Effects of metal doping on the catalytic performance of LaFe-based perovskites for CO2 hydrogenation to light olefins
MA Li-hai ,GAO Xin-hua ,ZHANG Jian-li ,MA Jing-jing ,HU Xiu-de ,GUO Qing-jie
(State Key Laboratory of High-effciency Utilization of Coal and Green Chemical Engineering, College of Chemistry &Chemical Engineering, Ningxia University, Yinchuan 750021, China)
Abstract: The catalytic behavior of K/LaFeBO3 (B =Cu, Zr, Al, Mn, Ni, and Zn) perovskite catalysts prepared by sol-gel and impregnation methods was investigated for CO2 hydrogenation to light olefins. The structure of various catalysts was characterized in detail by SEM, XRD, N2 adsorption-desorption, H2-TPR, CO2-TPD, TG, and XPS analysis. With the addition of Cu and Zn, the particles size decreased with high dispersion of Fe, while the exposed basic sites increased with lower hydrogen desorption temperature. The oxygen mobility in perovskites exhibited a considerable impact on catalytic activity and olefins selectivity, which considerably increased when Fe was substituted by Cu and Zn at the B site. Olefins were formed preferentially from oxygen species of the surface lattice with low binding energies (BEs). In addition, a faster diffusion rate of oxygen would lead to an enrichment of lattice oxygen species on the surface and increase the production of olefins.
Key words: CO2 hydrogenation;perovskite;LaFeBO3 catalyst;oxygen mobility
Carbon dioxide (CO2) capture and usage has received a lot of interest with the concerns of global warming and climate change. By integrating CO2capture and conversion, chemical looping combustion(CLC) presents a revolutionary strategy for reducing CO2emission and increasing its utilization. Chemical conversion of CO2by hydrogenation to methanol, light olefins (C=2−C=4) and other chemicals has attracted great attention. Especially, many progresses have been achieved for CO2selective hydrogenation to light olefins[1−4].
Two independent reactors make up the CLC system[5]. During the CLC process, fuel reduces metal oxide to a reduced state in a combustion reactor,whereas oxygen oxidizes the reduced state to an oxidized state in an air reactor. Fe-based perovskite composites can be used as oxygen carrier with promising CLC performance[4]. In addition, the reduced Fe-based composite in the outlet of combustion reactor can be applied for CO2hydrogenation via reverse water gas shift reaction (RWGS) and Fischer-Tropsch synthesis (FTS) to light olefins in a hydrogenation reactor. The hydrogenation-induced phase shift of iron oxides occurs between different reactors (Figure 1).The construction of oxygen carrying carrier and CO2activation bifunctional active centers of Fe-based oxygen carriers is the key to the coupling technology of chemical looping CO2capture and its utilization.
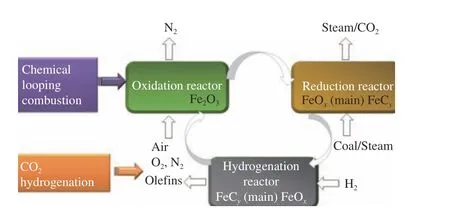
Figure 1 Schematics of chemical looping coupled hydrogenation process
The deactivation of metal oxide during numerous redox cycles is the key problem to be solved in CLC process[5,6]. A crucial point in developing chemical looping coupled with CO2hydrogenation systems is the selection of a high-performance oxygen carrier that can be reduced and oxidized repeatedly without deactivation. Fe-based catalysts have already been employed for the CO2hydrogenation reaction. CO2hydrogenation over Fe-based catalysts is generally based on a RWGS-FT process, during which CO is firstly generated by reverse water-gas shift (RWGS),followed by CO hydrogenation to various hydrocarbons via FT reaction[3]. However, the sole Febased catalysts always possess poor selectivity to light olefins and are prone to deactivate due to carbon deposition and sintering. Many metal promoters have been introduced for CO2conversion to increase the selectivity to light olefins[7]. For example, with the addition of copper, less methane was formed with higher activity[8]. Mn promoter, on the other hand, can considerably reduce the production of carbon deposition and improve catalyst stability[9]. By encouraging additional lattice oxygen vacancies and electron pairs for the catalyst, Zn can improve the activity of Fe catalysts[10]. The basicity of the catalyst surface can be adjusted by Al and Zr to ensure adequate CO2adsorption. The addition of Ni with a high hydrogenation capability also enhanced CO2conversion and the production of more active metals[7,11−13]. Additionally, alkali metals including sodium and potassium can be used as electron promoters to provide electrons to thedempty orbitals of iron or other metals, effectively promoting CO2adsorption while suppressing hydrogen adsorption. As a result, methane formation was suppressed to benefit light olefin production[14].
Perovskite oxides with ABO3structure can be considered as an acid-base catalyst with the presence of both metal (cationic) and oxygen (anionic) vacancies,which can tackle the problem of rapid deactivation caused by metal particle aggregation at high temperatures. Some perovskite oxides have been successfully applied for chemical looping coupling RWGS process[15,16]. It was suggested that the oxidation state mismatch in the ABO3structure causes charge imbalance and increases oxygen vacancy. The modification of transition metal on iron-based perovskite oxides enhanced the stabilization of the anomalous cationic oxidation state in the RWGS process by increasing the contact strength between active species and carriers. However, the specific surface area of perovskite oxides is always rather low with few active sites, which limits reactant absorbtion and thus decreased the activity. After reduction treatment and under reaction procedures, the perovskite is partially reduced. Reduced lattice cations move to the surface, where they exsolve and form metallic nanoparticles[17]. During CO2activation, C−O bond breaks on the catalyst surface, and the resulting oxygen atom diffuses into the bulk. Some studies have indicated that the nanoparticles formation by exsolution improved sintering stability due to “anchoring”, even at high reaction temperature.
Due to the complexity of the chemical compositions, topologies, and phases, the reduction and activation ability of perovskites with ABO3structure in CO2hydrogenation need to be further investigated. In this study, the catalytic behavior of LaFeBO3perovskite catalysts (B= Cu, Zr, Al, Mn, Ni, and Zn) in CO2hydrogenation was investigated. Detailed characterizations were used to establish the correlation between the catalytic performance and structure. The anchoring effect of reduced metal nanoparticles on oxygen mobility and olefin selectivity was also discussed.
1 Experimental
1.1 Catalysts preparation
Sol-gel and impregnation methods were used to prepare K/LaFeBO3(B =Cu, Zr, Al, Mn, Ni, Zn)catalysts. Typically, an adequate amount of various metal nitrates and citrate acid were dissolved in deionized water to form a mixed salt solution with molar ratios of La∶Fe=2 and Fe∶B=1. The prepared solution was then heated and stirred in 80 °C water bath for gel formation, which was then dried in an oven at 105 °C for 12 h. The dried samples were calcined at 400 °C for 1 h and 750 °C for 5 h, under static air with a ramp of 5 °C/min. The obtained perovskites were denoted as LaFeBO3(B=Cu, Zr, Al, Mn, Ni, and Zn).Finally, the samples were impregnated with potassium carbonate (2%).
1.2 Structure characterizations
Scanning electron microscopy (SEM, KYKY-2008B) was used to examine the morphology of all fresh and used catalysts at a voltage of 25 kV.
X-ray diffraction (XRD) patterns were conducted using monochromatic CuKα radiation and a Rigaku D/MAX2200PC spectrometer at 40 kV and 30 mA.The samples were scanned from 5° to 80° with scanning rate of 8(°)/min.
N2-physisorption measurement was carried out on a Micrometrics ASAP2460 instrument after all catalysts were vacuum purged at 300 °C for 3 h. The specific surface areas, average pore diameters, and pore volumes of the catalyst samples were obtained by Brunauer-Emmet-Teller and Barrett-Joyner-Halenda methods.
H2temperature-programmed reduction (H2-TPR)was tested using a Micromeritics AutoChem II 2920 chemisorption analyzer with a thermal conductivity detector (TCD). 50 mg of sample in a quartz tube was first flushed with He (30 mL/min) at 350 °C for 1 h to remove moisture before cooling to 50 °C. After that,the temperature was raised to 800 °C at a rate of 10 °C/min with a flow of 10% H2/Ar. The H2consumption was recorded simultaneously.
Temperature-programmed CO2desorption (CO2-TPD) was performed in-situ on a Micromeritics AutoChem II Chemisorption Analyzer. A 50 mg sample was reduced by 10% H2/Ar for 800 °C before being purged for 1 h with He (30 mL/min). After cooling in a He flow, the sample was pulse-adsorbed by CO2at 50 °C until saturation was obtained, followed by being purged with He for 1 h. The desorption signal was captured while the temperature was raised from 50 to 800 °C at a rate of 10 °C/min.
Thermogravimetric analysis was carried out using an SDT Q600 simultaneous thermal analyzer (TA instruments). Under CO atmosphere, the weight loss of the catalysts was measured from room temperature to 800 °C at a heating rate of 10 °C/min.
The elemental composition of the sample was analyzed by Agilent-5110 measurement as an energy dispersive X-ray spectrometer.
X-ray photoelectron spectroscopy (XPS) for all catalyst samples was performed with an AlKα X-ray source using a Thermo Scientific ESCALAB 250 spectrometer. The base pressure in the chamber was less than 2 × 10−8Pa. The C 1speak at 284.8 eV was used to calibrate binding energies (BEs) in relation to adventitious carbon.
1.3 Catalytic performance evaluation
The catalytic performance for CO2hydrogenation over various samples was tested in a stainless steel fixed-bed reactor (i.d.=8 mm; length=400 mm). 1 mL of catalyst and 2 mL of quartz sand were packed in the centre of the tube with a thermocouple in contact with the catalyst to measure the temperature of the catalyst bed. The perovskite was reduced for 4 h at 800 °C in H2flow prior to the catalytic test. CO2hydrogenation was carried out att=320 °C,p=2.0 MPa, GHSV=1000 h−1, and H2/CO2=3 (molar ratio). The gaseous products and reactants were detected using an online gas chromatograph (GC-9560) with a TCD and a flame ionization detector. The product selectivity was calculated on a carbon basis. CO2conversion (xCO2)and product selectivity (si) were calculated by Eqs. (1)and (2):
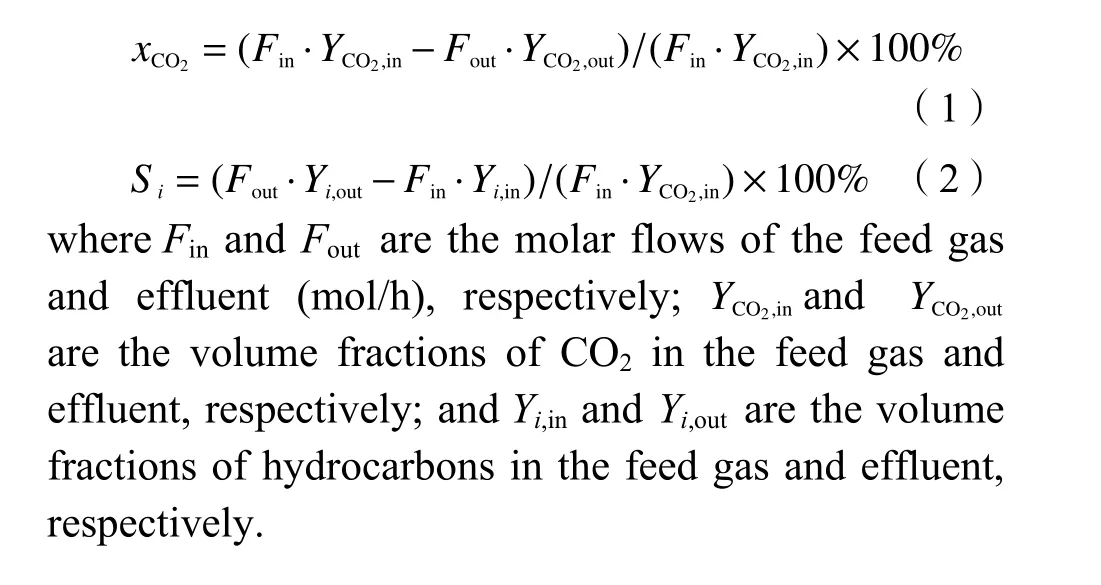
2 Results and discussion
2.1 XRD measurement
The typical formula for perovskite oxides is ABO3, with large ions occupying the 12-coordinated A sites (rA>0.90 Å) and transition-metal cations occupying the 6-coordinated B sites (rB>0.51 Å).Because of the extraordinary stability of the perovskite structure, many metals with differing oxidation states can be partially substituted for the cations in the A or B sites, resulting in structural defects such as cationic or anionic vacancies[18]. Figure 2 shows the diffraction peaks of LaFeO3perovskite structure (JCPDS 37-1493). When Cu, Mn, Zn, Al, and Zr are doped, the LaFeO3structure can be well preserved. However, the intensity of diffraction peaks is weakened and new phases including ZnFe2O4, FeAlO3, and La2Zr2O7are formed. In addition, doping Ni in LaFeO3caused the formation of NiFe alloy. The orthorhombic perovskite structures with the (121) characteristic reflection peak can be observed. Partial substitution of the B site results in a smaller pseudo cubic unit cell for perovskite structure with a slight shift to higher 2θdegree. As shown in Table 1, compared with LaFeO3,the crystalline size calculated by Scherrer equation decreased when different metals were doped. The grain size was found to be 14.8 nm when Mn was doped,while a higher grain size of 30.8 nm was obtained by Cu doping.
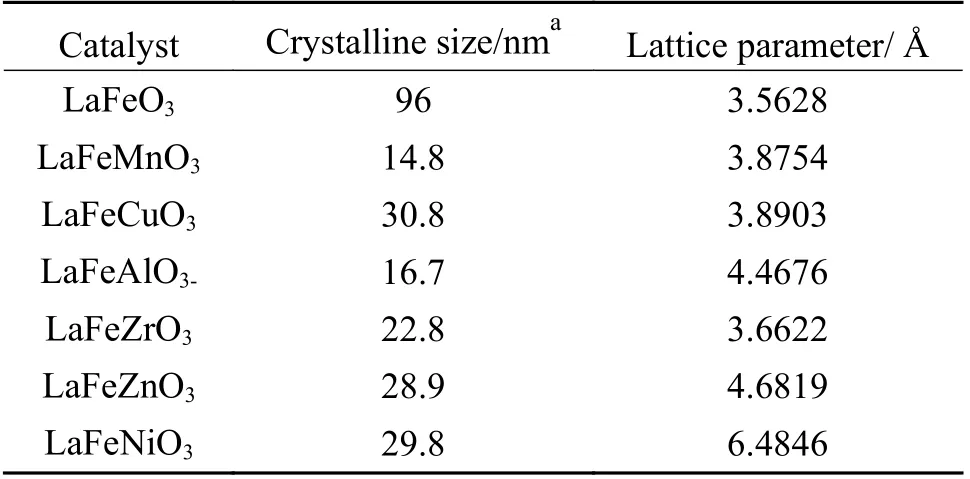
Table 1 Crystalline size of various catalyst samples
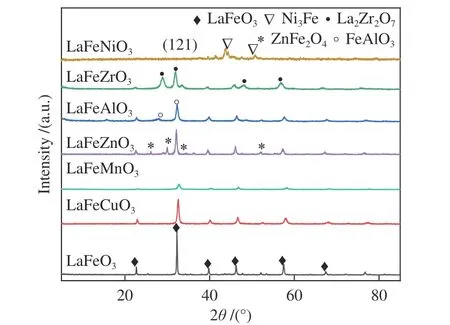
Figure 2 XRD patterns of the calcined catalyst samples
2.2 Textural properties
SEM images of the LaFeBO3catalysts revealed the existence of mesoporous structure (Figure 3), which was further evidenced by N2physisorption data(Figure 4). It can be seen that LaFeO3is made up of closely packed sphere-like nanoparticles with an average particle size of 80–100 nm (Figure 3(a), (b)).Small pores on the particle surface following Zr doping, as shown in Figure 3(f), may be caused by nitrate thermal decomposition. It produces flakes in which LaFeZnO3is surrounded with stacked and scattered particles between the lamellae after doping Cu (Figure 3(d)), Al (Figure 3(e)), and Zn (Figure 3(g)).Exsolved nanoparticles are evenly dispersed on the surface, which are formed from the perovskite lattice structure. For Zn doped materials, the surface active sites of LaFeO3are rougher with bubble pores, which is conducive to the adsorption of gas to a certain extent. It is expected to show higher catalytic hydrogenation activity[19,20]. The spherical particles became smaller after Ni doping with particle sizes of 20–30 nm (Figure 3(h)).
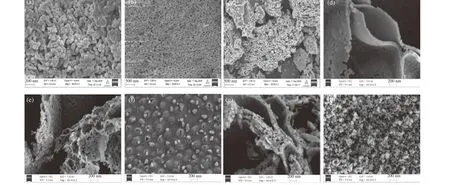
Figure 3 SEM images of LaFeO3 ((a), (b)), LaFeMnO3 (c), LaFeCuO3 (d), LaFeAlO3 (e),LaFeZrO3 (f), LaFeZnO3 (g) and LaFeNiO3 (h)
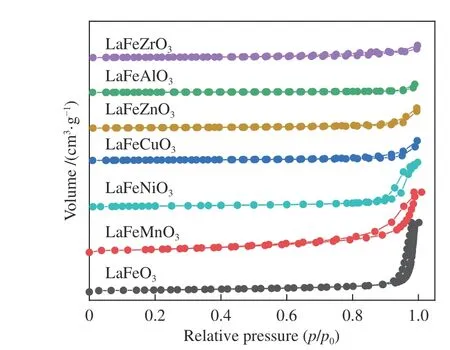
Figure 4 N2 adsorption-desorption isotherms of various samples
The LaFeBO3(B =Cu, Zr, Al, Ni, and Zn)catalysts exhibit low BET specific surface areas, as seen in Table 2. Metal doping promotes the formation of small grain size and small specific surface area except for LaFeMnO3sample, which is related to the weak crystallinity combined with XRD data. The samples with the addition of Zr or Zn have average pore diameters of about 10 nm, which is about a quarter of that of LaFeO3. The type II isotherm with H3 type hysteresis loop indicates the mesoporous structure(Figure 4) suggests the existence of mesoporous structure in perovskite. However, the hysteresis loop of the isotherm is small and the mesoporous structure is limited.
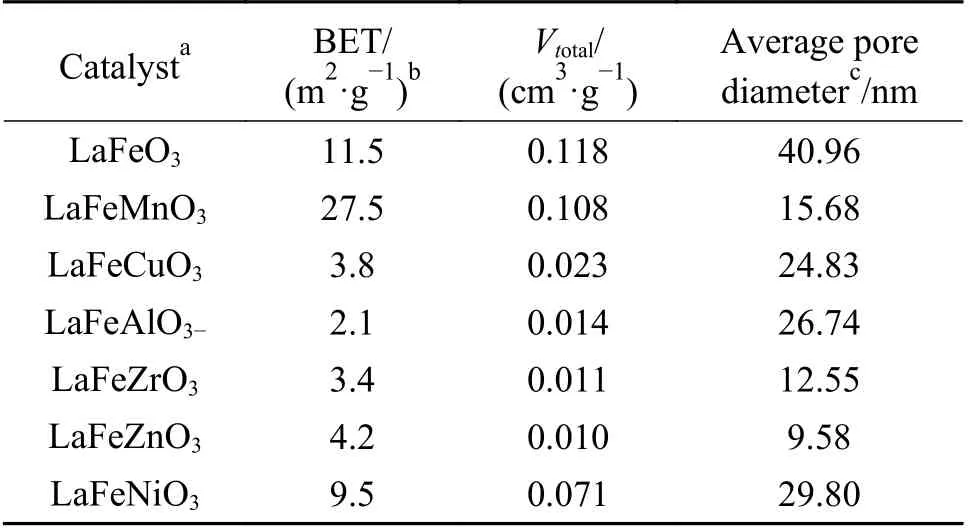
Table 2 Texture properties of LaFeBO3 catalyst samples
2.3 H2-TPR experiments
H2-TPR was employed to investigate the reducibility behavior of various perovskites. There are three peaks for the undoped LaFeO3at 388, 480, and 620 °C, which could be attributed to the reduction of Fe3+to Fe2+and then the reduction of Fe2+to Fe0,respectively (see Eq. (3))[21,22], indicating the first transformation of Fe2O3to Fe3O4and further reduction of FeO to Fe. In the case of metal iron-doped LaFeO3,the number of peaks was reduced (Figure 5). When Cu,Zr, Al, Mn, Ni, and Zn are added to the B-site of Febased perovskites, low valence Fe ions are formed (see Eq. (4)), which promotes the creation of oxygen vacancies. The impact will be similar if Fe3+is replaced with other metal ions.
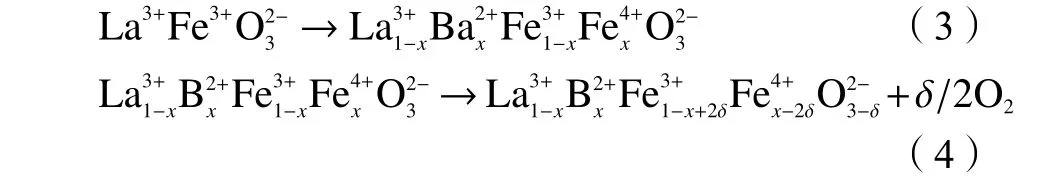
By replacing the lower valence B2+for Fe3+, the fraction of oxygen vacancy defects and the active oxygen quantity can be changed. By replacing B3+for Fe3+, the properties of transition metal cations and oxygen mobility can be significantly altered. When Ni2+and Cu2+were replaced for Fe3+, increased hydrogen consumption was observed. The materials provide more active oxygen at low temperatures due to the relatively simple redox reaction, which may be attributed to a quicker oxygen diffusion rate in the bulk with increased Fe substitution[23]. Fe is rather easy to be reduced after Cu doping, indicating that the LaFeCuO3catalyst contains more active oxygen at lower temperature. The LaFeCuO3catalyst has a quicker oxygen diffusion rate and effectively increases the desolvation of active metals, enhancing the catalytic activity of perovskites. Although the inclusion of Ni also enhances oxygen mobility compared with undoped LaFeO3, the H2consumption of Ni varies from Cu,which can be interpreted by different metal reduction ability under H2atmosphere. The maximum temperature of Al and Zr doped LaFeO3increased in the H2-TPR experiment (Figure 5). The presence of Fe and Zr/Al in the B-site of perovskite structure increases the amount of Fe ions while decreasing the number of oxygen vacancies, which is more likely to stabilize a high oxidation state[10,24].
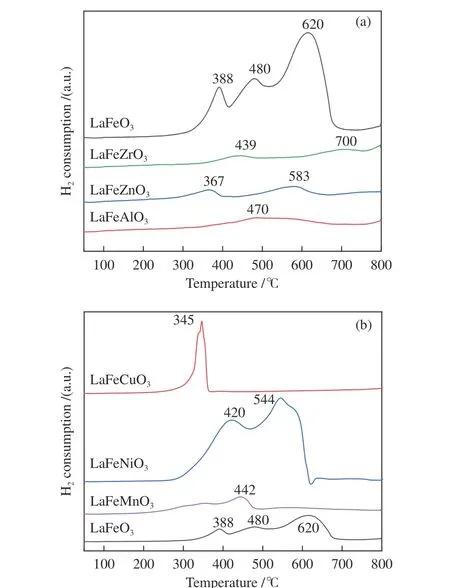
Figure 5 H2-TPR profiles of the LaFeBO3 samples
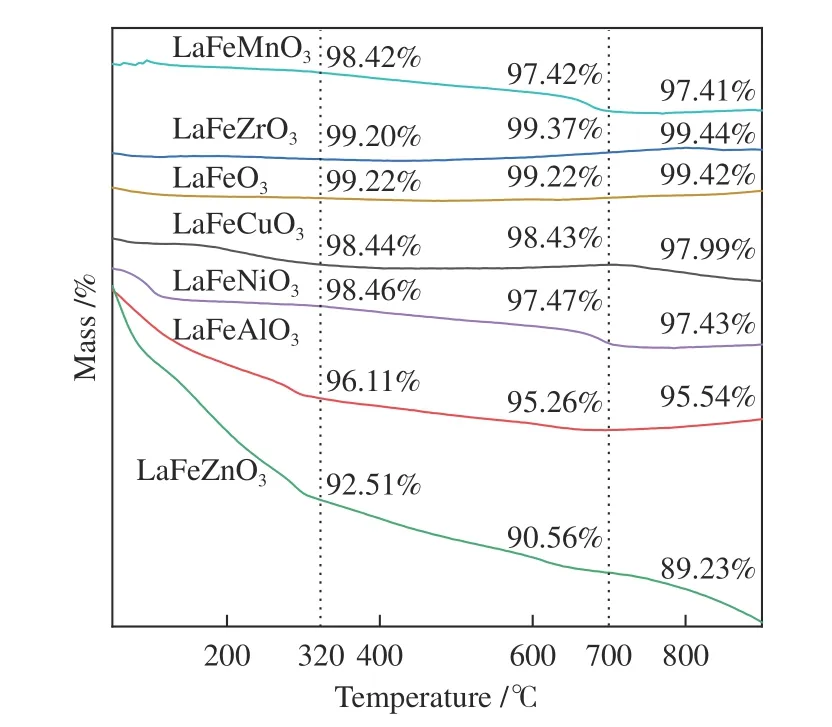
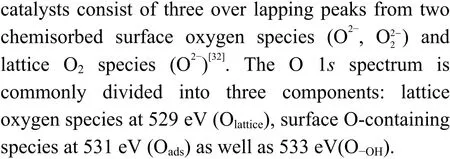
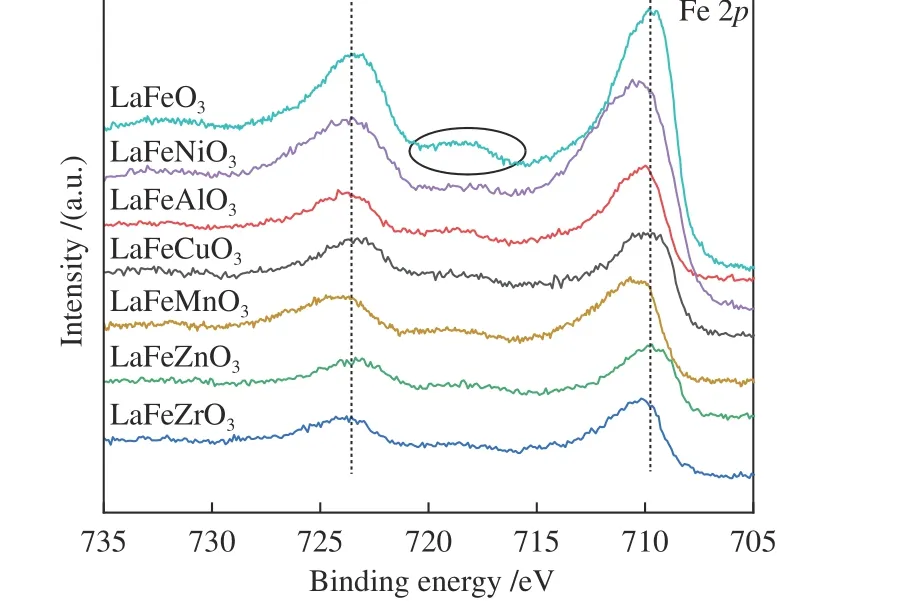
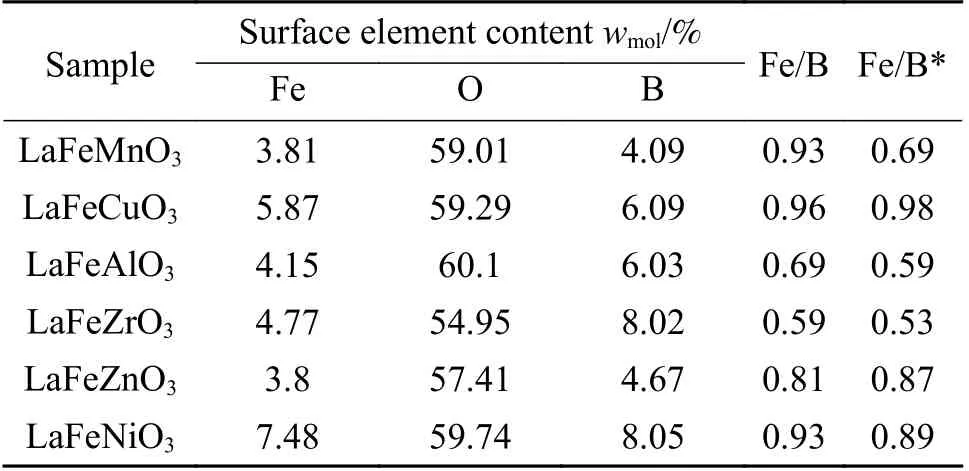
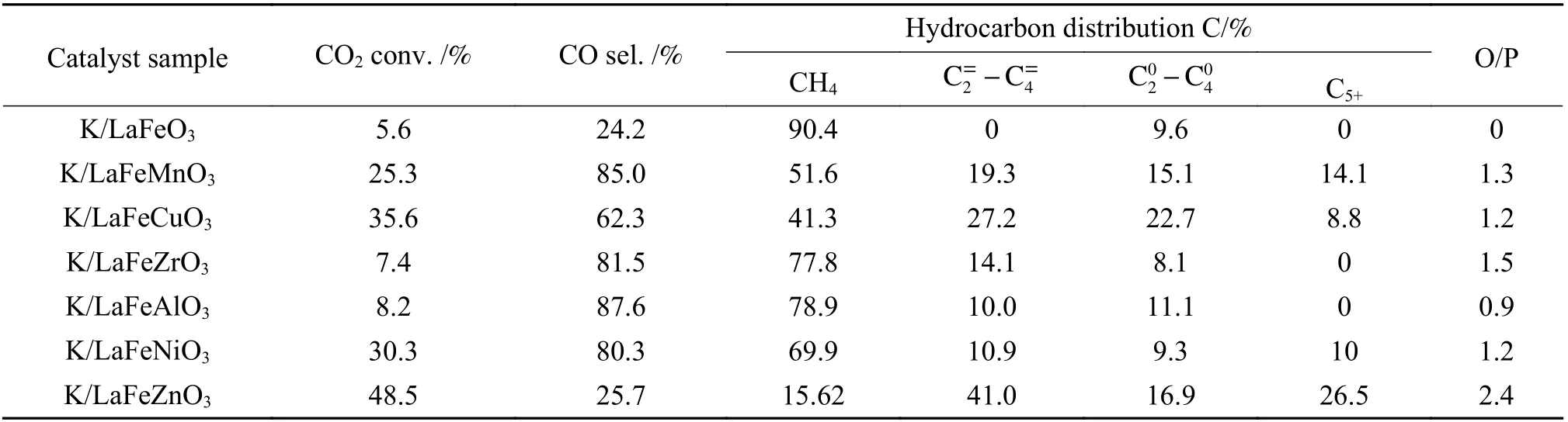
The peak region of the H2-TPR spectra is used to study the reduction behavior for each catalyst. The sequence of initial reduction temperature is LaFeCuO3 The CO2-TPD method was used to investigate the change in surface basicity of metal doped Fe based perovskite (Figure 6). Results revealed that doping Al,Mn and other elements at the B position showed a significant effect on the CO2desorption of perovskite.The CO2desorption curves can be divided into three stages. Weak CO2adsorption on the surface of perovskite samples was observed at low temperatures(50–150 °C). The second range is at 150–450 °C,indicating that CO2adsorption occurred at moderate basic sites. The third range is at about 450–750 °C,indicating that CO2was adsorbed on strong basic site of perovskite. When the B-site was doped with Zn, Zr,and Cu, the content of basic sites on the surface of the corresponding perovskite decreased slightly. Over LaFeNiO3, the amount of CO2adsorbed at weakly basic sites was almost disappeared. When Al and Mn were doped at the B site, the amount of adsorbed CO2was increased. Therefore, doping Al and Mn could enhance the surface basicity of perovskite[25], while doping Zn,Zr, Cu, and Ni will weaken the surface basicity[26]. Figure 6 CO2-TPD profiles of the catalyst samples The oxygen migration ability of doped metal perovskite was studied by characterizing the oxygen loss of the catalyst in CO atmosphere (see Figure 7).Except Mn doped LaFeO3, all other samples showed certain oxygen loss, especially for the Zn, Al and Cu doped samples. The properties of Zr doped and undoped samples are basically the same. The weight loss curve of Cu doped catalyst decreased slowly. By simulating the transformation of doped perovskite in reducing atmosphere, it can be judged from the slope in Figure 8 that their oxygen migration rate is greatly affected. When the active particles are exposed to the reducing gas, the active oxygen easily reacts with the reactants, and due to the chemical potential gradient,the oxygen anion penetrates from the bulk to the surface to improve the catalytic efficiency[22,27].Combined with the catalytic performance, it was found that the oxygen mobility or the generation ability of oxygen vacancy in perovskite had a significant effect on the catalytic activity and product selectivity. Figure 7 TG analysis of the catalyst samples Figure 8 Survey XPS profiles of LaFeBO3 samples Figure 9 XPS spectra of O 1s for different samples LaFeO3 (a), LaFeCuO3 (b), LaFeAlO3 (c), LaFeZrO3 (d), LaFeZnO3 (e), LaFeNiO3 (f), and LaFeMnO3 (g) It can be seen that the binding energy of O 1sin Fe based perovskite largely depends on the type of Bdoped metals, which has a great effect on CO2hydrogenation. The difference in the binding energy of O2−is due to the electronegativity difference of metal cations interacting with O anions. LaFeBO3perovskite catalysts contained lattice oxygen species with varying O 1sBEs and demonstrated a remarkably different selectivity of olefins in CO2hydrogenation, depending on the B site components[19]. In Figure 10, the Fe 2ppeaks in the perovskite are concentrated at 709−711 eV and 722−724 eV, which is attributed to Fe3+characteristic peak. Peak located at (717±0.4) eV belongs to the α-Fe2O3satellite peak. Affected by metal doping, the α-Fe2O3satellite peak disappears, thus the chemical environment around Fe changes greatly,which in turn affects the hydrogenation reaction behavior. Figure 10 XPS spectra of Fe 2p for different samples In conclusion, surface oxygen vacancies generated by the interaction of lattice oxygen with CO2were filled with adsorbed surface oxygen and bulk lattice oxygen species. As a result, oxygen mobility in perovskites has a considerable impact on the catalytic activity and product selectivity. The surface oxygen concentration or oxygen vacancy concentration, which is determined by the bulk oxygen concentration and the relative rate of lattice oxygen transport compared to the surface reaction, is a major determinant of the catalytic performance of perovskites in CO2hydrogenation[33].Besides, the chemical compositions and states of catalysts have been investigated in this study. These values were investigated and calculated from ICP and XPS spectra, as shown in Table 3. The bulk compositions of Fe and B species for LaFeBO3showed slightly lower surface concentrations of Fe and B species. The internal element content shows divergence from surface, which correlates the difference of Cu and Ni reduction ability in previous H2-TPR experiment explicitly. Table 3 Surface composition of the catalysts determined by XPS The catalytic performance for CO2hydrogenation is shown in Table 4. K/LaFeO3has the lowest activity with methane as the main product. However, with the addition of metal dopants, the catalytic activity increased. Metal doping is beneficial to expose more active sites and promote the CO2hydrogenation over LaFeBO3catalysts. Cu and Zn modified perovskite exhibit higher activity with 27%–41% of C2–C4olefins in total hydrocarbons. Table 4 Catalytic activity of different catalysts The LaFeBO3catalysts have a strong RWGS activity aside from Zn doping. CO selectivity reach as high as 80%−92%. The varied activities of LaFeBO3catalysts are most likely caused by the distinct characteristics of the lattice oxygen species, which are governed by the metal elements in the B sites.Nanoparticles and sintering are inhibited by the“anchoring” of the reducible lattice cations, which migrate and penetrate to form nanoparticles and diffuse on the surface of the materials[18,25]. The doped Zn and Cu boost the activation of oxygen anion in reducing gas and increase the number of exposed particles. Due to the presence of a chemical potential gradient, the active oxygen rapidly combines with it and permeates from the bulk phase to the surface, boosting catalytic activity and facilitating in the production of olefins. Sol-gel method was utilized to successfully prepare Cu, Zr, Al, Mn, Ni, and Zn-doped LaFeO3perovskites, and the potential of LaFeBO3perovskites as oxygen carriers for CO2hydrogenation was investigated. Increased catalytic activity are expected for LaFeBO3perovskites. Doping the catalyst with a second metal/metal oxide improved olefin selectivity by producing a highly active surface. The perovskites feature a tunable host lattice that allows for effective CO2adsorption, activation, and lattice oxygen migration, as well as the ability to improve catalytic activity. CO2conversion was facilitated by oxygen species in the surface lattice. Substituting Cu and Zn for Fe at the B site, in particular, considerably enhanced oxygen mobility and displayed high activity in CO2hydrogenation. In addition, Zn doped perovskite showed high selectivity to light olefins for CO2hydrogenation.2.4 CO2-TPD measurement
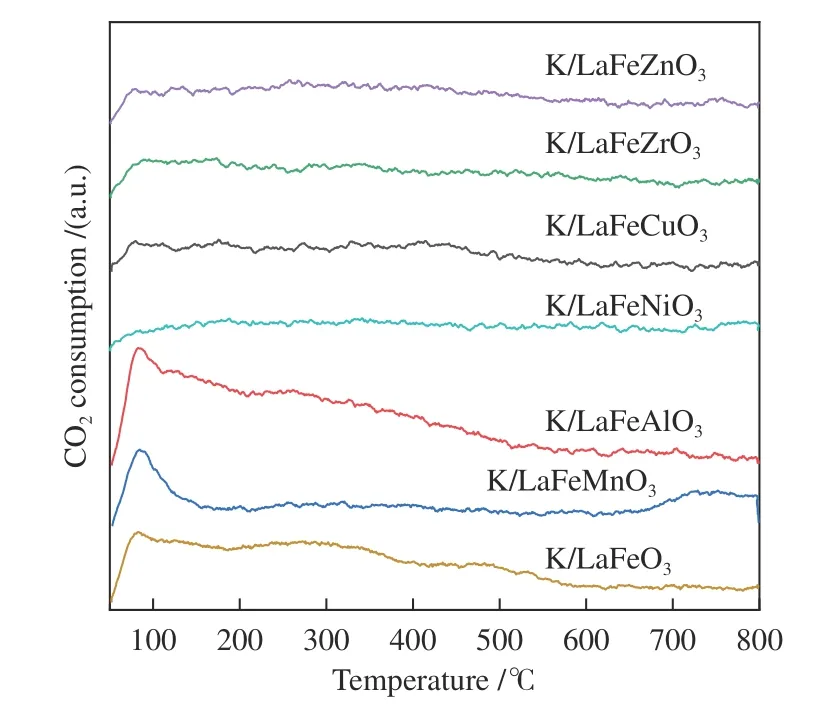
2.5 TG experiments
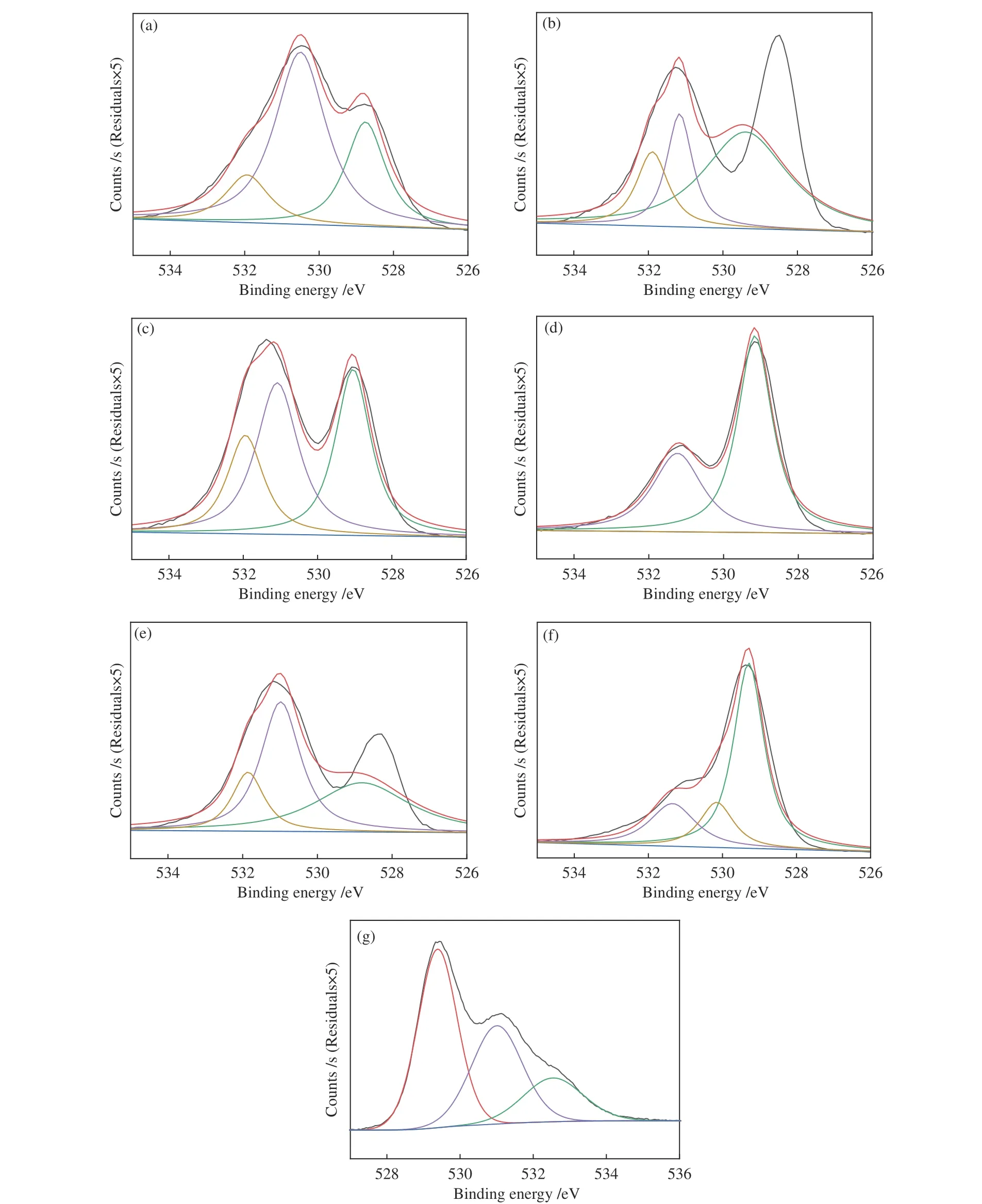
2.6 XPS measurement
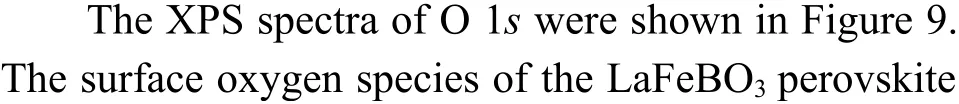
2.7 Catalytic performance
3 Conclusions

