磷酸铁锂废料中FePO4·2H2O提取及其杂质形成机理
黄 玲 张成智 谭 军 李穗敏
(季华实验室,佛山 528200)
0 Introduction
Lithium iron phosphate(LiFePO4,LFP)cathode has been widely used in electric vehicles due to its low cost,high safety,and long cycle life[1‑2].Approximate 13 000 t of LFP material was used for LFP batteries in 2014,due to the rapid development of EVs(electric vehicles)and HEVs(hybrid electric vehicles),the use of LFP material has risen to 124 000 t in 2020[3].As can be known,the average service life of LFP batteries is 8‑10 years,indicating that massive spent LFP batter‑ies will be generated during the next decades[4].LFP batteries are often called eco‑friendly green batteries because of no mercury,cadmium,lead,or other toxic heavy metals[1].However,LFP batteries consist of about 1.5% Li,15% Fe,9% P,15% organics,and 7% plas‑tic[5].These can also lead to serious safety issues and environmental pollution if not disposed of properly[6].Lithium recovery from LFP alone is hardly profitable due to the low lithium content in the spent LFP batter‑ies.Therefore,it is very critical to recycle other ele‑ments such as Fe and P from spent LFP batteries to improve the economic feasibility of LFP recycling.
Among the currently available recovery approach‑es for spent LFP batteries,there are two main ways of LFP battery recycling:direct regeneration and hydro‑metallurgical methods.In the direct regeneration pro‑cess,the waste LFP powders are usually treated by pyro‑metallurgical by adjusting the molar ratio of Li to Fe to P by metal salts[4].This process is quite quick and sim‑ple.However,the regenerated LFP materials often dis‑play poor electrochemical performances because of haz‑ardous elements such as Al and Cu[7].For the hydromet‑allurgy methods,various acids are used to leach waste LFP powders,including H2SO4,HCl,HNO3[7‑10],et al.Then the Li,Fe,and P are extracted in the form of Li2CO3,FeCl3,Li3PO4,Fe(OH)3,or FePO4·2H2O in the leachate.The above‑mentioned regeneration of LFP is effective but complex and high cost.In addition,the solution after leaching treatment is not purified,which will affect the purity of the final product.Some researchers have combined the above two methods to recycle waste LFP powders with a selective leaching‑calcining regeneration method.In this process,Li is firstly selective leaching by Na2S2O8,Fe2(SO4)3,acetic acid,et al.[7‑9],and then is extracted in the form of Li2CO3or Li3PO4in the leachate which has been puri‑fied.Meanwhile,Fe3+is employed to exchange the Li+and Fe2+during the leaching process,and then is com‑bined with PO43−and remains in the leaching residue in the form of FePO4.Then the leaching residue is cal‑cined to eliminate impurities,such as the acetylene black and polyvinylidene fluoride(PVDF),resulting in FePO4.However,this leaching‑calcining method still needs to be further optimized.For example,the selec‑tive leaching process needs to use expensive reagents and leads to high energy consumption.Significantly,iron phosphate obtained by the above methods cannot be used as the precursor of LFP because of uncertain impurities[11].
In our previous studies[12],we have successfully used NaOH solution and sulfuric acid as the leachate for the waste LFP powers and have obtained high‑purity Li‑Fe‑P leachate.We then recycled the Fe and P ele‑ments in the form of FePO4·2H2O at different pH and temperatures.However,the obtained FePO4·2H2O pow‑der shows different colors and exhibited different capacities,indicating that there are some impurity phases in FePO4·2H2O.In this work,potential(φ)‑pH diagrams of the Fe‑P‑Li‑H2O system were investigated,which have original thermodynamic guidance on the experiment process to prepare high‑purify FePO4·2H2O.Single‑factor experiments were performed to evaluate the influence of parameters such as pH value,tempera‑ture,and the initial molar ratio of Fe to P(nF∶enP).This FePO4·2H2O can be applied to synthesize LFP with excellent electrochemical performance.This optimal process with simple operation,low cost,and high‑added value is a feasible and promising way to recycle Fe and P from spent LFP batteries.
1 Experimental
1.1 Materials
The chemical reagents used in this work included phosphoric acid,ferric sulfate,30% hydrogen perox‑ide,and sodium hydroxide,all of which were of analyti‑cal grade.The waste LFP powders obtained from spent batteries mainly existed as 79.87% LFP,9.84% carbon,and 9.91% PVDF,and the main hazardous elements were 0.31% Al,0.01% Cu,0.05% Ni,and 0.20% F.This waste powders were first leached by NaOH solu‑tion and then H2SO4[12].The powders and 2 mol·L−1NaOH solution were placed into a continuously stirred tank reactor(CSTR).The leaching parameters were 40 ℃ of temperature,10∶1 of liquid to solid ratio,and 300 r·min−1.After 60 min of leaching,the slurry was filtered off and washed with high‑purity water.Alkali leaching was used to remove Al.Then,alkali leaching residue was leached by 2 mol·L−1H2SO4at 31 ℃ for 180 min.The liquid‑to‑solid ratio was 12∶1 and the mixture in the reactor was stirred at a speed of 400 r·min−1.Finally,an acidic leaching liquid was obtained.The composition of this acidic leaching solution wasshown in Table 1.As shown in Table 1,the molar ratio of Li to Fe to P was 1.028∶1.000∶1.033.
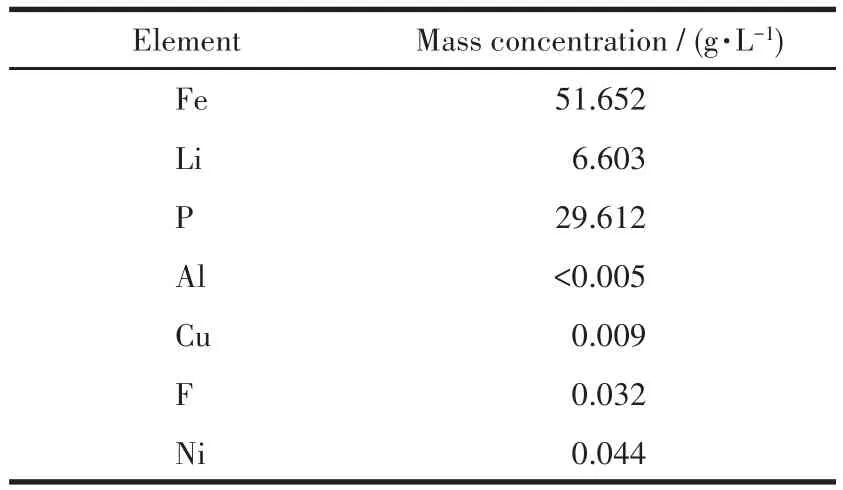
Table 1 Composition of leaching solution
1.2 Experimental procedure
The nF∶enPin the leaching solution was adjusted by analytical grade phosphoric acid and ferric sulfate,and Fe2+was oxidized to Fe3+by 30% hydrogen perox‑ide.This obtained solution was placed into a three‑necked round‑bottomed flask reactor which was equipped with an impeller stirrer.3 mol·L−1NaOH solution was slowly added into the reactor.A pH meter was used for online measure the pH of the solution in the reactor.Then this reactor was placed into an electro‑thermostatic water bath.The operational variables were set as follows:the end pH values of the reaction(1.5‑2.2),temperature(333 and 363 K)and nF∶enP(1∶1 and 1∶2).The obtained white slurry was aged for 3 h at 363 K,separated by filtration,and washed with a hot NaOH solution(353 K,1 mol·L−1)and distilled water,result‑ing in a white precipitate(ferric phosphate hydrate)and a colorless filtrate.The white precipitates were dried at 353 K for 24 h and then sintered at 973 K for 6 h under a flowing pure argon atmosphere to obtain FePO4powder.The white precipitates and sintered samples obtained at different conditions were marked and listed in Table 2.The resulting precursor P‑l1 and Li2CO3were mixed innF∶enLi=1∶1.05.The mixture was preheated at 500℃for 4 h and subsequently sintered at 820℃again for 12 h under a flowing gas mixture(95% N2and 5% H2)to obtain LFP materials(R‑LFP).
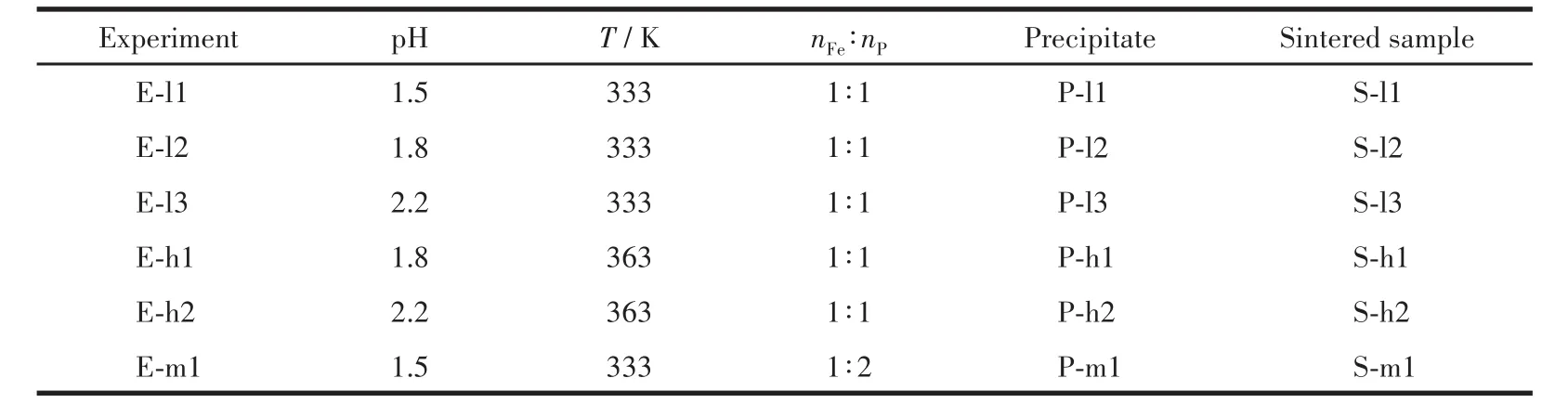
Table 2 Precipitation conditions and corresponding samples
1.3 Analytical methods
The contents of Fe,P,and Li in the filtrate were measured by inductively coupled plasma atomic emis‑sion spectroscopy(ICP‑OES,Themo Fisher,iCAP7000 Plus,1300 W,Ar flow of 12 L·min−1).The FePO4sam‑ples were prepared for ICP‑OES analysis to evaluate the concentration of Li,P,Al,Cu,and Ni.The content of Fe in samples was determined by dichromate titra‑tion.The change in pH of the solution was determined by a pH meter(333‑423 K,Shanghai INESA).The structure of samples was identified by X‑ray diffraction(XRD,Bruker D8 Discover,40 kV,40 mA)with Cu Kα radiation(λ=0.154 18 nm)at 4(°)·min−1from 10°‑90°in two thetas.The thermal properties of precipitates were analyzed by thermogravimetry(TG,TA TGA 550)at a heating rate of 10 K·min−1and within a temperature increase range of 298‑973 K in N2flow of 20 mL·min−1.
The charge/discharge was performed through the battery test system(LAND,CTR2001A)in the galvano‑static mode.Cathode materials were prepared by the mixed slurry,which comprised 80% LFP,10% conduc‑tive material,and 10% PVDF in N‑methyl pyrrolidone solvent(NMP,A.R.).And then,the slurry was evenly cast onto an Al‑foil and dried in a vacuum oven at 100℃for 12 h.CR2032 cells were assembled in a glove box full of Ar,and lithium foil was used as the counter electrode.The electrolyte was 1 mol·L−1LiPF6,which was dissolved in ethylene carbonate(EC),dieth‑yl carbonate(DEC),and dimethyl carbonate(DMC)with a volume ratio of 1∶1∶1.Electrochemical tests were performed between 2.7 and 4.2 V at 0.2C(1C=180 mA·g−1)at 298 K.
2 Results and discussion
2.1 φ⁃pH diagrams of the Fe⁃P⁃Li⁃H2O system
In this work,the precipitation process was basedbook=360,ebook=178on the Fe‑P‑Li‑H2O system,and the probable species that exist in the solution including Fe2+,Fe3+,Li+,Fe(OH)+,Fe(OH)2+,Fe(OH)2+,Fe(OH)4−,PO43−,HPO42−,H2PO4−,H3PO4,Fe,Fe(OH)2,Fe(OH)3,LFP,FePO4·2H2O,Fe3(PO4)2·8H2O,Li3PO4,and LiH2PO4.All the thermodynamic data cited in the study were calculated from the evaluation reviews in Ref.[13‑22],and the calcu‑lated thermodynamic data is shown in Table 3.This inventory leads to 32 equilibrium equations which are listed in Table S1(Supporting information).The stan‑dard free energy(ΔfGT⊖)of different reactions can be obtained according toAccording to the method of Ref.[13‑14,18],the φ‑pH formu‑lations of different reactions at 298 and 363 K were cal‑culated and listed in Table S1.In the diagram below,solid lines represent the Fe‑P‑Li‑H2O system.Every line in the diagram indicates that a species exists in equilibrium with the adjacent ion or solid species at the particular specified activity.Then φ ‑pH diagrams for the Fe‑P‑Li‑H2O system were drawn at a pressure of 0.1 MPa and activity of 1.0.
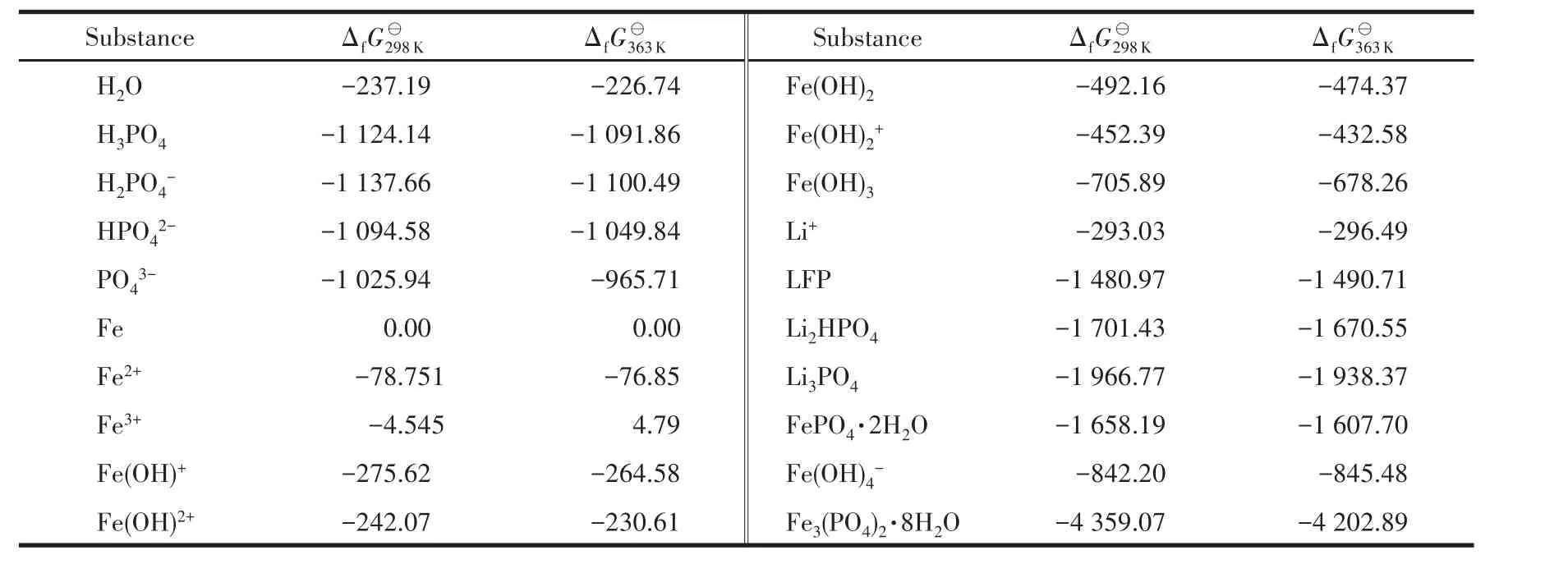
Table 3 Standard free energy(ΔfGT⊖)of main substances of Fe⁃P⁃Li⁃H2O system kJ·mol−1
According to Fig.1,the dashed lines in the dia‑grams represent the limits of the thermodynamic stabil‑ity of water.The main value of a stability diagram is that it provides a quick visual representation of the electrochemical constraints on the stability of species in the given aqueous system related to the pH values.It is shown that FePO4·2H2O resides in the northwestern corner of the diagram(Fig.1).The implication is that,to support the precipitation of Fe and P in the form of FePO4·2H2O,a relatively oxidizing environment(high potential)and relative acidic solution(pH<5.9)must be provided at 298 K.In reducing environment,Fe3(PO4)2·8H2O and LFP replace FePO4·2H2O as the stable Fe(Ⅱ)species.In acidic to a neutral solution,the stability species is LFP and Fe(OH)3.In the basic solu‑tion(pH>7.2),Fe(OH)2replaces LFP under reducing conditions,and Fe(OH)3exists under oxidizing condi‑tions.Li3PO4is stable in the basic solution.With increasing temperature,the stable region of solid spe‑cies changes significantly.A comparison of Fig.1a and 1b indicates that the FePO4·2H2O stability field shrinks with the increase the temperature;also,the Fe3(PO4)2·8H2O species has disappeared from Fig.1a and ferric hydroxo(Fe(OH)4−)species appears from Fig.1b.The trends shown in Fig.1 are consistent with those reported by Jing and Kumar[11,21].The implication is that,to support the precipitation of Fe and P in the form of FePO4·2H2O,a more positive potential condi‑tion(φ>0.416 V)and lower acidic solution(pH<5.0)must be provided at 363 K.Similarly,Fe(OH)2and Fe(OH)3species stability fields shrink.It is obviously observed that the equilibrium line of FePO4·2H2O/LFP moves towards lower pH and positive potential with rising temperature,resulting in the enlargement of the stability domain of LFP.This demonstrates that increasing temperature is not beneficial to the process of FePO4·2H2O precipitation but the conditions of reac‑tion kinetics are more favorable at high temperatures.
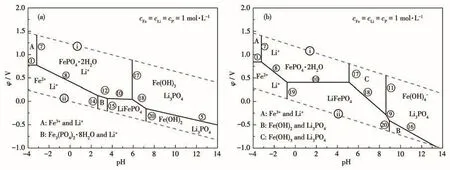
Fig.1 φ‑pH diagrams for the Fe‑P‑Li‑H2O system of different temperatures:(a)298 K and(b)363 K
2.2 Preparetion of FePO4·xH2O in the Fe⁃P⁃Li⁃H2O solution
Fig.2a shows the XRD of the iron phosphate hydrate precipitates(FePO4·xH2O).The broad humps between 2θ=22°to 38°were observed in precipitates and no obvious crystalline peak was found,indicating that all precipitates were amorphous.To characterize the phase of samples,all precipitates had been sintered at 973 K for 6 h,and then XRD was taken for these samples and the results are shown in Fig.2b‑2d.The main diffraction peaks of all samples matched well with the standard pattern of FePO4(PDF No.84‑0876),and the diffraction peaks were strong,demonstrating the main component of FePO4.While the end pH value of the reaction was over 1.5,an impurity peak was observed at 28.9°,and XRD phase analysis shows that it is Fe3PO7(PDF No.76‑1761).This diffraction peak of impurity was stronger with increasing the end pH value of the reaction,meaning that the impurity content of the sample was increasing.According to Fig.1,the Fe3PO7species did not appear in the φ‑pH diagram of the Fe‑P‑Li‑H2O system,but Fe(OH)3did.However,the solubility product constant(Ksp)of iron phosphate hydrate precipitates(FePO4·2H2O,9.91×10−16,298 K)is much larger than the one of Fe(OH)3(4.0×10−38,298 K)[21‑22],which makes the recovery of high purity iron phosphate hydrate precipitates challengeable.As a result,FePO4·xH2O synthesized by the pH with 1.5‑2.2 contained a small amount of Fe(OH)3.During the sinter‑ing process,the reaction equations can be expressed as:
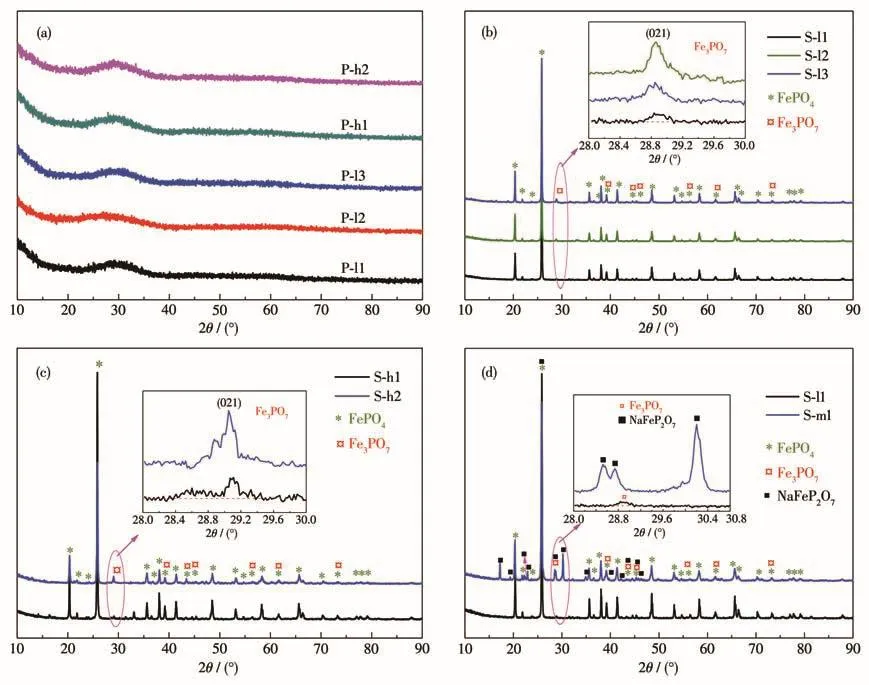
Fig.2 XRD patterns of(a)precipitates and FePO4obtained via sintering iron phosphate hydrate which was precipitation(b)at 333 K,(c)at 363 K,and(d)with different nFe∶nPand the corresponding enlarged patterns
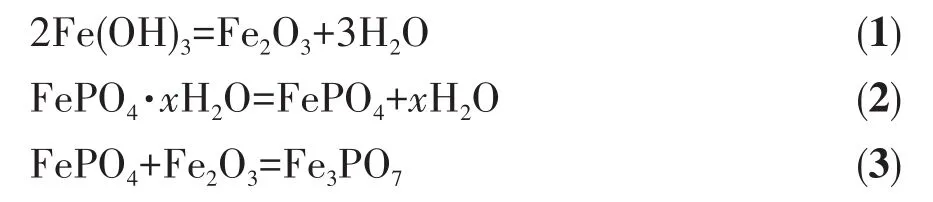
And Eq.3 is consistent with the report by Yang[23].
The total reaction equation can be expressed as:
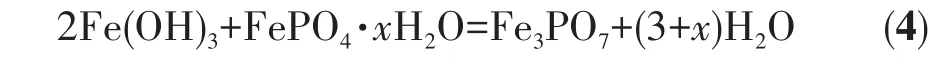
The Fe3PO7content of sintered samples can be calculated by Fe and P content and is listed in Table 4.As shown in Table 4,the Fe3PO7content of the samples increased as the end pH value of the reaction increased.Suppose there was no crystal water in precipitates,that is,x=0.The ratio of FePO4to Fe(OH)3in precipitates was calculated by Eq.4,and the result is listed in Table 5.With increasing the end pH value of the reac‑tion from 1.5 to 2.2,Fe(OH)3content of FePO4increased from 0.04% to 2.26%.The Kspof Fe(OH)3is much smaller than the Kspof FePO4·2H2O[14],resulting that Fe3+reacting preferentially with OH−rather than PO43−.Thereby,the precipitation of Fe(OH)3is much easier than FePO4·xH2O.Furthermore,Fe3PO7was formed at the expense of FePO4during the sintering process,which would further reduce the purity of FePO4.As shown in Fig.1,it is the area of a stable region of FePO4·2H2O at a pH of 1.5‑2.2 in an oxidizing environ‑ment.Based on the principle of thermodynamic equilib‑rium,Fe(OH)3can be converted to FePO4·2H2O.But aging for the reaction slurry could not improve the puri‑ty of the product because of a slow rate of conversion.To synthesize iron phosphate for battery materials,the nFe∶nPof iron phosphate hydrate should be controlled at 0.97‑1.02[24].Thereby,the end pH value of the reac‑tion should be below 1.80 at 333 K and 1.5 at 363 K.Then nFe∶nPof precipitates obtained was 1.001 ‑1.019,and the recovery rate of Fe and P was about 62% to 76%.Obviously,the Fe(OH)3content was increasing sharply with the increasing temperature of the reaction.For example,Fe(OH)3content was 0.30% in P‑l2 syn‑thesized at 333 K while it was 2.26% in P‑h2 synthe‑sized at 363 K.This means that temperature is also one of the essential conditions to avoid generating Fe(OH)3during the co‑precipitation process.
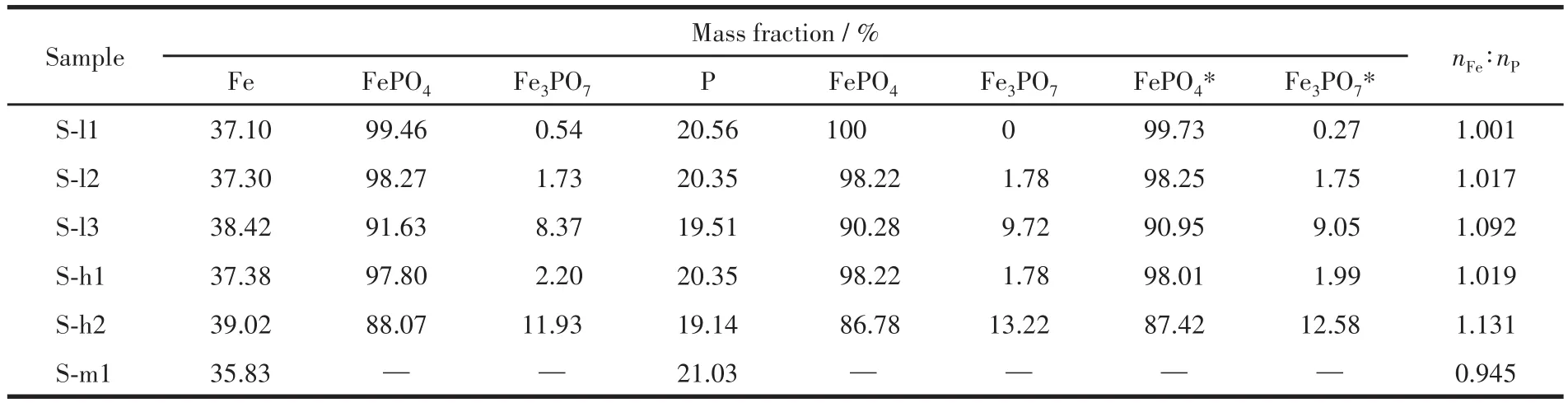
Table 4 Phase composition of sintered samples
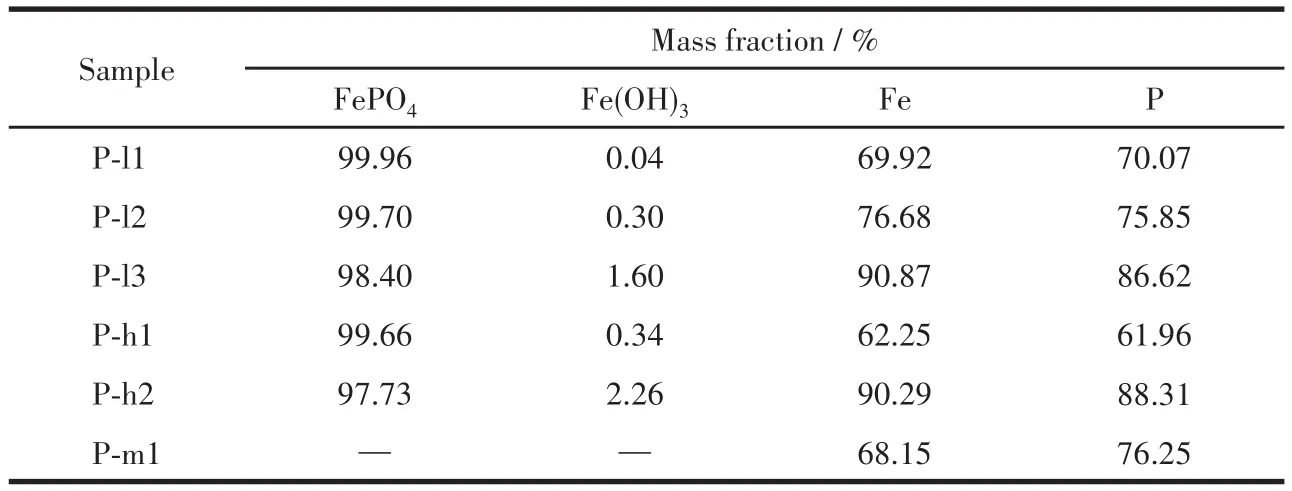
Table 5 Phase composition of precipitates and recovery rate of Fe and P
Fig.2d exhibits the XRD patterns of FePO4pow‑ders synthesized at different nFe∶nPin initial solution.From the patterns,the main diffraction peaks of the samples matched well with the FePO4phase(PDF No.84‑0876).Some other peaks of impurity phases were observed in samples besides Fe3PO7(PDF No.76‑1761),and XRD phase analysis shows that it is attribut‑ed to NaFeP2O7(PDF No.80‑1475).The intensity of NaFeP2O7diffraction peaks was much stronger than that of Fe3PO7,suggesting that NaFeP2O7content was higher than Fe3PO7in S‑m1.During the precipitation process,when nFe∶nP=1∶2,some H3PO4could react with NaOH to form NaH2PO4,which would further react with FePO4·xH2O and produce NaFeP2O7during the sintering process[25‑27].The reaction equations can be expressed as:
NaH2PO4+FePO4·xH2O=NaFeP2O7+(1+x)H2O (5)The Fe3PO7and NaH2PO4content of S‑m1 can be cal‑culated by Fe and P content(Table 4‑5).The S‑m1 sample consisted of 90.81% FePO4,9.14% NaFeP2O7,and 0.44% Fe3PO7.Suppose there was no crystal water in P‑m1,that is,x=0.The percentages of FePO4,NaH2PO4and Fe(OH)3were 95.66%,4.31% and 0.03% in P ‑m1,respectively.Therefore,nFe∶nPin ini‑tial solution is one of the factors affecting FePO4·xH2O precipitation in the solution medium.
To further determine the crystal water content in precipitates,the precipitates were analyzed by TG.The TG and DTG‑curves of the recycled precipitates are shown in Fig.3.A turning point was observed at 373 K for sample P‑l1(the one of P‑l2 was at 367 and 384 K for P‑h1).The weight loss from 298 K to this turning point was 0.92% for P‑l1(0.67% for P‑l2 and 5.35% for P‑h1),indicating that the sample has some absorbed water.After this point,there are continuous quality changes in the temperature range between this turning point and 723 K,which gives one distinct peak(DTG curve).In this part,the peak temperature of the deriva‑tive thermogravimetric(DTG)curves was 423 K for P‑l1(429 K for P‑l2 and 419 K for P‑h1),suggesting that FePO4·xH2O loses its crystal water(Eq.2).And the weight loss was 20.08% for P‑l1(19.60% for P‑l2 and 20.74% for P‑h1 respectively),which are consistent with those reported[24].Suppose the precipitates were pure FePO4·xH2O,and then x in FePO4·xH2O was 2.08 for P‑l1,2.03 for P‑l2,and 2.15 for P‑h1,which is consistent with the theoretical value of 2.00.Based on the above analysis,the chemical formula of precipi‑tates obtained at 333 and 363 K was FePO4·2H2O,in accordance with the literature[11,28‑29].There is one dis‑tinct peak in the range of 723‑873 K,and the loss of weight was 0.56%‑0.70%,which is the decomposition of Fe(OH)3(Eq.1)and the reaction of Eq.3.
The composition and the impurity content of pre‑cipitates are listed in Table 6.When nFe∶nP=1∶1 and pH≤1.8 at 333 K or pH≤1.5 at 363 K,the purity of recycled FePO4·2H2O was over 99.73% and the crystal water content was 19.60%‑20.74%,which is consistent with of iron phosphate for battery materials standard[22].
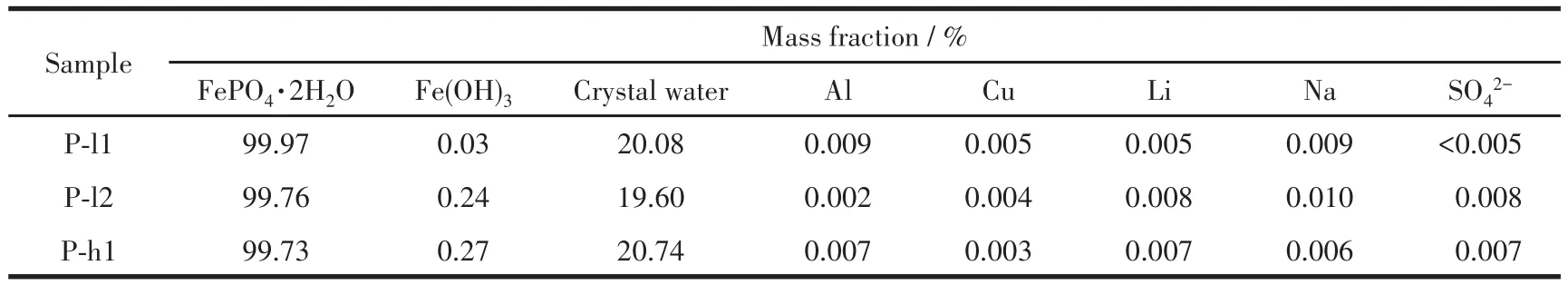
Table 6 Compositions phase and content of hazardous elements in precipitates
Fig.3d‑3f shows the images of FePO4·2H2O syn‑thesized at 333 and 363 K.With increasing pH value and temperature of the reaction,FePO4·2H2O color var‑ied from white to yellow.For the obvious difference,the increase of Fe(OH)3which is reddish‑brown pow‑der,is a reasonable explanation.
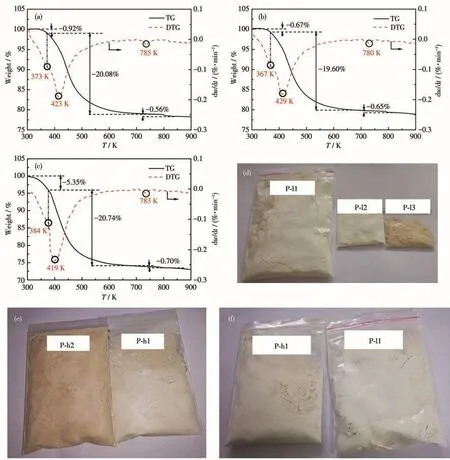
Fig.3 TG curves and DTG curves for the precipitates from 298 to 973 K:(a)P‑l1,(b)P‑l2,and(c)P‑h1;(d‑f)Images of precipitates
Fig.4 shows an electrochemical performance com‑parison between R‑LFP and LFP obtained from Great Power Energy&Technology Co.,Ltd(G‑LFP).The ini‑tial discharge capacity of pristine R‑LFP and G‑LFP were 154.1 and 156.4 mAh·g‑1,with a coulombic efficiency of 95.9%,and 94.9%,respectively.After charge‑discharge for four cycles,R‑LFP delivered a discharge capacity of 155.8 mAh·g−1,which is still a little lower than G‑LFP.That may be due to trace impu‑rity in R‑LFP,which reduces the electrode activity and increases the electrode impedance.Fig.4b shows that R‑LFP and G‑LFP displayed a discharge capacity of 150.8 and 151.4 mAh·g−1,and showed excellent capac‑ity retention of 96.79% and 96.80% after 100 cycles at 0.2C respectively.Numerous efforts have proved that the morphologies and microstructure of LFP are one of the main factors affecting its electrochemical perfor‑mance[11,21].It suggests that technology parameter in the co‑precipitation process is very important to the mor‑phologies and microstructure of FePO4·2H2O,which could improve the cycle performance of LFP.
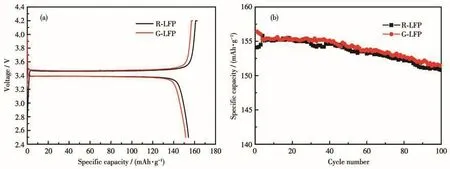
Fig.4 (a)Charge/discharge curves and(b)cycling performance of LFP
3 Conclusions
In this study,φ‑pH diagrams of the Fe‑P‑Li‑H2O system are drawn by thermodynamic calculation meth‑od with variable temperature.The results indicate that FePO4·2H2O stable field shrinks as the temperature increases from 298 to 363 K.Therefore,a more posi‑tive potential condition(φ>0.416 V)and a lower acidic solution(pH<5.0)is essential for the precipitation of Fe and P in the form of FePO4·2H2O.It suggests that when the initial molar ratio of Fe and P in the reaction solutions was controlled at 1∶1,the final pH value of the reaction was suggested to be 1.5 at 333 K to obtain an amorphous and white FePO4·2H2O with a crystal water content of 20.08% and the atomic ratio of Fe/P was 1.001.The purity of this as‑prepared FePO4·2H2O was 99.97%,where the main impurity present was Fe(OH)3.The content of Fe(OH)3was increasing with the pH value and temperature.Meanwhile,this FePO4·2H2O has few hazardous elements and can be utilized as LPF battery materials with a discharge capacity of 154.1 mAh·g−1and a capacity retention of 96.79% at 0.2C after 100 cycles,which greatly improves the eco‑nomic efficiency of recycling the spent LFP battery.
Supporting information is available at http://www.wjhxxb.cn

