Improving plasma sterilization by constructing a plasma photocatalytic system with a needle array corona discharge and Au plasmonic nanocatalyst
Bin ZHU(朱斌),Qiwei LI(李其玮),Yanan GAO(高亚楠),Yan YAN(闫妍),Yimin ZHU(朱益民),∗ and Li XU(徐力)
1 Laboratory of Plasma Catalysis,College of Environmental Sciences and Engineering,Dalian Maritime University,Dalian 116026,People’s Republic of China
2 Novel Energy Materials and Catalysis Research Center,Shanwei Institute of Technology,Shanwei 516600,People’s Republic of China
Abstract Efficient sterilization by a plasma photocatalytic system(PPS)requires strong synergy between plasma and photocatalyst to inactivate microorganisms while suppressing the formation of secondary pollutants.Here,we report that a PPS constructed from a needle array corona discharge and Au/TiO2 plasmonic nanocatalyst could remarkably improve the sterilization of Escherichia coli(E.coli)and alleviate formation of the discharge pollutant O3.At 6 kV,the combination of corona discharge and Au/TiO2 achieves sterilization efficiency of 100% within an exposure time of 5 min.At 5 kV and an exposure time of 8 min,the presence of Au/TiO2 improves sterilization efficiency of the corona discharge from 73% to 91% and reduces the O3 concentration from 0.38 to 0.04 ppm,whereas the presence of TiO2 reduces the sterilization efficiency and O3 concentration to 66% and 0.17 ppm,respectively.The Au/TiO2 in the PPS enables a uniform corona discharge,enhances the interaction between plasma, E.coli and nanocatalysts,and suppresses the formation of O3.Further,the Au/TiO2 can be excited by ultraviolet–visible light emitted from the plasma to generate electron–hole pairs,and thus contributes to the formation of reactive radicals and the oxidative inactivation of E.coli.The PPS constructed from a needle array corona discharge and Au-based plasmonic nanocatalyst provides a promising approach for developing high-efficiency sterilization techniques.
Keywords:plasma photocatalysis,sterilization,corona discharge,Au/TiO2 nanocatalyst,synergetic effect
1.Introduction
Nowadays,efficient sterilization techniques are widely required for surface sterilization and air purification[1,2].Conventional sterilization methods(mainly autoclave and chemical disinfectants)commonly suffer from limitations of high temperature and toxicity,and the need for novel approaches is urgent.Consequently,various sterilization techniques(ozone,radiation,plasma,etc)have been studied by researchers[3–5].Among these techniques,cold plasma is one of the most promising approaches for overcoming the sterilization challenge[6,7].Compared with the conventional sterilization methods,cold plasma possesses features of fast response and high reactivity at low temperature(even at room temperature),enabling rapid and efficient sterilization processes without elevated temperatures.
To date,plasma sterilization has been extensively investigated in the fields of environment and biomedicine,including air and water sterilization[8,9],microbial inactivation[10]and living tissue treatment[11].To achieve highefficiency plasma sterilization,the performances of different plasmas(low-pressure plasmas and atmospheric-pressure plasmas)have been measured,and the mechanisms for plasma sterilization deeply explored[12,13].During the past 50 years,many low-pressure plasma sterilization systems,such as capacitively coupled plasma,inductively coupled plasma and microwave plasma,have been investigated by various groups,and some commercial plasma sterilization setups have been successfully developed[6].More recently,atmospheric-pressure plasmas have drawn increasing attention for sterilization because they can perform at low input power without the need for complex vacuum systems,and possess much wider application fields than low-pressure plasmas[14,15].It has been reported that the inactivation effect of plasma on microorganisms mainly originates from the two aspects:(1)ultraviolet(UV)irradiation from plasma destructs deoxyribonucleic acid and ribonucleic acid of microorganisms;(2)UV irradiation,energetic particles and reactive radicals(such as O and OH)in plasma directly erode microorganisms.Given these sterilization mechanisms,great efforts have been devoted to improve sterilization efficiency by designing and constructing atmospheric-pressure plasma systems[16–18].Nevertheless,plasma sterilization still encounters the challenge of relatively low sterilization efficiency.Furthermore,for plasma sterilization operating in air,the formation of discharge byproducts(such as O3and NOx)is inevitable,which tends to cause secondary pollution during the sterilization process.
Plasma photocatalysis,which features anin situcombination of discharge and photocatalysis,provides a solution to the challenges of plasma sterilization.A plasma photocatalytic system(PPS)has been intensively investigated for the removal of low-concentration volatile organic compounds(VOCs)from indoor air[19–21].Compared with plasma alone,a PPS can improve sterilization because of the synergistic effect between plasma and photocatalysts.The photocatalysts located in the plasma region act as the reaction sites of surface catalytic reactions,which facilitate the formation of highly reactive species[22,23].The light emitted from the discharge directly contributes to the inactivation of microorganisms.The light can also excite photocatalysts to generate plenty of electron–hole pairs,which benefits the formation of reactive species over the photocatalyst surface and thus boosts sterilization.Further,the presence of photocatalysts in the plasma offers a number of catalytic sites for O3decomposition[24,25].This not only provides plenty of reactive oxygen species for the sterilization,but also suppresses the generation of secondary pollutants.Hence,the problems encountered by plasma sterilization probably are tackled by the PPS.To our knowledge,few works on sterilization in a PPS have been reported.
In this work,we constructed a PPS by integrating a needle array corona discharge with a highly active Au/TiO2plasmonic nanocatalyst[26–28],and employed the PPS in the sterilization of the typical microorganismE.coli(ATCC 25922).It was demonstrated that the PPS can improve sterilization efficiency and suppress the generation of secondary pollutants relative to the corona discharge.To unravel the enhanced mechanism,optical and electrical diagnoses of the PPS as well as nanocatalyst characterization were performed.
2.Experiment
2.1.Plasma reactor
Figure 1 shows schematic diagrams of the plasma reactor configuration.Briefly,five stainless-steel hollow needles(outer diameter 3 mm,thickness 0.3 mm)fixed on a stainless-steel plate(diameter 37 mm,thickness 0.5 mm)were used as the high-voltage electrodes.Five needles obeyed the following distribution:one needle was located at the center of the plate;the other four needles were distributed around the center needle with a distance from it of 8 mm;the surrounding needles kept the same distance from their neighboring needles.A circular stainless-steel mesh(diameter 30 mm,thickness 0.5 mm)with a hole diameter of 1.4 mm was used as the ground electrode.The high-voltage electrode and ground electrodes were placed into a quartz cylinder(inner diameter 50 mm,thickness 5 mm,height 20 mm)to make the plasma reactor,and the top and bottom of the quartz cylinder were both sealed with a polymethyl methacrylate(PMMA)plate.The distance from the needles of the tip to the mesh(dNM)was changeable(5–12 mm).For the sterilization,E.coliwere coated onto a Petri dish,and the mesh electrode was placed over the Petri dish with 1 mm gap from the mesh surface to theE.coli.The gas was first introduced into the reactor from the upper PMMA plate,then it flowed through the five hollow needles into the discharge region.The plasma can effectively treatE.coliduring the sterilization process due to the small distance between the mesh electrode and theE.coli.
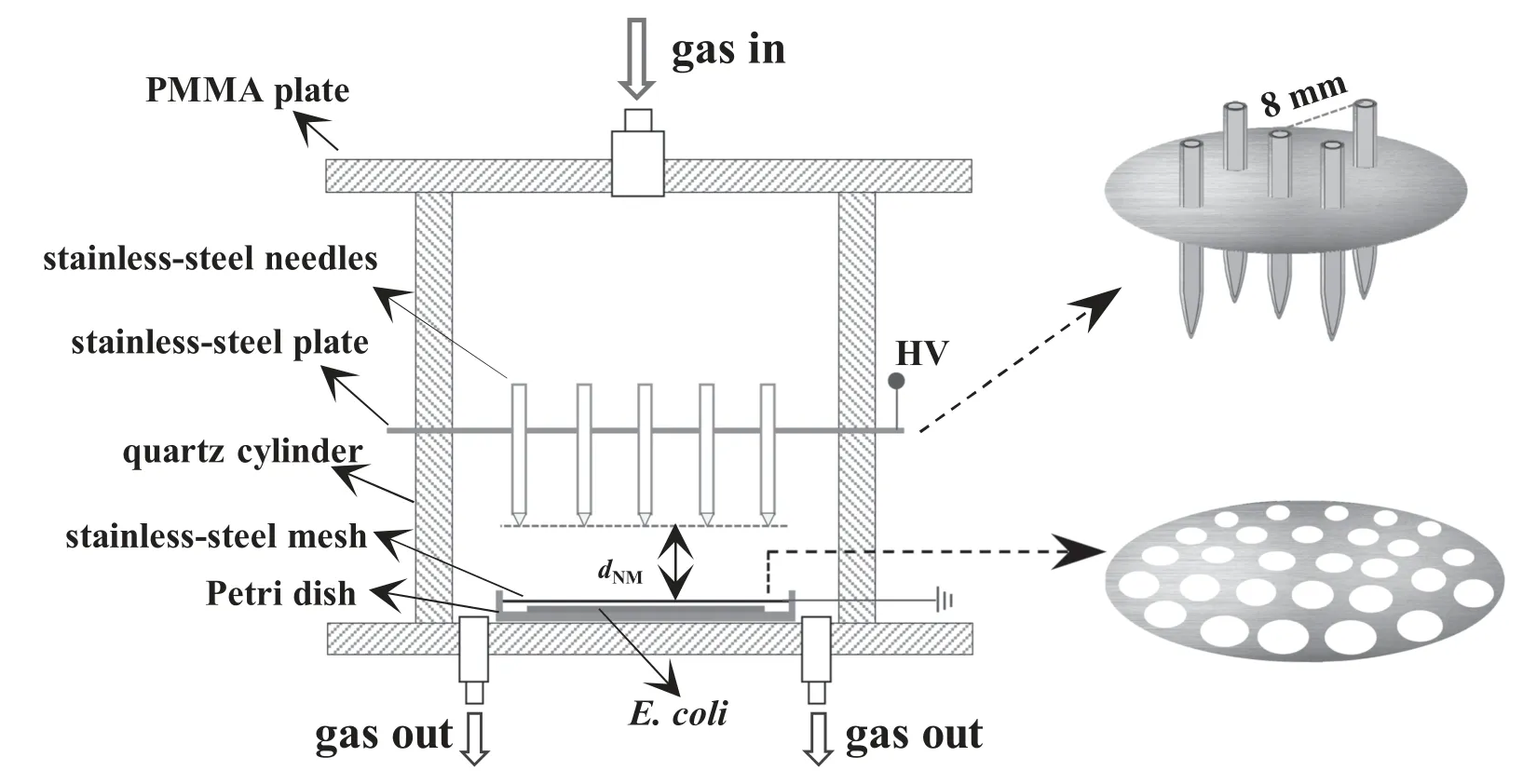
Figure 1.Schematic diagram of the plasma reactor configuration.
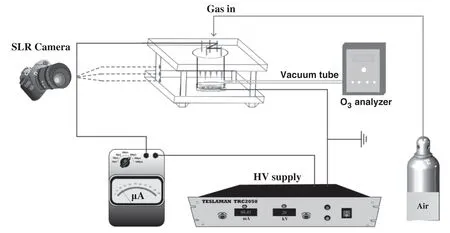
Figure 2.Schematic diagram of the experimental setup.
A schematic diagram of the experimental setup is shown in figure 2.The plasma reactor was supplied with a negative(−50 to 0 kV)DC power supply(TRC2025,Dalian Teslaman,China).A micro-ampere meter(C65,Harbin Sadade Electric Technology,China)was connected into the circuit to measure the discharge current,and the voltage was recorded by an oscilloscope(DS2302A,Rigol)equipped with a highvoltage probe(P6015,Tektronix).The discharge photographs were taken by a digital camera(Canon 6D)with an exposure time of 15 s,and the emission spectra were acquired by a spectrometer(Acton SP 2500i,Acton,USA).The camera and spectrometer were all located in a direction perpendicular to the needles.A mass flow controller(MFC D08-4F,Sevenstar Electronics Co.,Ltd,China)was used to control the flow rate of gas introduced into the plasma reactor.Simulated air(20%O2,balance N2)of 100 ml min−1was injected into the plasma reactor through the hollow needles and flowed through the stainless-steel mesh electrode during the discharge(see figure 1).All discharge experiments were performed at atmospheric pressure and a temperature of 27 °C.
2.2.Nanocatalysts preparation and characterization
In this work,the Au/TiO2plasmonic nanocatalyst was prepared with a modified impregnation method using TiO2nanocatalyst(Degussa P25,powder)as the support[29].Briefly,an aqueous solution of HAuCl4(2.43×10−2mol l−1)was first impregnated into the TiO2support with a nominal Au loading of 1 wt%.Then,the impregnated sample was aged for 12 h,and washed with the deionized water and aqueous ammonia solution(pH 8)sequentially.Finally,the sample was dried at 80 °C for 6 h to obtain the as-prepared Au/TiO2.To construct the PPS,the nanocatalysts were integrated with the stainless-steel mesh via a dip-coating method[29].First,18 mg of nanocatalyst powder was added to 1 ml of deionized water and sonicated for 20 min.Then the slurry was coated on the mesh,and dried at 150°C for 1 h to obtain the coating.The weight of the coating was 13±2 mg.The PPS integrated with the Au/TiO2is denoted as PPS(Au/TiO2).For comparison,we also constructed a PPS(TiO2)from a combination of TiO2and a needle array corona discharge.
X-ray photoelectron spectroscopy(XPS;ESCALAB250,Thermo VG,USA)with a monochromatized Al Kα(1486.6 eV)x-ray source operating at 15 kV was applied to measure the surface chemical states of the nanocatalysts.The measurements were performed in the analytical chamber at 10−8Pa after evacuating the samples in the fore-chamber of the XPS spectrometer.The peak of C 1s(284.6 eV)was used to calibrate the binding energies.
Optical properties of the nanocatalysts were analyzed by ultraviolet–visible diffuse reflectance spectra(UV–vis DRS)using a spectrometer(UV-2600,Shimadzu)in the wavelength range of 200–800 nm.Barium sulfate was used as a background reference.Photoluminescence(PL)spectra were obtained with a spectrophotometer(F-7000,Hitachi,Japan)with a 150 W xenon lamp light source and monochromatic light excitation at 300 nm.
Scanning electron microscopy(SEM)was performed to obtain the morphology of theE.coli.Before the measurement,theE.colisuspension was first transferred onto a square silicon slice(5 mm×5 mm×0.6 mm)and dried at 37°C for 1 h.Then,the silicon slice was exposed to plasma under different discharge conditions.After exposure,the silicon slice was placed into a phosphate buffer solution of glutaraldehyde for 24 h to immobilize theE.coli.Subsequently,the silicon slice was washed by phosphate buffer saline solution(0.03 mol l−1)and alcohol solution to obtain the desired sample.Finally,the slice was coated with an ultra-thin layer of platinum by ion sputtering and observed with an electron microscope(S-800,Hitachi)and a JSM-7800F field emission SEM operating at 5 kV.
2.3.Sterilization evaluation
As illustrated in figure 1,E.colisterilization was performed by locating the mesh electrode over Luria–Bertani(LB)medium in a culture dish.To ensure the intimate interaction of plasma,E.coliand nanocatalysts,the air was introduced into the PPS through the hollow needles.Before the sterilization,E.colisuspension was coated on the surface of the LB medium.Subsequently,E.coliwere sterilized by the plasma.Finally,the sterilizedE.coliwere incubated at 30°C for 24 h with the purpose of evaluating their survival rate.The sterilization efficiency(η)of the PPS was evaluated by counting the colony-forming units(CFUs)(equation(1))
where CFUexposeddenotes the number of CFUs for bacteria exposed to the plasma,whereas CFUcontrolis the number of CFUs of unexposed bacteria.
The concentration of O3during the sterilization was also monitored with an O3analyzer(Mini-Hicon,USA)at the outlet of the plasma reactor(see figure 2).
3.Results and discussion
3.1.Discharge characteristics
Figure 3(a)showsI–Vcharacteristic curves of the PPS(Au/TiO2)at variousdNMvalues.It can be seen that the discharge current increases with increasing voltage at differentdNM,and an increase indNMleads to an increase in the discharge current at the same voltage.At the same voltage,the corona discharge with smalldNMfacilitates the achievement of a high discharge current.However,a smalldNMis prone to generate an unstable spark discharge at high discharge voltages.Therefore,we chose a moderatedNMof 8 mm for the sterilization in this work.In figure 3(b),theI–Vcharacteristic curves between the corona discharge and PPS are compared.Moreover,typical discharge photographs for the PPS(Au/TiO2)are illustrated in insets of figure 3(b).The nanocatalysts present in the PPS show an obvious influence on the corona discharge.Compared with the pure corona discharge,the discharge current of the PPS(TiO2)is decreased because the TiO2(semiconductor material)over the mesh electrode suppresses the conduction of discharge current between the needles and the mesh electrode.In contrast,the PPS(Au/TiO2)exhibits a higher discharge current than the pure corona discharge.This is because highly dispersed metallic Au nanoparticles on the TiO2surface facilitate an expansion of plasma over the mesh electrode,leading to an increase in the discharge current at the same discharge voltage.For the PPS(Au/TiO2),the expansion of plasma over the mesh electrode is reflected by the discharge photographs(insets of figure 3(b)).A faint light is observed around the needle tips at a low voltage of 4.1 kV,and the discharge region spreads to the mesh electrode with increasing voltage to 4.8 kV.With further rising voltage to 5.7 kV,the discharge region becomes much brighter,and an obvious expansion of plasma over the mesh electrode can be observed(see inset of figure 3(b)).The discharge characteristic of the PPS(Au/TiO2)could enhance the interaction between plasma,E.coliand Au/TiO2,which would benefit sterilization.Figure 3(c)shows the typical emission spectra of the PPS(Au/TiO2).Emission lines of N2in the UV–vis region are clearly observed,indicating that the corona discharge can directly inactivate theE.coliby UV light.Furthermore,UV–vis light emitted from the plasma would excite the plasmonic nanocatalysts to generate electron–hole pairs,which contribute to the formation of reactive radicals and thus the oxidative inactivation ofE.coli.The gas temperature of the discharge is evaluated via SPECAIR fitting[30,31].In figure 3(d),SPECAIR fitting of the measured N2(C3Пu→B3Пg)spectrum for the PPS(Au/TiO2)is illustrated.The rotational temperature of the PPS(Au/TiO2)is about 310 K at a discharge voltage of 5.9 kV.This suggests that the sterilization effect of the PPS(Au/TiO2)onE.colioriginates from energetic electrons,reactive radicals and light radiation rather than the high temperature.
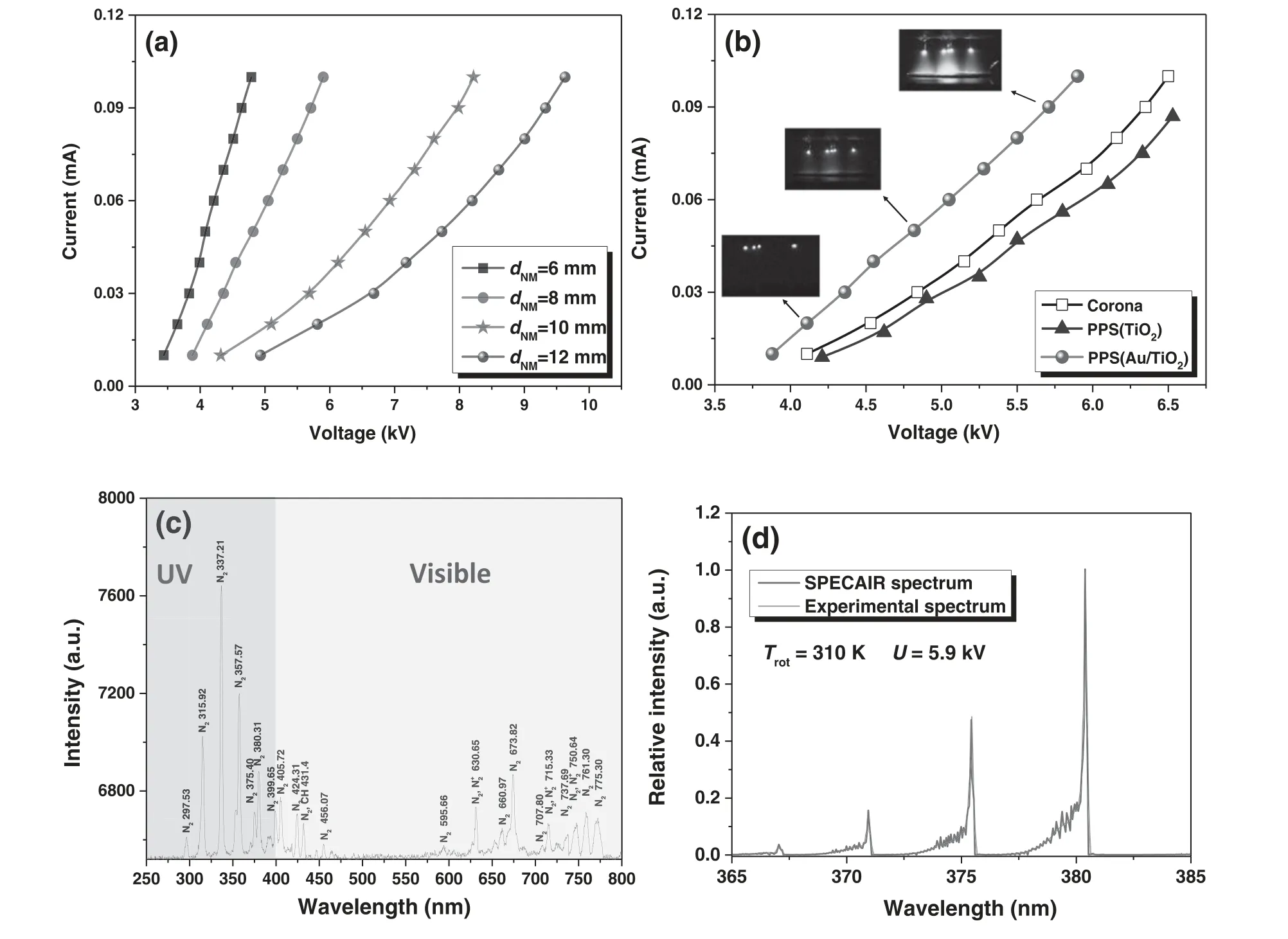
Figure 3.(a)I–V characteristics of the PPS(Au/TiO2).(b)I–V characteristics of different corona discharge systems at the same dNM of 8 mm.(c)Emission spectra of the PPS(Au/TiO2).(d)SPECAIR fitting of the measured N2 C3Пu →B3Пg spectrum for the PPS(Au/TiO2)at voltage of 5.9 kV.Insets of(b)are discharge photographs for the PPS(Au/TiO2)at a dNM of 8 mm.
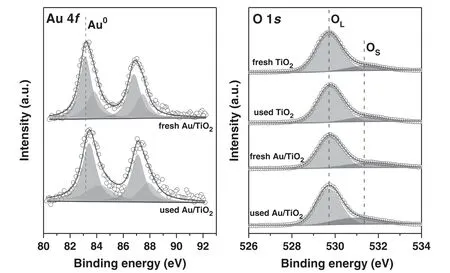
Figure 4.XPS spectra of Au 4f and O 1s for fresh nanocatalysts before and after the sterilization process.
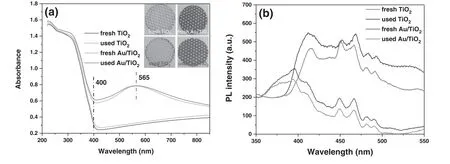
Figure 5.UV–vis DRS(a)and PL spectra(b)for the nanocatalysts before and after the sterilization process.The insets in(a)are photographs of the mesh electrodes coated by fresh and used nanocatalysts.The used samples were taken from the PPS after 8 min continuous sterilization.
3.2.Surface and optical properties of the nanocatalysts
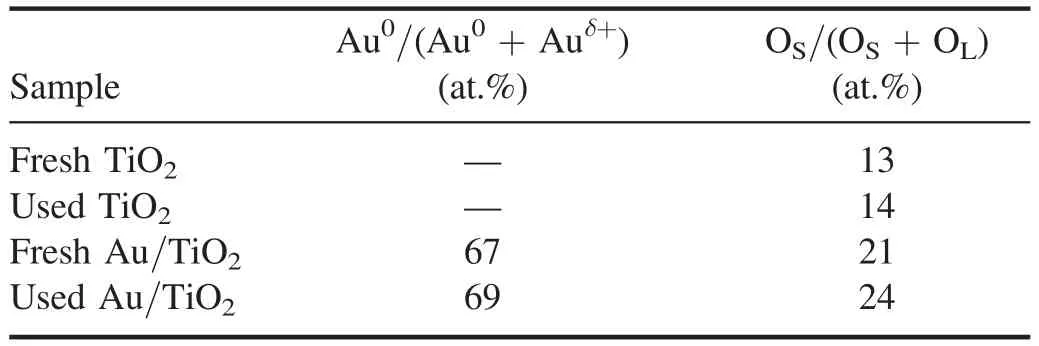
Table 1.XPS analysis of Au 4f and O 1s based on figure 4.
To understand the influence of the surface chemical state of the nanocatalysts onE.colisterilization,XPS measurement were performed for the nanocatalysts before and after the sterilization.In figure 4,the Au 4f and O 1s binding energies are deconvoluted,and the contents of metallic Au(Au0)and surface oxygen(OS)are summarized in table 1.The Au/TiO2possesses a high content of Au0(>60 at.%)before and after the sterilization process.This facilitates the expansion of plasma over the mesh electrode[32]and thus the interaction between plasma and Au/TiO2during the discharge.Furthermore,the presence of Au nanoparticles would create numbers of Au|TiO2interfaces to generate highly active oxygen species,thus promoting the oxidative inactivation processes.After the sterilization,the binding energy of Au 4f in the used Au/TiO2exhibits a slight blue shift(see figure 4)because of the formation of oxygen species at Au|TiO2interfaces.Figure 4 also shows that the O 1s spectra for the nanocatalysts are deconvoluted into OSand lattice oxygen(OL).According to table 1,for fresh samples,the introduction of Au nanoparticles onto the TiO2surface makes OScontent increase from 13 at.% to 21 at.%,indicating the favorable formation of OSspecies at Au|TiO2interfaces.This suggests that the PPS(Au/TiO2)would exhibit great advantages over the PPS(TiO2)in the oxidative inactivation ofE.coli.It is worth noting that the samples used possess higher OScontent than the fresh samples,which is probably due to the adsorption of OSon the nanocatalyst surface during the discharge.
We further measured UV–vis DRS and PL spectra of the nanocatalysts with the purpose of evaluating their response to light emitted from the discharge.Due to the existence of TiO2,strong absorption bands for UV light(wavelength<400 nm)can be observed for all the nanocatalysts(see figure 5(a)).Further,metallic Au nanoparticles in the Au/TiO2can exhibit localized surface plasmon resonance(LSPR)under visible light irradiation[33,34],making an obvious absorption band for the Au/TiO2appear at about 565 nm.This absorption is also evidenced by the extinction features of the nanocatalysts.According to the insets in figure 5(a),the mesh electrodes coated with TiO2and Au/TiO2present as pale white and dark purple coatings,respectively.Figure 5(a)also illustrates that the used Au/TiO2still strongly absorbs visible light,indicating good stability during the sterilization.Actually,the performance of nanocatalysts in photocatalysis is not only related to the generation of electron–hole pairs but also depends on the lifetime of the electron–hole pairs[35].PL spectra of the nanocatalysts show that the electron–hole pairs formed on Au/TiO2possess a much longer lifetime than those of TiO2(see figure 5(b)),since an intense PL peak corresponds to a short electron–hole pair lifetime.Here,the migration of the electrons to Au nanoparticles contributes to the prolonged lifetime of the electron–hole pairs on Au/TiO2[36],which suggests that Au/TiO2can more efficiently drive the oxidative inactivation processes.
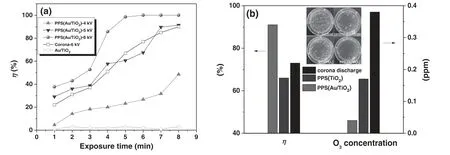
Figure 6.Sterilization efficiency(η)of the PPS(Au/TiO2)as a function of exposure time at different discharge voltages(a)and comparison of corona discharge,PPS(TiO2)and PPS(Au/TiO2)(b)at a voltage of 5 kV and exposure time of 8 min.The insets in(b)are photographs of the unexposed E.coli and the E.coli exposed to corona discharge and PPS.
3.3.Sterilization evaluation
Figure 6 shows the sterilization performances of the corona discharge,PPS and Au/TiO2.From figure 6(a),it can be seen that the increase of both discharge voltage and exposure time have positive roles in promoting η of the PPS(Au/TiO2).This is because the increase in voltage and exposure time would enhance the interaction of plasma,E.coliand Au/TiO2.TheE.colican be totally inactivated in the PPS(Au/TiO2)within 5 min at a voltage of 6 kV.However,after 8 min continuous sterilization,the PPS(Au/TiO2)can only obtain an η of 91% and 49% at voltages of 5 kV and 4 kV,respectively.It is noted that total inactivation ofE.colican be achieved by prolonging the exposure time even at relatively low discharge voltages.The PPS(Au/TiO2)can totally sterilize theE.coliwithin 15 min and 30 min at 5 kV and 4 kV,respectively.This suggests that the sterilization efficiency of the PPS(Au/TiO2)could be effectively improved by changing the process conditions(e.g.exposure time,discharge voltage).To demonstrate the synergistic effect between the corona discharge and Au/TiO2,figure 6(a)also shows η of the corona discharge and Au/TiO2.At 6 kV,η for the pure corona discharge increases from 22% to 90% with increasing exposure time from 1 min to 8 min.The Au/TiO2cannot sterilize theE.coliwithout the presence of a discharge.Apparently,the PPS(Au/TiO2)exhibits much better performance than the sum of corona discharge and Au/TiO2,indicating a synergy between the corona discharge and Au/TiO2.To confirm the advantage of the PPS(Au/TiO2)in the sterilization,the performances of corona discharge,PPS(TiO2)and PPS(Au/TiO2)at 5 kV and an exposure time of 8 min are compared in figure 6(b).Under the same conditions,pure discharge,the PPS(TiO2),and PPS(Au/TiO2)show distinct performance in the sterilization.Compared with the unexposedE.coli(insets of figure 6(b)),the corona discharge obtains an η of 73%,while the PPS(Au/TiO2)increases η to 91%.Nevertheless,the PPS(TiO2)only achieves an η of 66%.This implies that the performance of the PPS in the sterilization greatly depends on the nanocatalysts.Figure 6(b)also compares the concentration of O3for different discharge systems in the sterilization process.The corona discharge can generate 0.38 ppm O3,whereas O3concentrations for the PPS(TiO2)and PPS(Au/TiO2)are 0.17 ppm and 0.04 ppm,respectively.This suggests that the presence of nanocatalysts(especially Au/TiO2)in the discharge system could effectively suppress the formation of the secondary pollutant O3.In addition,NOxwas not detected during the sterilization,which might be attributed to the relatively weak discharge intensity of the corona.

Figure 7.SEM photographs of E.coli:unexposed control(a)and exposed to corona discharge at 6 kV for 2 min(b),4 min(c)and 6 min(d).
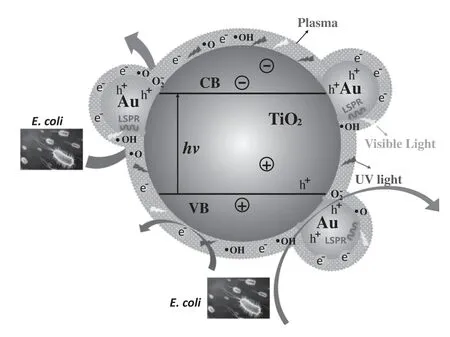
Figure 8.Schematic diagram of the sterilization occurring in the PPS(Au/TiO2).
3.4.Enhancement of the sterilization mechanism in the PPS(Au/TiO2)
The application of plasma in the sterilization ofE.colihas been intensively investigated by researchers[37–39].It has mostly been accepted that large numbers of energetic electrons and reactive radicals in the plasma can directly damage and inactivate theE.coli[7].Moreover,the UV light emitted from the discharge would irradiate the surface of theE.coli,which contributes to the sterilization[7,39].The PPS also possesses the above two sterilization routes.As shown in figure 7,at the same discharge voltage,the influence of the PPS(Au/TiO2)on the morphology ofE.coligradually becomes obvious as the exposure time is increased.The energetic electrons and reactive radicals as well as the UV light irradiation cannot effectively damageE.coliwithin a short exposure time(<4 min),and thus the morphology of theE.colishows no obvious change after the sterilization(see figure 7).When the exposure time is increased to 4 min,damage to theE.colican be observed,and ruptures,holes and deformations appear in the SEM photographs.Notably,with increasing the exposure time to 6 min,the interaction between plasma andE.colican be enhanced,and thus the ruptures,holes and deformations can be more clearly observed in figure 7.
This study demonstrates that the PPS(Au/TiO2)achieves an improved sterilization performance compared with the pure corona discharge,and the enhancement mechanism mainly originates from the interaction between corona discharge and Au/TiO2.A schematic diagram of the sterilization ofE.coliis illustrated in figure 8.From figure 8,it can be seen that the positive effect of the PPS(Au/TiO2)onE.colisterilization lies in the following aspects.First,Au/TiO2in the plasma modulates the discharge characteristics,which facilitates the generation of energetic electrons and radicals[32,40].The Au nanoparticles on the Au/TiO2surface extend the plasma region over the mesh electrode and increase the discharge current(see figure 2(b)).This endows the PPS(Au/TiO2)with a much higher density of energetic electrons and radicals,benefiting the interaction between electrons,radicals andE.coli(see figure 8).In contrast,without the presence of Au nanoparticles,TiO2over the electrode suppresses the increase in the discharge current and the generation of electrons and radicals,and thus leads to reduced sterilization efficiency.Second,the Au/TiO2could absorb UV–vis light emitted from the discharge to allow electron–hole recombination(see figure 5).However,the corona discharge and PPS(TiO2)can only utilize UV light.This suggests that the PPS(Au/TiO2)more efficiently drives the generation of radicals and boosts the oxidative inactivation ofE.coli.Third,the presence of nanocatalysts in plasma can modulate the distribution of products,accelerating the decomposition of undesired O3(see figure 5(b)).In particular,the Au/TiO2creates larger numbers of active Au/TiO2interfaces for O3decomposition[32],which not only provides numerous highly reactive oxygen species for the oxidation inactivation ofE.colibut also suppresses the formation of the secondary pollutant O3.
4.Conclusion
A PPS(Au/TiO2)was constructed via the integration of a needle array corona discharge and Au/TiO2plasmonic nanocatalyst with the purpose of promoting sterilization efficiency.The strong synergistic effect between the corona discharge and Au/TiO2enables improved sterilization efficiency as well as a reduced O3concentration compared with the corona discharge and the PPS(TiO2).At 5 kV and an exposure time of 8 min,the presence of Au/TiO2improves the sterilization efficiency of the corona discharge from 73% to 91%,while reducing the O3concentration from 0.38 to 0.04 ppm.The presence of Au/TiO2makes the corona discharge extend over the mesh electrode,and thus enhances the interaction between plasma,E.coliand Au/TiO2.The LSPR effect of Au nanoparticles makes Au/TiO2respond to light within the UV–vis region,enabling the utilization of light emitted from the discharge and thus the efficient formation of reactive radicals via photocatalytic reactions.Further,the Au|TiO2interfaces of the Au/TiO2provide active catalytic sites for the generation of oxidative species and the decomposition of O3during the discharge.Thus,the oxidative inactivation ofE.coliis enhanced and the formation of O3is suppressed in the PPS(Au/TiO2).The improved performance of PPS(Au/TiO2)in the sterilization provides a promising strategy for developing a high-efficiency sterilization technique.
Acknowledgments
We gratefully acknowledge financial support from National Natural Science Foundation of China(Nos.52041001,21808024),Natural Science Foundation of Liaoning Province(No.2020-MS-126)and Special Foundation for Key Fields of Colleges and Universities in Guangdong Province(No.2021ZDZX4094).
 Plasma Science and Technology2023年1期
Plasma Science and Technology2023年1期
- Plasma Science and Technology的其它文章
- Development of miniaturized SAF-LIBS with high repetition rate acousto-optic gating for quantitative analysis
- Rotor performance enhancement by alternating current dielectric barrier discharge plasma actuation
- A nanoparticle formation model considering layered motion based on an electrical explosion experiment with Al wires
- Focused electron beam transport through a long narrow metal tube at elevated pressures in the forevacuum range
- A study of the influence of different grid structures on plasma characteristics in the discharge chamber of an ion thruster
- High-resolution x-ray monochromatic imaging for laser plasma diagnostics based on toroidal crystal
