Breath-by-breath measurement of exhaled ammonia by acetonemodifier positive photoionization ion mobility spectrometry via online dilution and purging sampling
Lu Wang , Dandan Jiang , Lei Hua , Chuang Chen , Dongming Li ,Weiguo Wang , Yiqian Xu , Qimu Yang , Haiyang Li ,*, Song Leng
a CAS Key Laboratory of Separation Science for Analytical Chemistry, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian,116023,China
b Health Management Center, The Second Affiliated Hospital of Dalian Medical University, Dalian,116023, China
c University of Chinese Academy of Sciences, Beijing,100049, China
d Dalian Key Laboratory for Online Analytical Instrumentation, Dalian,116023, China
e Dalian Engineering Research Center of Breath Diagnostic Technology, Dalian,116023, China
Keywords:
Photoionization ion mobility spectrometry
Acetone modifier
Exhaled ammonia
Breath analysis
ABSTRACT Exhaled ammonia (NH3) is an essential noninvasive biomarker for disease diagnosis.In this study, an acetone-modifier positive photoionization ion mobility spectrometry(AM-PIMS)method was developed for accurate qualitative and quantitative analysis of exhaled NH3 with high selectivity and sensitivity.Acetone was introduced into the drift tube along with the drift gas as a modifier, and the characteristic NH3 product ion peak of (C3H6O)4NH4+ (K0 = 1.45 cm2/V·s) was obtained through the ion-molecule reaction with acetone reactant ions (C3H6O)2H+ (K0 = 1.87 cm2/V·s), which significantly increased the peak-to-peak resolution and improved the accuracy of exhaled NH3 qualitative identification.Moreover,the interference of high humidity and the memory effect of NH3 molecules were significantly reduced via online dilution and purging sampling, thus realizing breath-by-breath measurement.As a result, a wide quantitative range of 5.87-140.92 μmol/L with a response time of 40 ms was achieved, and the exhaled NH3 profile could be synchronized with the concentration curve of exhaled CO2.Finally, the analytical capacity of AM-PIMS was demonstrated by measuring the exhaled NH3 of healthy subjects, demonstrating its great potential for clinical disease diagnosis.
1.Introduction
Ammonia (NH3) is a key component of biological processes in almost all organs,and the dynamic balance state of ammonia in the body is largely dependent on the liver and kidneys [1,2].The liver and kidney dysfunction will decrease the abilities to convert NH3to urea and to excrete NH3and urea, resulting in an increased accumulation of blood ammonia and urea nitrogen [3-5].The excess urea then penetrates the gastrointestinal tract through the mucosal capillaries and is decomposed into CO2and NH3by ureaseproducing bacteria in the gastrointestinal tract, which aggravates hyperammonemia and azotemia.These pathophysiological changes are reflected in the blood ammonia concentration.However, the measurements of blood ammonia concentrations are greatly affected by sampling, transport and storage conditions of blood specimens [6].
Fortunately, the partial pressures of NH3in alveolar gas and arterial plasma are almost equal,and NH3exchange occurs quickly through the alveolar interface, which can be detected in exhaled breath[7].When NH3generation and removal are imbalanced,the concentration of exhaled NH3changes.Thus, it is of great clinical value to detect exhaled NH3to determine disease status such as in cirrhosis, hepatic encephalopathy [4,8], chronic kidney failure,renal hemodialysis[5,9,10],Helicobacter pylori infection[11,12],and halitosis [13,14].
The relatively low concentration of NH3in exhaled gas(11.74-117.43 μmol/L for healthy people [15]), the strong adsorbability of NH3, and the complex composition and 100% relative humidity (RH) of exhaled gas necessitate extremely high requirements of the analytical technique for accurate detection of exhaled NH3.To date, a series of analytical techniques have been exploited to measure exhaled NH3, such as photoacoustic spectroscopy [16-19], chemical or optical NH3sensors [20-24], and selected ion flow tube mass spectrometry [25,26].However, the high price, bulkiness, or complex operation limit the clinical applications of these techniques.
With its advantages of rapid detection,portability,and ease of operation and maintenance, ion mobility spectrometry (IMS) has been widely used in environmental pollution monitoring [27],clinical disease diagnosis [28], and explosives and drug detection[29].Jazan et al.[30] measured exhaled NH3by using a corona discharge (CD) IMS for normal subjects and patients with renal failure.The linear dynamic range was 0.70-47.56 μmol/L for standard NH3samples with a limit of detection (LOD) of 0.39 μmol/L.Huang et al.[31] developed a63Ni source IMS to determine trace concentration of NH3in the atmosphere.The63Ni and the CD sources generated the same reactant ions, (H2O)nH+,and thus the same product ions, (H2O)nNH4+, were reported for NH3[30,31].However, both the reactant ion and the product ion are very sensitive to humidity,which affects the production rate of ion, the drift time of the ion peak, and the quantification of NH3concentration.To resolve these issues, Li et al.[27] developed a dopant-assisted photoionization(DAPI)-IMS by using 2-butanone as the dopant.Reactant ions (C4H8O)2H+were generated in the DAPI source.The product ion (C4H8O)NH4+was specifically and sensitively detected against a background of high humidity and complex matrices.With a LOD of 0.23 μmol/L and a quantitative range of 1.17-46.97 μmol/L, DAPI-IMS was used for atmospheric NH3measurement via on-site monitoring of the day-to-day emission profiles in a toilet.
In this study, acetone-modifier positive photoionization ion mobility spectrometry (AM-PIMS) was developed for breath-bybreath measurement of human exhaled NH3.Acetone was introduced into the drift tube as a modifier to improve the peak-to-peak resolution(Rp-p)between the reactant ion peak and the product ion peak.Furthermore, an online dilution and purging sampling strategy was employed to eliminate the interference of exhaled breath moisture and the memory effects.Thus, the breath-bybreath measurement of exhaled NH3was realized.Finally, the performance of the proposed method was demonstrated by measuring the exhaled NH3concentration of healthy people receiving physical examination.
2.Experimental
2.1.AM-PIMS
IMS distinguishes ionized analytes in gas phase according to the characteristic ion mobilities of different ions under a constant electric field.The home-built AM-PIMS generally consisted of a sampling system,a photoionization source,and an ion drift tube,as shown in Fig.1.The drift tube consisted of dozens of stacked stainless-steel rings and polytetrafluoroethylene (PTFE) rings.The whole lumen is divided into a reactant ionization region and an ion drift region by a tyndall-powell gate (TPG) with the lengths of 2.5 cm and 7.7 cm, respectively.The gate opening pulse was set at 50 μs.A radio-frequency vacuum ultraviolet (VUV) lamp (Steven Sepvest Corporation,Haymarket,VA,USA)with a photon energy of 10.6 eV was used as the photoionization source in the IMS drift tube.The drift tube temperature was controlled at 130°C,and the electric field was set at 600 V/cm.The reactant ions were generated near the VUV lamp and reacted with the sample molecules in the ionization region.The product ions were separated in the drift region and detected by a Faraday plate.
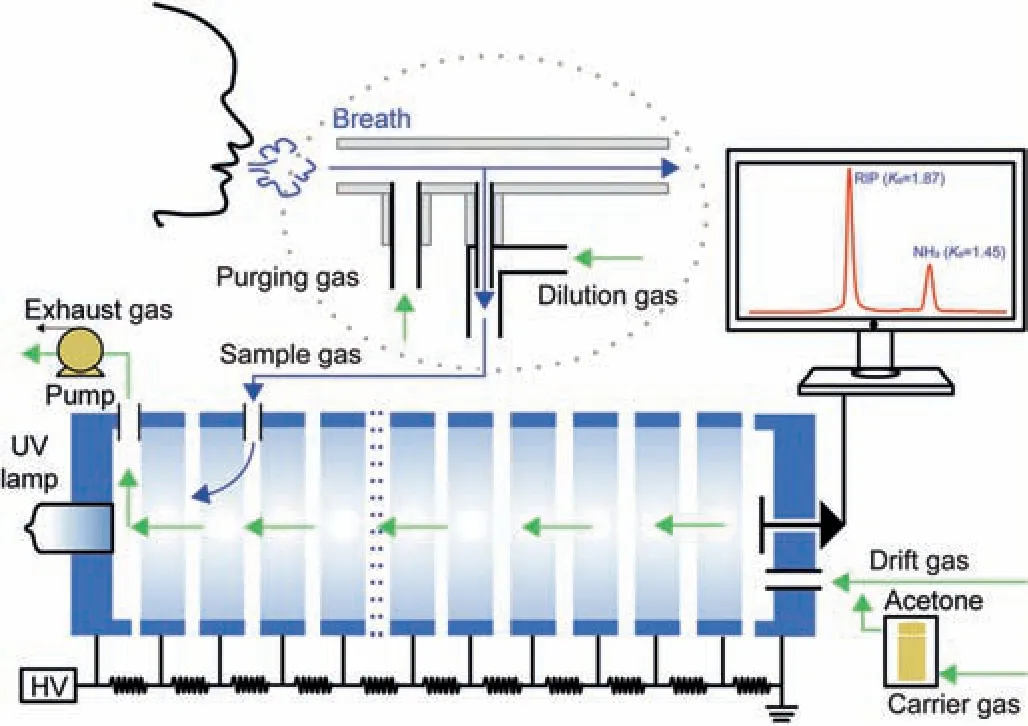
Fig.1 .Schematic drawing of acetone-modifier positive photoionization ion mobility spectrometry (AM-PIMS) and the exhaled NH3 measurement by online dilution and purging sampling.
A unidirectional gas flow mode was adopted in this study, as indicated by the green arrows in Fig.1.The purified gas was filtered through a molecular sieve and used as carrier gas,drift gas,dilution gas,and purging gas.Exhaled breath sample gas was introduced into the ionization region viaa gas inlet near the TPG,and acetone modifier reagent molecules were introduced via a drift gas inlet by carrier gas.Then,all the gases were pumped out of the drift tube.The flow rates of the carrier gas, drift gas, and exhaust gas were set at 100 mL/min,500 mL/min,and 800 mL/min,respectively,by three mass flow controllers.Thus,negative pressure in the drift tube allowed the breath sample gas to enter the ionization region automatically.
A hollow cylinder tube made of quartz was used as the main exhalation tube,as shown as dashed ovals in Fig.1.The quartz tube was 8 cm long, with an outer diameter of 1.2 cm and an inner diameter of 1 cm.A gas inlet and a gas outlet were provided at either end,and the exhaled breath samples were collected through a disposable plastic mouthpiece connected to the gas inlet.There are two hollow openings near the gas inlet of the quartz tube,namely,the purging gas inlet and the sampling port,with an inner diameter of 0.4 cm.Exhaled breath samples were extracted in the quartz tube through the sampling port, mixed with dilution gas,and introduced to the AM-PIMS through a PTFE tube of 10 cm in length and 0.25 cm in diameter.The dilution gas was introduced at the top of the PTFE tube.It serves two different purposes.For one thing, the dilution gas effectively dilutes the humidity of the exhaled sample.For another, the sample gas is surrounded by the dilution gas to reduce the contact between NH3molecules and the inner wall of the PTFE tube wall; thus the interference of NH3adsorption is eliminated.Purging gas was introduced from the side of the quartz tube into the sampling device to clean the quartz tube and ensure that the detection was not affected by NH3in the environment.The flow rates of the online dilution and purging gas were set at 150 mL/min and 2,000 mL/min, respectively.During exhaled breath sampling, the effect of the exhaled NH3concentration measurement with the purging gas could be negligible as the flow rate of the exhaled breath is much higher than that of the purging gas.A CO2sensor (C200, National Medical Co., Ltd.,Shaoxing,China)was placed at the gas outlet of the quartz tube to provide a reference profile to determine whether the exhalation was eligible during the real-time measurement of exhaled NH3.The standard NH3gas was measured without purging gas.
The subjects exhaled normally through a disposable plastic mouthpiece,and the cross-infection of subjects was avoided.All the gases in the drift tube, including the acetone modifier, were exhausted from the outlet to the outdoors by a pump.Therefore,neither the operator nor the subject was exposed to danger.
2.2.Sample preparation
Acetone was purchased from Kermel Chemicals Co., Ltd.(Tianjin, China).As a modifier, acetone was placed inside a 5-mL glass vial(Agilent Technologies Co.,Ltd.,Palo Alto,CA,USA)with a 0.1 cm diameter hole on the top of the seal.Then,the vial was placed into a stainless steel bottle with carrier gas at a flow rate of 100 mL/min,and the headspace was purged to generate a constant acetone modifier concentration of 22.38 mmol/L at 25°C.Dry NH3standard gas(0%RH)of 352.30 μmol/L in N2was purchased from Dalian Da'te Gas Company (Dalian, China).A series gas samples with different NH3concentrations were prepared by mixing the standard gas with the purified air.The RH of NH3samples could be adjusted by changing the humidity of purified gas as described [32].A dewpoint hygrometer (DP300, CS Instrument GMH, Harrislee, Germany)was placed at the sample gas inlet of the IMS to measure and record the humidity of the sample gas.
2.3.Calculations
The reduced mobility (K0) of the target analytes in the IMS system was calculated via Eq.(1).
Where Kt0and Ks0are the reduced mobilityies of the target and the standard analyte,respectively.ttdand tsdare the drift time of the target and the standard analyte, respectively.The dimer ions of dimethyl methylphosphonate (DMMP), (DMMP)2H+, with a K0of 1.40 cm2/V·s and drift time of 5.21 ms were selected as the calibration standard based on the commonly used method.
2.4.Online analysis of breath sample from volunteers
Seven volunteers(Group 1)recruited from our laboratory were on an unrestricted diet intake.Group 2 consisted of 85 healthy volunteers from the Health Management Center of the Second Affiliated Hospital of Dalian Medical University (52 males, 33 females,age:49±11 years,body mass index:24.8±3.4 kg/m2).They were asked to fast for at least 8 h before the measurement, to strictly control oral hygiene, to avoid physical exercise, and to abstain from smoking for at least 2 h.The volunteers were asked to relax and completely exhale in a normal posture for breath sampling.They were required to exhale calmly into a quartz tube via a disposable mouthpiece.The collection was repeated at least five exhalations for each volunteer,and the results were averaged.This study was approved by the Ethics Committee of the Second Affiliated Hospital of Dalian Medical University (No.V02, 20,210,715),and all the volunteers signed informed consent forms.
3.Results and discussion
3.1.Effect of acetone-modifier introduction mode on exhaled NH3 qualitative identification
Different introduction flow modes of the acetone reagent molecules had a great effect on the qualitative identification of NH3.Therefore,the influence of the two introduction flow modes on the detection of 14.68 μmol/L NH3(0%RH)was tested and compared,as shown in Fig.2.In both flow modes, acetone reagent molecules(ionization energy(IE)=9.7 eV,proton affinity(PA)=812 kJ/mol)underwent direct photoionization and self-protonated process successively, and eventually a large number of acetone dimer ions(C3H6O)2H+were generated as the reactant ions with a drift time of 3.90 ms and a K0of 1.87 cm2/V·s (R1-R3).

Fig.2 .Two different introduction flow modes of acetone reagent molecules.(A)In flow mode A,NH3 and acetone were introduced via two gas inlets close to the Tyndall Powell gate in ionization region.(B)In flow mode B,acetone was introduced via the drift gas inlet with drift gas.The ion mobility spectra of 14.08 μmol/L NH3 were shown in(C)flow mode A and (D) flow mode B.RIP: reactant ion peak.
In flow mode A,as shown in Fig.2A,acetone reagent molecules were directly introduced into the ionization region via the gas inlet near the TPG, and the exhaled gas was introduced via the sample gas inlet between the gas outlet and the reagent molecule inlet.The NH3product ion with drift time of 3.68 ms and a K0of 1.98 cm2/V·s was assigned as (C3H6O)NH4+generated by the proton transfer reaction between the reactant ions(C3H6O)2H+and NH3molecules[27],as shown in Fig.2C and R4.The peak position of(C3H6O)NH4+appeared before the reactant ion peak (RIP) in mode A, and difference in the drift time between the two peaks was 0.22 ms.
In flow mode B,acetone reagent molecules were introduced via the drift gas inlet,as shown in Fig.2B.As acetone was distributed in the whole drift tube, a cluster reaction between the NH4+ion and acetone molecules occurred in the drift region.Thus, a characteristic product ion peak of NH3appeared after the RIP with a drift time of 5.16 ms and a K0of 1.45 cm2/V·s was assigned as(C3H6O)4NH4+(R5),as reported in reference[33].The difference in the drift time between the (C3H6O)4NH4+and RIP peaks was 1.26 ms.
According to Eq.(2), the Rp-pof the NH3product ion peak and RIP in flow mode B was calculated to be 7.27, which exhibited an improvement factor of 6.5 over that in flow mode A (Rp-p= 1.08).Here,td1and td2are the drift time of the two ion peaks,and FWHM1and FWHM2are the full width at half maximum(FWHM)of the two ion peaks, respectively.The enhancement of Rp-peliminated the interference of adjacent high-intensity RIP peak on the NH3product ion peak quantification, which could greatly improve the accuracy of qualitative identification of exhaled NH3.
Moreover, there were no interference peaks in the IMS spectra for exhaled NH3analysis,as shown in Fig.S1.The other components in the exhaled gas did not interfere with the qualitative determination of NH3.Therefore, acetone is highly selective for the qualitative determination of exhaled NH3as a modifier in positive photoionization IMS, and the acetone modifier introduction flow mode B was chosen in this study for its improved resolution.
3.2.Online dilution sampling to reduce the influence of exhaled humidity
Humidity is a non-negligible interference factor in breath analysis.Water molecules were involved in the proton transfer reactions with acetone reactant ions and NH3molecules, presenting a challenge for exhaled NH3measurements.Thus,the influence of humidity ranging from 0% RH to 100% RH on the qualitative and quantitative detection of NH3with direct sampling and dilution sampling was explored, as shown in Fig.3.
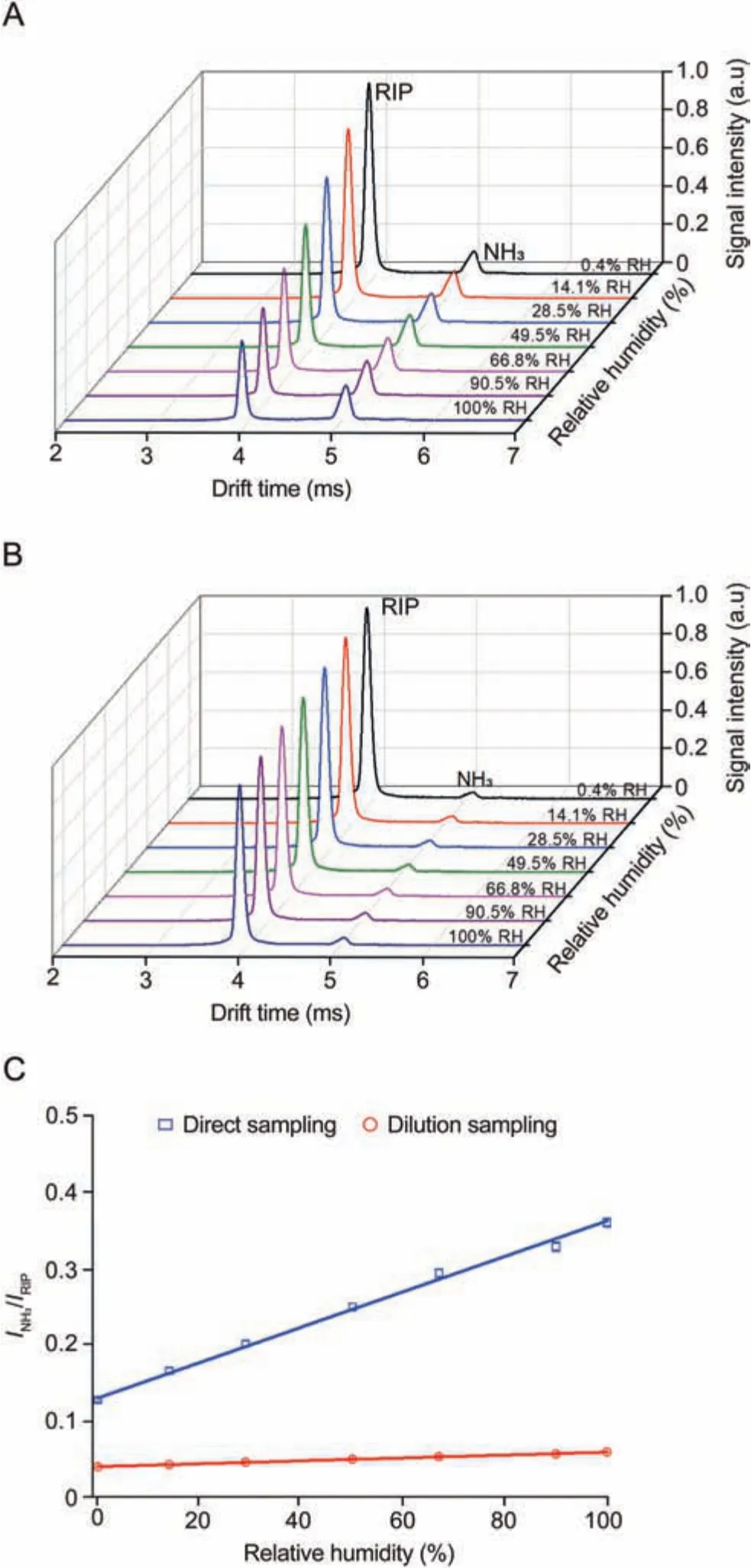
Fig.3 .Ion mobility spectra of 5.87 μmol/L NH3 at different relative humidity (RH, %)with(A)direct sampling and(B)dilution sampling,respectively.(C)Comparison of the quantitative factor(INH3/IRIP)of 5.87 μmol/L NH3 at different RH under direct sampling and dilution sampling.RIP: reactant ion peak.
Direct sampling means that the exhaled breath gas (100% RH)was directly sampled into the ionization region at a flow rate of 200 mL/min without any sample dilution.As shown in Fig.3A,with the increase of the RH of sample gas,the signal intensity of the RIP extremely reduced due to the absorption of VUV photons by water molecules, while the signal intensity of the NH3product ion peak increased significantly.
Although the proton affinity of water (PA = 691.0 kJ/mol) is lower than that of acetone, as shown in R6, water clusters(PA = 827.0 kJ/mol) with higher proton affinity could react with protonated acetone(PA=853.6 kJ/mol)in the ionization region due to the high humidity of the exhaled breath, as shown in Table S1[27,34].
Moreover, the proton affinity of NH3was higher than that of the water cluster (H2O)n, as reported in Ref.[34].As a result, the signal response of NH3was improved.However, when the sample gas entered the drift region, the humidity was almost zero.Sufficient acetone was introduced into the drift region, where a high concentration of acetone dominated.The hydrated NH3ion reacted with the acetone molecules.Thus, all the NH4+and its hydrates disappeared, and a new ion, (C3H6O)4NH4+, appeared and was formed through reaction in R7 and R8.
Therefore, the product ion species of the NH3in the spectra remained unchanged,but the signal intensity of the NH3product ion peak varied considerably with the RH of sample gas in the direct sampling strategy.However, during the actual exhalation detection process, the RH of the exhaled gas varied from 80% to 100%.It is challenging to quantify exhaled NH3accurately,and it is necessary to reduce the interference of the humidity.A method of online dilution sampling was developed to minimize the effect of sample humidity.After mixing with the online dilution gas at a flow rate of 150 mL/min,the RH of the exhaled NH3sample gas at a flow rate of 50 mL/minwas diluted by a factor of 4.The signal intensities of the RIP and NH3peaks could be maintained stable with the online dilution, as shown in Fig.3B.Thus,the influence of humidity in the ionization region was effectively reduced.Furthermore, to correct the influence of the change in ion intensity in the initial reaction [35], the ratio of the analyte and RIP signal intensity(INH3/IRIP)was adopted as the quantitative response factor of NH3for different concentrations.
Fig.3 C displays the relationship between the quantitative response factor (INH3/IRIP) and humidity for direct sampling and online dilution sampling.With the increase in the RH of the sample gas, the quantitative ratio increased in the direct sampling mode(blue line),while the quantitative ratio remained almost unchanged in the online dilution sampling mode (red line).As a result, combined with the online dilution gas, the interference of humidity in exhaled breath on the accurate measurement of exhaled NH3could be greatly reduced without additional dehumidification equipment.
3.3.Online purging sampling to eliminate NH3 memory effect
Although exhaled NH3can be accurately and qualitatively identified,it is difficult to achieve breath-by-breath measurements due to the adsorption of NH3.The residual NH3on the inner wall of PTFE tube affects the accuracy of the subsequent measurement.An online purging sampling strategy was developed to eliminate the memory effect of NH3.The effect of the flow rates of purging gas was investigated at five flow rates (0, 500,1,000,1,500, and 2,000 mL/min).Fig.4A shows the real-time variation curves of the exhaled NH3quantitative factor (INH3/IRIP) during a single breath at different purging gas flow rates.The actual duration of a single exhalation is approximately 3 s.The NH3signal intensity increased rapidly at the beginning of exhalation at all purging gas flow rates; however, the variation in the NH3signal intensity exhibited significant differences at the end of exhalation.When the flow rate of the purging gas was zero,the NH3signal intensity remained at a high level after the end of the exhalation due to the adsorption of NH3,and a strong background signal of NH3from the atmosphere was present before the start of exhalation.The quantitative factor(INH3/IRIP)was approximately 0.10 and was almost unaffected by the increasing purging gas flow rate,as shown in Fig.4B.The reason was that the flow rate of exhalation gas was more than 15,000 mL/min.The dilution effect of the purging gas on the exhaled NH3concentration was negligible even at 2,000 mL/min.In addition,the response time of the NH3signal from the initial signal increased until it returned to the baseline decreased from more than 40 s to 3.1 s when the flow rates of purging gas increased from 0 to 2,000 mL/min,as shown in Fig.4C.The signal intensity response profile of exhaled NH3coincided with the exhalation time until the flow rate of the purging gas reached 2,000 mL/min.It is of particular significance when detecting exhaled NH3in routine clinical applications inwhich a large number of patients may be involved.The results verified that the proposed method could significantly reduce the interference caused by NH3adsorption.
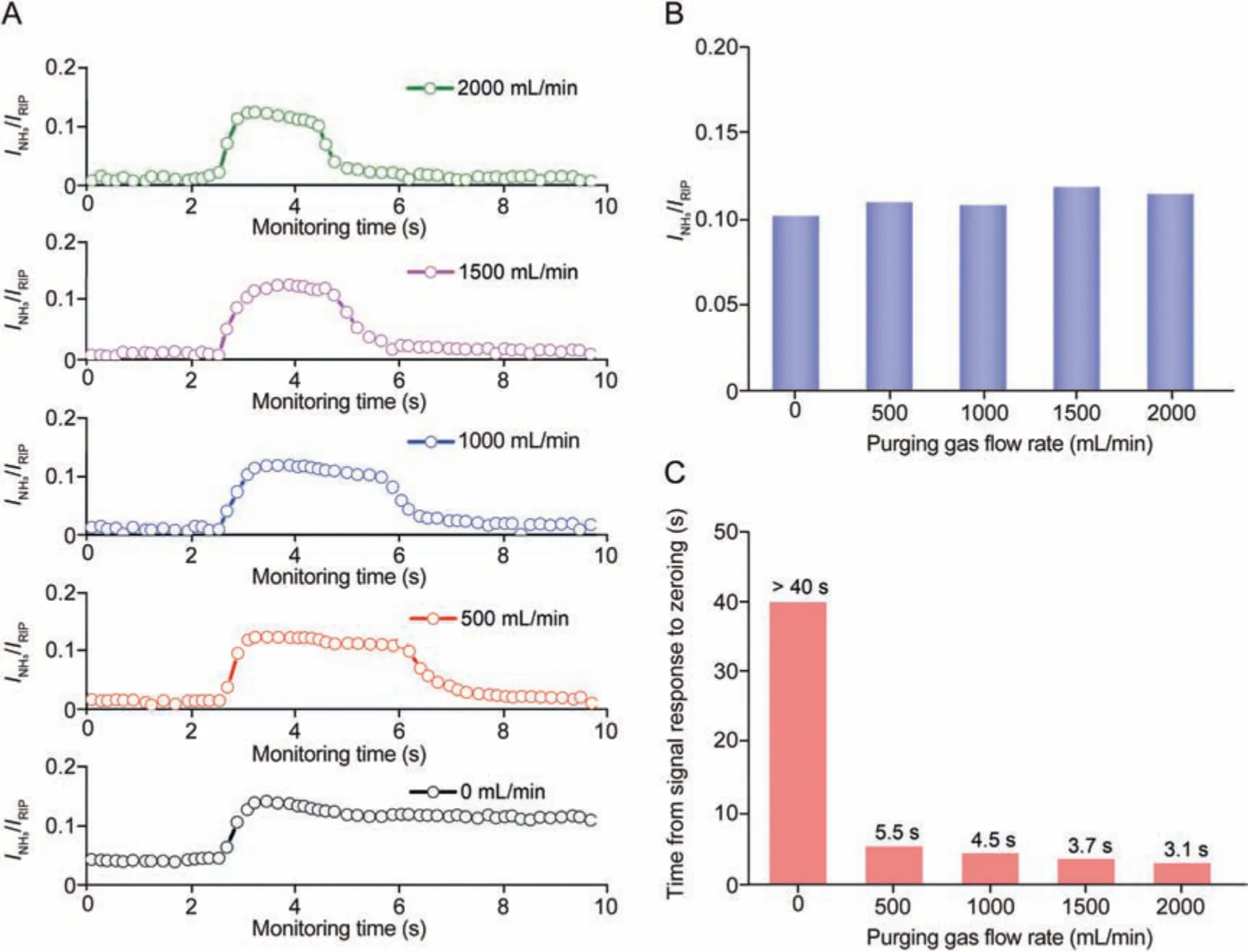
Fig.4 .(A)Real-time curves of the exhaled NH3 quantitative factor(INH3/IRIP)and(B)maximum signal response factor in each exhalation,and(C)the time from signal response to to baseline for a single exhalation at different purging gas flow rates (from zero to 2,000 mL/min).
3.4.Quantitative standard curve for NH3
To simulate the condition of high humidity of exhaled breath,NH3standard samples with 100% RH were used to obtain a quantitative standard curve.The quantitative factor INH3/IRIPwas adopted as the quantitative response factor.Considering the kinetics of the ion molecule reaction inside the ionization region [36], the quantitative model can be expressed simply by Eq.(3).
The calibration curve y=0.96(1-e-0.004x)in Fig.S2 was used to calibrate the exhaled NH3concentrations in range of 5.87-140.92 μmol/L.As shown in Figs.S2B and C, for the continuous monitoring of 11.74 μmol/L and 46.97 μmol/L NH3with 100% RH, the relative standard deviations (RSDs) of the quantitative factor (INH3/IRIPwere 2.3% and 5.8%, respectively, indicating that AM-PIMS had good stability and repeatability for the measurement of exhaled NH3.Furthermore, the LOD (signal-to-noise (S/N) = 3)was 1.29 μmol/L, which was low enough for the clinical exhaled NH3analysis.
A performance comparison of the existing techniques for exhaled NH3detection is listed in Table 1 [1,18,19,22-26,30].Among these existing techniques, including spectroscopy, sensor,mass spectrometry, and IMS, AM-PIMS exhibited the advantage of real-time sampling and online detection ability for exhaled NH3with a response time of only 40 ms and a LOD of 1.29 μmol/L.

Table 1 Comparison of AM-PIMS with other techniques for the measurement of exhaled NH3.
3.5.Accuracy, repeatability, and stability
To evaluate the accuracy and applicability of the AM-PIMS, a recovery experiment was performed on human exhaled breath.Each measurementoftheexhaledsamplewasperformedafteranoralrinse with water to reduce the dynamic variability in the exhaled NH3concentration.As shown in Table S2, the recoveries of exhaled NH3were 94.8%-96.1%, and the relative errors ranged from -2.5% to-2.2%.The experimental results demonstrated the good accuracy,repeatability, and stability of AM-PIMS for exhaled NH3measurements.
3.6.Breath-by-breath measurement of exhaled NH3 in healthy volunteers
The continuous monitoring curve of exhaled NH3is presented in Fig.5A, which demonstrated the rapid response and real-time analysis properties of the method.The concentration curve of the exhaled CO2in Fig.5B was used to reflect the complete process for each exhaled NH3measurement, and was also used as a reference profile for the breath-by-breath measurement of exhaled NH3concentrations.The exhaled NH3signal increased rapidly during the initial phase and varied with the exhalation phase.At the end of each exhalation, the NH3signal intensity decreased sharply to the baseline.The rapid response of each exhalation and the stable concentrations of exhaled NH3demonstrated that the method was reliable and effective.
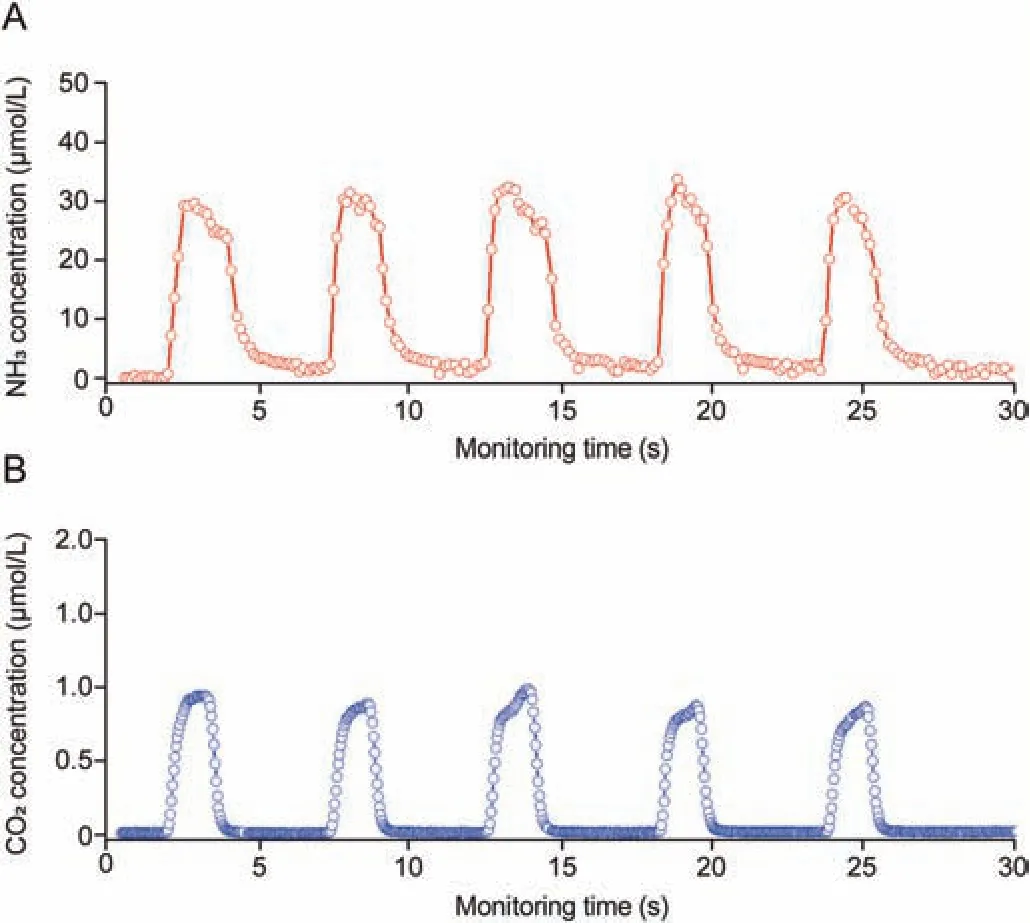
Fig.5 .(A) Concentration profile of the exhaled NH3 was synchronized with (B) the concentration curve of the exhaled CO2 for five consecutive exhalations.
To test the applicability of the AM-PIMS,two sets of exhaled NH3experiments were performed.First,the diurnal variation of exhaled NH3at different time of day was measured in seven volunteers(Group 1).The exhaled NH3concentrations varied substantially depending on the time of day, as shown in Fig.6, which might be related to an increase in the metabolic rate.The human body's metabolic rate peaks between 6 and 9 a.m.and remains relativelyactive from 9 to 12 a.m.There is also a slight increase in metabolism in the afternoon.The increased dietary intake and protein decomposition lead to a gradual increase in exhaled NH3concentration during the day.In addition, it has been reported that exhaled NH3concentrations are correlated with exercise load and oral pH level [26].
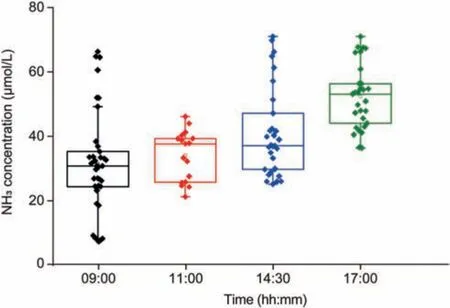
Fig.6 .Box-whisker plots of diurnal variation of exhaled NH3 concentrations for seven volunteers.
The concentration of exhaled NH3is greatly influenced by diet and metabolic rate.As a biomarker in the human body, the measurement of the exhaled NH3concentration should be performed after fasting to ensure the accuracy of the results.Therefore, to minimize the effects of diet and diurnal variations, subjects who underwent an early morning (7:30-9:30 a.m.) fast were selected for exhaled NH3detection.The histogram in Fig.7A shows the frequency distribution of exhaled NH3for healthy individuals in Group 2, exhibiting an approximately normal distribution.The concentrations of exhaled NH3(10.86-81.79 μmol/L) in this population were well within the limit of quantification(5.87-140.92 μmol/L)in all exhaled samples analyzed.The average concentration of exhaled NH3was 38.10±14.62 μmol/L with a 95% confidence interval (95% CI) of 16.68-65.94 μmol/L in this population, which was in line with that of the previous report [15].As shown in Fig.7B,the exhaled NH3concentrations were divided into two groups according to gender.A two-tailed Mann-Whitney U test was performed to examine the difference in distribution between the two groups, which showed relatively higher concentration in males (42.33 ± 14.62 μmol/L) than that in females(31.41 ± 12.10 μmol/L), with P <0.001.
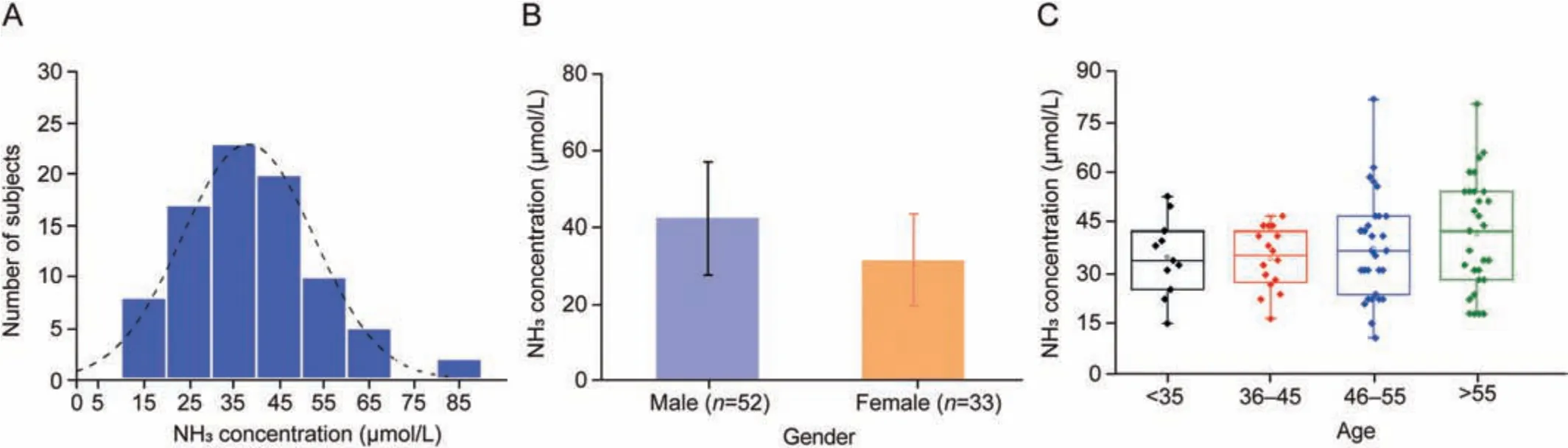
Fig.7 .(A) Frequency histogram of exhaled NH3 concentrations for healthy people with fasting.The average concentration of the exhaled NH3 was 38.10 ± 14.62 μmol/L, and the range was 10.86-81.79 μmol/L.(B) Exhaled NH3 concentrations grouped by gender.(C) Box plots of exhaled NH3 concentrations grouped based on age levels.
The box plot distribution of exhaled NH3concentrations grouped by age level shown in Fig.7C indicated a slight age-related change in exhaled NH3.The concentrations of exhaled NH3were significantly different in people younger than 35 years than in those older than 45 years old.The work of Turner et al.[37]indicated that breath NH3concentration levels had a positive relationship with age at a factor of 1.2 per decade year.These changes are attributed to normal physical aging.Aging is associated with hepatic microcirculation disturbances,accompanied by elevated hepatic vascular resistance and increased portal vein pressure [38,39].Kidney function is also affected by the complex aging process, and the glomerular filtration rate has been reported to decrease steadily with age increasing, and the progression of this process might be affected by the co-existence of diseases as well [40,41].Although the exhaled NH3concentration increases during healthy aging,the overall concentration remains in the normal range.Since there is no parenchymal organic damage evident in healthy aging,the function of the urea cycle and NH4+excretion is maintained at normal levels by the body's compensatory mechanisms.
4.Conclusions
As an essential component of biological processes and a potential biomarker for disease diagnosis, NH3produced by human metabolism can quickly enter exhaled gas for rapid and noninvasive sampling and detection.It is of great clinical value for exhaled NH3detection to determine the disease status.AM-PIMS combined with online dilution and purging sampling was developed for accurate qualitative and quantitative analysis and breath-by-breath measurement of exhaled NH3.The Rp-pof the NH3product ion peak and RIP was significantly improved by acetone modifier introduction from the drift tube, which could greatly improve the accuracy of qualitative identification of exhaled NH3.The humidity interference of exhaled gas, and the memory effects of NH3molecules were eliminated through combination with online dilution and purging sampling.AM-PIMS exhibited the advantages of realtime sampling and online detection ability for assessing the exhaled NH3concentration with a response time of only 40 ms, a LOD of 1.29 μmol/L, and a wide quantitative range of 5.87-140.92 μmol/L.The feasibility and efficiency of AM-PIMS were verified by the measurement of exhaled NH3in healthy subjects receiving a physical examination in a hospital,and demonstrated a reference concentration range of 16.68-65.94 μmol/L (95% CI) in healthy population.In this paper, the exhaled NH3measurement relied on the exhaled CO2concentration profile for quality control.However, the respiratory rate and depth were not precisely controlled in this work, which might affect the measurement of exhaled NH3concentration.Further studies will focus on the standardization of the respiratory parameters.In the future,lowercost and smaller AM-PIMS for accurate measurement of exhaled NH3will be used as a simple and point-of-care method for monitoring the variation of blood ammonia concentration, which may play an important role in real-time supervision of curative effect of dialysis and early identification and rapid diagnosis of hepatic encephalopathy.
CRediT author statement
Lu Wang: Validation, Investigation, Formal analysis; Visualization, Writing - Original draft preparation;Dandan Jiang: Conceptualization, Methodology, Visualization, Writing - Original draft preparation, Reviewing and Editing;Lei Hua: Conceptualization,Writing - Reviewing and Editing;Chuang Chen:Resources, Methodology; Visualization;Dongming Li: Resources, Data curation,Visualization;Weiguo Wang: Resources, Methodology; Visualization;Yiqian XuandQimu Yang: Investigation, Formal analysis;Haiyang Li: Conceptualization, Writing - Reviewing and Editing;Resources, Supervision;Song Leng: Resources; Supervision,Validation.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant Nos.: 22027804, 21974141, and 21904125),Natural Science Foundation of Liaoning Province(Grant Nos.: 2022-MS-019 and 2022-MS-016), Science and Technology Innovation Foundation of Dalian (Grant No.: 2022JJ13SN096),Dalian Institute of Chemical Physics(Grant Nos.:DICP I202141 and DICP I202144), and 1 + X Program for Large Cohort Study-Clinical Research Incubation Project, The Second Hospital of Dalian Medical University (Project No.:2022DXDL01).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2023.02.008.
 Journal of Pharmaceutical Analysis2023年4期
Journal of Pharmaceutical Analysis2023年4期
- Journal of Pharmaceutical Analysis的其它文章
- Neutrophil elastase: From mechanisms to therapeutic potential
- Development of a CLDN18.2-targeting immuno-PET probe for non-invasive imaging in gastrointestinal tumors
- High-throughput transcriptional profiling of perturbations by Panax ginseng saponins and Panax notoginseng saponins using TCM-seq
- A chiral metal-organic framework {(HQA)(ZnCl2)(2.5H2O)}n for the enantioseparation of chiral amino acids and drugs
- Recent applications and chiral separation development based on stationary phases in open tubular capillary electrochromatography(2019-2022)
- Quantitative characterization of cell physiological state based on dynamical cell mechanics for drug efficacy indication
