Combination of transarterial radioembolization with atezolizumab and bevacizumab for intermediate and advanced staged hepatocellular carcinoma: A preliminary report of safety and feasibility
Qin Yu, Yting Wng, Ethn Unghusri, Mikin Ptel, Divy Kumri, Thuong Vn H,Anjn Pilli, Chih-yi Lio, Osmn Ahme
a Department of Radiology, University of Chicago Medical Center, University of Chicago, Chicago, IL, 60637, USA
b Hematology and Oncology, Ascension Providence Hospital, Southfield, MI, 48075, USA
c Division of Gastroenterology,Hepatology,and Nutrition,Department of Medicine,University of Chicago Medical Center,University of Chicago,Chicago,IL,60637,USA
d Hematology and Oncology, Department of Medicine, University of Chicago Medical Center, University of Chicago, Chicago, IL, 60637, USA
Keywords:
ABSTRACT Purpose: The IMbrave150 Phase III trial demonstrated the superiority of atezolizumab and bevacizumab (Atezo/Bev) over sorafenib for unresectable hepatocellular carcinoma (HCC).The present study aims to evaluate the feasibility of TARE in combination with Atezo/Bev for the treatment of intermediate and advanced staged HCC.Methods: A retrospective review at a single institution was performed between May 2021 and December 2022.Patients who received TARE using yttrium-90 (Y90) with concomitant or sequential Atezo/Bev systemic treatment were included.The following outcomes were retrieved: overall survival (OS), radiologic tumor response,progression-free survival, technical adverse events related to TARE, and toxicity based on the National Cancer Institute–Common Terminology Criteria for Adverse Events version 5.0.
1.Introduction
Immuno-oncology has witnessed a recent paradigm shift in the management of cancer due to its ability to utilize the innate and adaptive immune system to initiate an antitumor response.1In the management of hepatocellular carcinoma (HCC), however, initial immunotherapy trials CheckMate-459 and KEYNOTE-240 failed to show the superiority of Nivolumab and Pembrolizumab over the standard of care, respectively.2,3The ability of the tumor to create an immunosuppressive microenvironment or other similar mechanisms to evade the immune system have been offered as reasons for ineffective results with immunotherapy in certain malignancies.4Combined radiation therapy and immune checkpoint inhibitors have been shown to induce synergistic effects in treating solid tumors.5Recent studies on transarterial radioembolization(TARE)demonstrate safety in combining yttrium-90(Y90)with nivolumab, ipilimumab and/or pembrolizumab for HCC and metastatic cancers to the liver.6,7The recent landmark IMbrave150 Phase III trial demonstrated the superiority of atezolizumab and bevacizumab(Atezo/Bev)over sorafenib in unresectable HCC,establishing it as a new first line immunotherapy for unresectable HCC.8In the Atezo/Bev arm however, 58.6% of patients developed disease progression during a median follow-up time of 8.9 months.Whether the addition of TARE to this systemic regimen can enhance the antitumor response and potentially prolong survival remains less well-known.This preliminary study was performed to evaluate the safety and feasibility of combining TARE and the Atezo/Bev regimen for intermediate and advanced stage HCC.

Fig.1.Patient-level treatment sequence relative to first radioembolization(0 mo).Stars mark radioembolization treatment.Time from diagnosis to last follow-up is shown in gray.Atezo/Bev treatment duration is shown in blue.
2.Methods
2.1.Patient selection
This retrospective study was approved by the institutional review board.Ten patients (9 male, 1 female) with unresectable HCC received TARE and Atezo/Bev between May 2021 and December 2022.A cut-off of 90 days between TARE and Atezo/Bev was implemented in accordance with previous literature.6Eligibility for TARE was determined on a case-by-case basis based on a multidisciplinary team involving medical oncologists, diagnostic radiologists, interventional radiologists, and hepatobiliary surgeons.
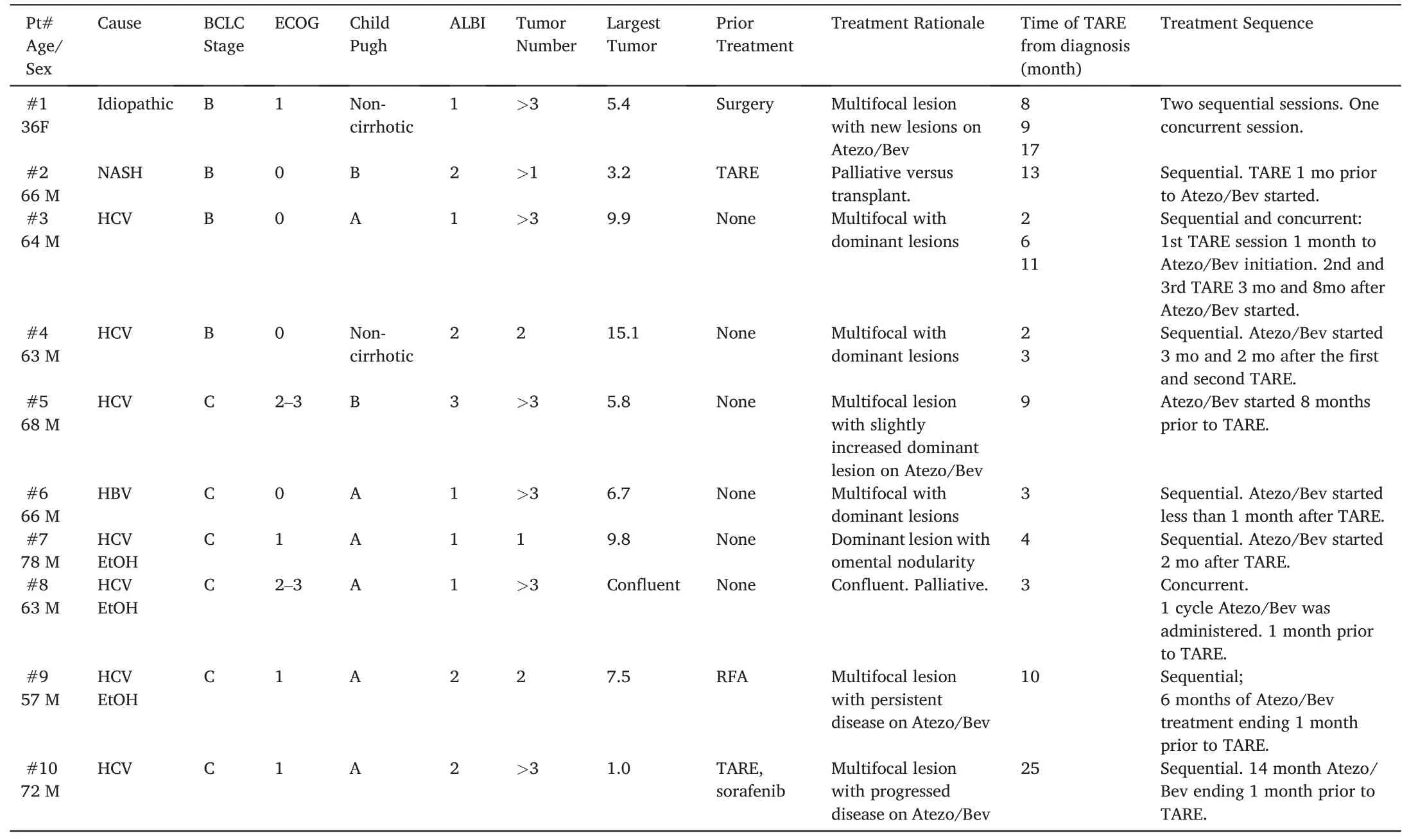
Table 1 Baseline characteristics of included patients.ALBI: Albumin-Bilirubin Grade.BCLC: Barcelona clinic liver cancer.ECOG: Eastern Cooperative Oncology Group Performance Score.HBV:hepatitis B virus.HCV: hepatitis C virus.RFA: radiofrequency ablation.TARE: transarterial radioembolization.

Table 2 Radioembolization technique, dose, treatment response, and toxicity.CR: complete response.LSF: lung shunt fracture.NA: data not available.PR: partial response.rRHA: replaced right hepatic artery.SD: stable disease.
2.2.Treatment
Each cycle of Atezo/Bev treatment consists of Atezolizumab (1200 mg)and Bevacizumab(15 mg/kg)intravenously on day 1 over a 21-day cycle.8All patients underwent endoscopy for variceal screening 6 months prior to Atezo/Bev administration (5/8 [62.5%] cirrhotic patients with non-bleeding grade 1 esophageal varices; no varix in 3/8 [37.5%] patients).TARE treatments consisted of two separate outpatient visits with initial mapping angiography and subsequent administration within a week as previously described,9and dosimetry was calculated using the medical internal radiation dose (MIRD) model.A total of 15 treatment sessions were administered during the study period.All patients were treated with glass microspheres (Therasphere; Boston Scientific, Marlborough, Massachusetts) except one treated with resin microspheres(Sirtex Medical, Wilmington Massachusetts, patient #8).Bevacizumab was held 2–3 weeks prior to mapping phase.Post-TARE systemic therapy was initiated/resumed in 1–2 months.“Sequential” treatment was defined as TARE administered prior to the initiation or after completion of Atezo/Bev.The regimen was considered “concurrent” if Atezo/Bev was administered prior to and after TARE administration.Per patient treatment sequence is depicted in Fig.1.
2.3.Outcomes assessment and survival

Fig.2.Progression-free survival from the time of radioembolization.
Baseline and clinical follow-up evaluations were performed per standard institutional clinical pathways.Laboratory data and imaging results were collected at baseline,6–12 weeks post TARE,and every 3–6 months thereafter.Tumor response was evaluated with computed tomography or magnetic resonance imaging.Target tumor response was characterized for tumors treated by TARE according to the modified Response Evaluation Criteria in Solid Tumors(mRECIST)and compared to the most recent study prior to TARE.Disease control was defined as the rate of tumors that achieved complete response (CR), partial response(PR),or stable disease(SD).Objective response(OR)was defined as the rate of tumors with CR or PR.Time to target tumor progression and nontarget tumor progression was calculated.Progression free survival(PFS),defined as disease progression regardless of intrahepatic or extrahepatic progression was calculated based on Response Evaluation Criteria in Solid Tumors 1.1 criteria,and overall survival(OS)were calculated from TARE using the Kaplan-Meier method.
2.4.Adverse events
Toxicity was assessed at 1 and 3 months after TARE administration based on the National Cancer Institute–Common Terminology Criteria for Adverse Events(CTCAE)version 5.0.The serum laboratory values of interest included alkaline phosphatase, aspartate aminotransferase,alanine transaminase,total bilirubin,albumin,hemoglobin,white blood cell count and platelet count.Clinical toxicity of interest included nausea,vomiting, loss of appetite, diarrhea and abdominal pain.Reasons for post-TARE systemic therapy delay or hold were also collected.
3.Results
Of the 10 patients included in this study,9(90%)were male,with a median of 65 (range 36–78) years (Table 1).The largest tumor per patient ranged from 1.0 to 15.1 cm.The most common etiology of liver disease was HCV(70%).Six(60%)patients had BCLC stage C disease(3 portal vein tumor thrombus, 3 metastatic disease), whereas the remainder were BCLC-B (40%).Nine (90%) patients presented with multifocal liver lesions.One patient had a solitary liver lesion with omental nodularity.A total of 15 sessions of TARE were administered(Fig.1).Sequential administration of Atezo/Bev and TARE was adopted in 12/15(80%)TARE sessions.Atezo/Bev and TARE were concurrently administered in 3/15(20%)sessions.Child Pugh score,ECOG status,and ALBI scores are listed in Table 1.A total of 21 doses were prescribed,with a median administered dose of 156 Gy;14(66.7%)doses were delivered in a segmental fashion, whereas 7 (33.3%) were lobar.The median cumulative prescribed dose per patient was 404 Gy.
The median clinical follow-up was 12 months with a range of 4–17 months.Excluding patient #8 with confluent disease and patient #10 without imaging follow-up after TARE, a total of 12 dominant tumors were targeted with TARE.CR and PR were achieved in 9 (75%) and 3(25%)tumors,respectively(Table 2).The 3 tumors with PR in patient#1 progressed at follow-up with a de novo tumor at 2 months, and subsequent treatment with repeat TARE resulted in CR for at least 7 months.Non-target tumor progression occurred in 4/10 patients during followup.Median progression time was not reached (Fig.2), with a 6-month PFS of 78.8% (95%CI: 36.5–93.9%) and 12-month PFS of 66.7%(28.2–87.8%).An example case is depicted in Fig.3.
Patient #8 developed G3 transaminitis and G2 hypoalbuminemia within 3 months post-TARE.This patient presented with confluent and bilobar hepatic tumor burden with TARE and Atezo/Bev administered as a palliative intent.The patient opted for hospice after 1 cycle of Atezo/Bev and TARE.Patient 5 who progressed on Atezo/Bev and then received TARE developed proximal muscle weakness one-month post-TARE,associated with elevated creatine kinase and bilateral thigh edema on MRI, which was attributed to immunotherapy related myositis.As a result, Atezo/Bev was held until creatine kinase returned to normal limits,and then switched to Lenvatinib.However,this patient developed severe fatigue, diarrhea and impaired cognition, requiring intermittent holding and dose reduction.Dose reduction or withhold occurred in two patients who received TARE first and Atezo/Bev subsequently for multifocal and extrahepatic disease.Bevacizumab was held 5 months post-TARE in patient#2 due to confusion,which was felt to be related to hepatic encephalopathy versus transient ischemic attack (TIA).Bevacizumab dosage was reduced at 12 months post-TARE in patient#7 due to weight-loss.
Two patients died during follow-up in hospice due to disease progression,4 and 10 months after TARE,respectively.The median survival time was not reached(Fig.4).OS rates were 90.0%(95%CI:9.5–98.5%)and 77.1% (95%CI: 34.5–93.9%) at 6 months and 12 months,respectively.

Fig.3.A) Right hepatic enhancing mass measures 9.9 × 9.7 cm (Patient #3).B) Initial mapping angiogram shows tumor supply primarily from the right hepatic artery.Initial treatment session planning with segment 6 dose 142 Gy and right lobar dose 92 Gy.Second treatment session with 251 Gy lobar dose.Third treatment session with 121 Gy lobar dose.C)Intraoperative catheter-directed computed tomographic angiography shows yttrium-90 microsphere uptake by the tumor.D)Five months post-radioembolization follow-up shows reduced tumor size and enhancement, measuring 6.8 × 5.8 cm, compatible with partial response.E) Eight months post-radioembolization shows absence of enhancement without a viable tumor.The cavity measures 5.4 × 4.9 cm.
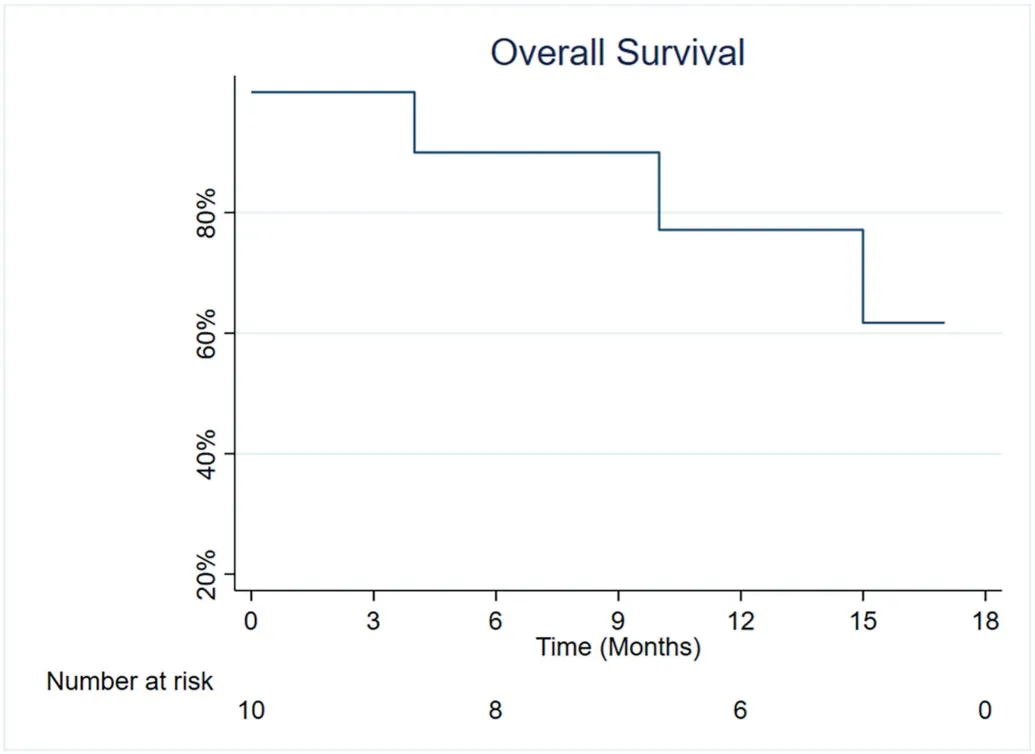
Fig.4.Overall survival from the time of radioembolization.
4.Discussion
The IMbrave150 demonstrated the superiority of Atezo/Bev over sorafenib for unresectable HCC with a median PFS of 6.8 versus 4.3 months.The synergistic effects can be attributed to atezolizumab's reversal of T-cell suppression via blockage of PD-1 and B7-1 interaction and bevacizumab's inhibition of vascular endothelial growth factor mediated immunosuppression.10,11TARE has been shown to increase peripheral interleukin, intratumoral lymphocyte activation gene 3-positive CD4+tumor-infiltrating lymphocytes, CD3+T cells, regulatory T cells,and inflammatory(PD-L1+and HLA-DR+)monocytes,suggesting a potential synergistic effect when combined with immune checkpoint inhibitors.12,13This study provides preliminary data on the radiological response of Y90 and Atezo/Bev treatment, given that target tumor control was achieved in all cases with the intention to treat(9/9)rather than palliative intent(n=1).Among 4 patients with stage B tumor burden,3(75%) were downstaged to within Milan criteria.Observed OS rates of 90.0%and 77.1%at 1-year and 2-year,respectively,were higher than the 84.8% and 67.2% from the Imbrave150 Atezo/Bev cohort.Based on these preliminary data, the addition of TARE to Atezo/Bev could be considered in the following scenarios as seen from this present study:1)Concurrent or sequential treatment for multifocal tumor with single or oligodominant lesions;2)Sequential treatment for multifocal tumor with limited response to initial Atezo/Bev; 3) Solitary or few dominant liver lesions with limited extrahepatic disease;4)initiate locoregional therapy(TARE)in a timely fashion while patients undergo endoscopy for variceal workup/treatment prior to Atezo/Bev initiation.
The present study also suggests an acceptable safety profile with the addition of TARE to the Atezo/Bev regimen.In the present study, one patient with confluent tumor burden developed G3 transaminitis and hypoalbuminemia,likely related to TARE to a limited hepatic reserve and disease progression (ECOG 2–3 baseline).Another patient developed weakness and myositis, which is a known side effect of immunotherapy.14Bevacizumab was held in one patient (Child-Pugh B) after TARE due to worsened altered mental status, which might have been related to aggravated hepatic encephalopathy or TIA.The latter was considered a contraindication for the use of bevacizumab.Approximately 15%of patients from the IMbave150 trial treatment group discontinued treatment due to side effects.8The most commonly encountered G3 or above side effects associated with Atezo/Bev were hypertension(15.2%),elevated AST (7%), and elevated ALT (3.6%).There was no report of included patients receiving TARE from the IMbrave150 trial.8Approximately 48% of patients from the Atezo/Bev group received at least 1 prior local therapy with 39%transarterial chemoembolization and 14%radiofrequency ablation; 10% of patients received external radiation therapy.Based on the evidence of other systemic treatment agents,higher adverse events were observed when lobar TARE was added.15Nonetheless,the use of segmental TARE has gained increasing popularity and has been shown to decrease hepatotoxicity by preserving functional liver reserves,a technique used during 66.7%of deliveries in the present study.16,17Further, recent evidence also demonstrated efficacy in Y90 induced hypertrophy with lobectomy and modified lobectomy,16which provides bridging resection as potential options for patients with initially unresectable HCC with a plan to receive Atezo/Bev.A careful discussion with patients and among multidisciplinary team members regarding goals of care is necessary in these circumstances.
The present study is limited by its retrospective nature,small sample size and short-term follow-up.Additionally,patients were heterogeneous in terms of tumor burden and prior treatment.Further, the lack of a comparison group also limits the assessment of the safety and effectiveness of this combined regimen against TARE or Atezo/Bev alone.The last but not the least, the optimal sequence of Atezo/Bev and TARE administration is yet to be determined to induce synergistic effects.Post-TARE serum analysis found only a temporary alteration of T lymphocyte levels,suggesting that combined immunotherapy should administered at a certain time point to optimize synergistic effects.12From a technical perspective, as anti-VEGF agents may weaken vessel walls, TARE following initiation of Atezo/Bev could increase the risk of procedural complications such as vessel dissection during TARE mapping and treatment.18
In summary, this small patient cohort demonstrated preliminary safety, feasibility, and initial efficacy of TARE in combination with Atezo/Bev for unresectable HCC.Further prospective and comparative studies with large sample sizes are warranted to determine long term efficacy.
Data availability statement
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
Author contribution
Conceptualization:QY,OA.
Data curation:QY,EU,
Formal analysis:QY,YW.
Funding acquisition:MP,OA.
Investigation:QY,YW,EU.
Methodology:QY,YW,EU.
Project administration:QY,OA.
Resources:MP,DK, TVH,AP,CL,OA.
Software:QY,YW.
Supervision:OA.
Validation:MP,DK, TVH,AP,CL,OA.
Visualization:QY,YW.
Writing-original draft:QY,YW,EU,MP,OA.
Writing-review& editing: All.
Ethical approval
The study was approved by the ethics committee of University of Chicago Medical Center.All clinical practices and observations were conducted in accordance with the Declaration of Helsinki.Informed consent was obtained from each patient before the study was conducted.
Patient consent
Written informed consent was obtained from patients for publication of these case reports and any accompanying images.
Declaration of competing interest
We declare that we do not have any commercial or associative interest that represents a conflict of interest in connection with the work submitted.
 Journal of Interventional Medicine2023年4期
Journal of Interventional Medicine2023年4期
- Journal of Interventional Medicine的其它文章
- Mechanisms and therapeutic strategies to combat the recurrence and progression of hepatocellular carcinoma after thermal ablation
- Overview of peripheral arteriovenous malformations: From diagnosis to treatment methods
- Embolization of brain arteriovenous malformations with squid co-polymer embolic material: Initial experience
- A novel cerebrovascular drug-coated balloon catheter for treating symptomatic intracranial atherosclerotic stenosis lesions:Study protocol for a prospective, multicenter, single-arm, target-value clinical trial
- Argon-helium cryoablation treatment of undifferentiated pleomorphic sarcoma of the thyroid: A case report and literature review
- “Guidezilla” extension catheter combined with balloon technique for treating pulmonary artery stenosis caused by Takayasu arteritis
