the best yield. Also, this group took DFT to prove the rationality of this method.
5 Synthesis of oxazolidinones from CO2 and dihalogenated compounds
Macé et al.[128]broke the rules by first proposing a one-pot reaction of fixing carbon dioxide with dibromo-alkane in atmospheric carbon dioxide and at lower temperature (Figure 11). By regulation of the temperature and pressure, it was finally determined that the reaction could get quantified yield at 60 °C with 2-(tert-butyl)-1,1,3,3-tetramethylguanidine (2-tBuTMG)as a catalyst for 20 h. The reaction mechanism was proved by using DFT method. Furthermore, the reaction was extended to larger rings which synthesized more drug molecules.
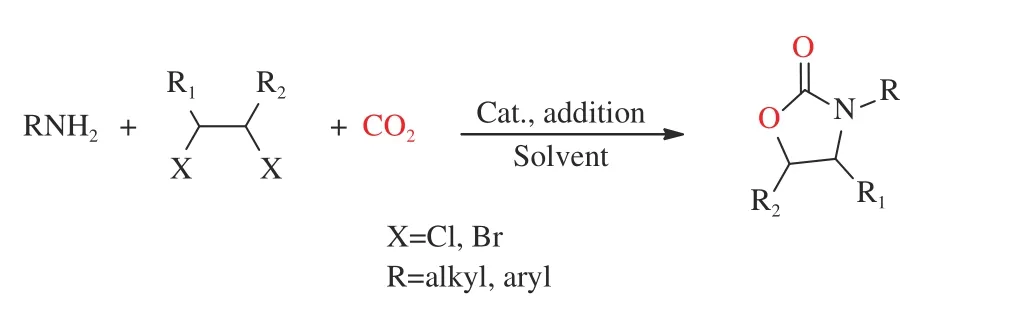
Figure 11 A route to synthesize oxazolidinones from dihalogenated compounds[128](with permission from Wiley-VCH)
With the continuous pursuit of green catalyst,[Bmim]OAc/Cs2CO3/Bu4NBr are used as catalyst to catalyze the three-component reaction of dichloroalkanes and amines as well as carbon dioxide without solvent at atmosphere pressure at 70 °C in 4 h[129]. Various amines incorporating naphthalenyl amines and other amines with steric resistance substituent can convert to the corresponding oxazolidinone successfully with excellent yield. To our surprise, larger membered rings were applicable in this method through replacing 1, 2-dichloroethane by longer chain. Recently, Chen’s group[130]discovered a one-pot reaction of dichloroaklanes and amine using superbase DBU as catalyst under CO2atmosphere Taking cyclohexylamine and 1, 2-dichloroethane as the initial reactant, it was found that the best yield was obtained under 1.0 MPa of CO2pressure at 80 °C within 12 h. Secondary amines was suitable for the reaction system, and it can get satisfactory yield. The possible mechanism of the reaction was explored by the control experiments. In the proposed mechanism,DBU coupled with CO2, and amine obtained the intermediate. O or N nucleophile can achieve two different pathways. Eventually, the DBU could be regenerated, and DBU was still able to catalyze other reactions, as shown in Figure 12.
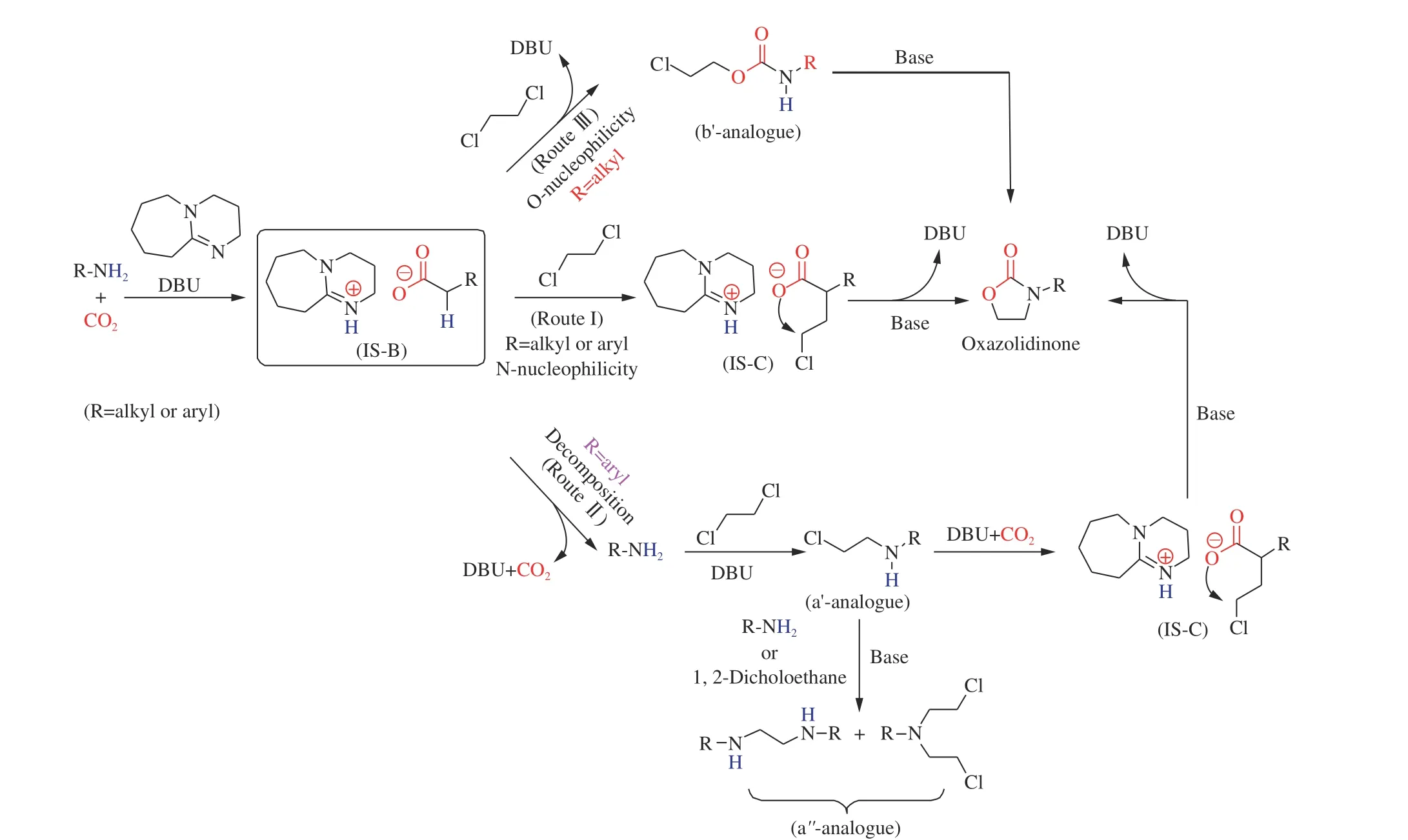
Figure 12 Plausible mechanism for DBU catalyzed synthesis of oxazolidinones from CO2, amines and 1,2-dichloroethane[130](with permission from Royal Society of Chemistry)
6 Summary and outlook
In conclusion, we have summarized the main progress on the synthesize of oxazolidinones from the reaction of carbon dioxide and other complexes. Metal catalysis and organic catalysis have been abundantly reported in this field, but it is still highly expected to produce more effective and greener methods for the production of oxazolidinones. In addition, a large-scale production in industry was required due to the extensive application of oxazolidinones in agriculture,medicine and daily life. But the currently methods can rarely achieve this goal, so further exploration of the synthesis of oxazolidinones was necessary. Further investigation on this field would be focused on the design of more effective and environmentally friendly catalytic systems and new reactions.

