Chryseobacterium/Elizabethkingia species infections in children
Aysun Yahşi, Gülsüm İclal Bayhan, Tuğba Erat, Ahmet Yasin Güney, Seval Özen, Kübra Konca, Belgin Gülhan,Saliha Kanık Yüksek, Aslınur Özkaya Parlakay
Department of Pediatric Infectious Diseases, Ankara City Hospital, Ankara, Turkey
ABSTRACT Objective: To investigate the clinical and epidemiological features and outcome of Chryseobacterium and Elizabethkingia spp.infections in children, together with antimicrobial susceptibilities.
KEYWORDS: Chryseobacterium; Elizabethkingia;Flavobacteriaceae; Weeksellaceae
1.Introduction
Chryseobacterium and Elizabethkingia are rare pathogens in children from the Weeksellaceae family[1].The taxonomy of these organisms is confusing because of frequent changes.Some species,including Flavobacterium meningosepticum, were reclassified as Chryseobacterium in 1994[2].In 2005, Chryseobacterium (C.)meningosepticum and C.miricola were transferred to a new genus on the basis of phylogenetic characteristics named Elizabethkingia after the microbiologist who first described them[3].While Chryseobacterium and Elizabethkingia species are considered from the Flavobacteriaceae family, they have been transferred to the family Weeksellaceae[1].C.indologenes, C.odoratum, C.multivorum, C.breve, C.gleum, Elizabethkingia (E.) meningoseptica,and E.anophelis are frequently reported pathogenic members of the Weeksellaceae species.Chryseobacterium and Elizabethkingia species are aerobic, nonfermenting, oxidase-positive, Gram-negative rods commonly found in the water, soil, and environment[2,3].
C.indologenes and E.meningoseptica are the most common pathogenic species and can cause a wide range of clinical presentations and a high mortality rate[2].Although E.meningoseptica most commonly causes neonatal meningitis, it can also cause many other diseases such as sepsis, pneumonia,endocarditis, septic arthritis, and skin infection[4].C.indologenes can cause serious infections such as ventilator-associated pneumonia,bacteremia, and catheter-related blood-stream infections, as well as multidrug-resistant nosocomial infections and hospital outbreaks in pediatric and adult patients[5,6].
Identifying these microorganisms is important due to inherent resistance to multiple antibiotics that are typically prescribed for empiric treatment of Gram-negative bacterial infections, including extended spectrum β-lactam agents and aminoglycosides, and the high mortality rates of inadequately or improperly treated infections[4].Unfortunately the data on infections related to these microorganisms, especially in pediatric patients, is currently inadequate.
The aim of the current study was to document the clinical patterns,risk factors, and outcomes of pediatric patients infected with Chryseobacterium and Elizabethkingia spp.at our hospital, as well as the antimicrobial susceptibility of the strains isolated from these patients, so as to reveal the different characteristics of these two microorganisms which are of the same family.
2.Subjects and methods
2.1.Study design and population
This retrospective study was conducted at the Ankara City Hospital, which is a tertiary pediatric hospital in Turkey.Patients under 18 years of age with isolates of Chryseobacterium and Elizabethkingia spp.between March 2014 and March 2022 were included in the study.Each case was reviewed by a pediatric infectious disease specialist; isolates discarded due to contamination or colonization, as determined according to the clinical picture, were excluded from the study (Figure 1).The clinical samples collected were blood (either from a peripheral vein or through a central venous catheter), endotracheal aspirate, sputum, cerebrospinal fluid, pus, and urine.Data obtained from the records included age,sex, unit, duration of hospitalization (before culture positivity),presence of prior antibiotic treatment, history of surgery in the last month, underlying diseases, presence of colonization with resistant microorganisms, the use of indwelling catheters (such as central venous catheter, mechanical ventilation, urinary catheterization,tracheostomy, or percutaneous endoscopic gastrostomy tube),trimethoprim-sulfamethoxazole (TMP-SMX) prophylaxis, time to negative culture, laboratory results, microbiology data, antibiotic treatment, and outcome.In infected patients, the empiric treatment was changed according to the antibiotic susceptibility test results after the detection of Chryseobacterium and Elizabethkingia spp.The data were evaluated separately for the two microorganisms and a comparison was made.
2.2.Definitions
Colonization was defined as the first stage of microbial infection at the appropriate portal of entry.The presence of microorganisms in or on a host together with growth and multiplication but without clinical symptoms of infection was accepted as colonization.Colonized patients were not included in the study[6].
Significant bacteremia was defined as growth of Chryseobacterium and Elizabethkingia spp.in blood cultures as well as the clinical manifestation of signs of the systemic inflammatory response syndrome.Sepsis was defined as life-threatening organ dysfunction caused by a dysregulated host response to infection, and classified according to laboratory and clinical findings[7].Catheter-related bloodstream infection, nosocomial pneumonia, urinary tract infection, surgical site infection, and skin and soft tissue infection were defined according to the Centers for Disease Control and Prevention guidelines[8].
2.3.Microbiologic identification
Samples were inoculated on routine 5% Sheep Blood agar and MacConkey agar 5% Sheep Blood agar (BioCell, Turkiye)and MacConkey agar (BioCell, Turkiye).After 16-24 hours of incubation at 37 ℃, the growing isolates were identified with VITEK®MS (bioMérieux, France).Antimicrobial susceptibility profiles of the isolates of Chryseobacterium and Elizabethkingia were determined by VITEK®2 Compact (bioMérieux, France),and interpreted based on the Clinical and Laboratory Standards Institute’s criteria for other non-Enterobacteriaceae[9].
2.4.Ethical approval
The Ethics Committee of Ankara City Hospital approved the study with reference number 2022/E2-22-1566.
2.5.Statistical analyses
All statistical analyses were conducted using SPSS software(version 25; IBM, Chicago, IL).The data of the patients were collected retrospectively from the hospital records.Normality of distribution of the continuous variables was measured with the One-Sample Kolmogorov-Smirnov Test.The continuous variables with a normal distribution were expressed as mean±SD and compared using Student’s t-test, while the variables with a non-normal distribution were expressed as median (min-max) and compared using the Mann-Whitney U test.Categorical variables were compared using the χ2test or Fisher’s exact test.In all analyses, a P value <0.05 was considered to indicate statistical significance.
3.Results
3.1.Population
Chryseobacterium and Elizabethkingia spp.were isolated in 49 patients.Twenty-two of these patients were female (44.9%), and 27 were male (55.1%).The median age was 14 (0.21-185.0) months.Of the 49 cases, 29 (59.2%) were identified as Elizabethkingia and 20 (40.8%) as Chryseobacterium.There was no outbreak during the study period.
An underlying disease was present in 44 (89.8%) of these patients.These diseases consisted of malignancies in 21 patients,cardiovascular disorders in 13 patients, neuromotor disorders in 5 patients, chronic lung diseases in 3 patients, and renal disorders in 2 patients.Eighteen (36.7%) patients were hospitalized in the pediatric hematology oncology unit, 16 (32.7%) in the infant unit,10 (20.4%) in the intensive care unit, 3 (6.1%) in the toddler unit,and 2 (4.1%) in the neonatal service.There were 33 patients with a central venous catheter and 9 with a urinary catheter; 8 patients who were hospitalized in the intensive care units were mechanically ventilated; 6 patients had a tracheostomy and 12 patients had a percutaneous endoscopic gastrostomy tube.Of the 29 patients with Elizabethkingia infection, 25 had a central venous catheter, while only 8 of the 20 patients with a Chryseobacterium infection had a central venous catheter (86.2% vs.40.0%, P=0.001).None of the patients were receiving total parenteral nutrition.Fifteen (30.6%)patients had undergone surgery in the last month.Twenty-one patients (42.9%) diagnosed with leukemia were receiving TMPSMX prophylaxis and this rate was significantly higher in patients with Elizabethkingia infections (55.2% vs.25.0%, P=0.036).
There were 3 (6%) coinfections with other bacteria in the entire study group.No repetitive culture positivity was detected.The median duration of hospitalization before culture positivity was 25 days (1-204 days).Elizabethkingia infections were significantly more common than Chryseobacterium infections (P=0.007).The median length of hospital stay (before culture positivity) of patients infected with Elizabethkingia was longer than patients infected withChryseobacterium (28 days vs.9.5 days, P=0.007).There were 42(85.7%) patients with a history of antibiotic use in the last month,and the rate was significantly higher with Elizabethkingia infections(96.6% vs.70.0%, P=0.009).A total of 31 (63.2%) patients had used carbapenem antibiotics in the last month, and this was again significantly more common with Elizabethkingia infections(P=0.005) (Table 1).There was no significant difference between the other antibiotic groups regarding infections due to the two microorganisms.
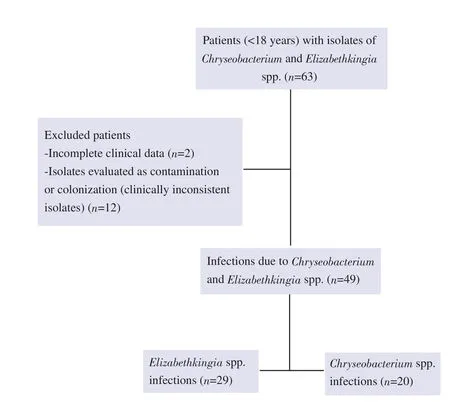
Figure 1.Flowchart of the selection process.
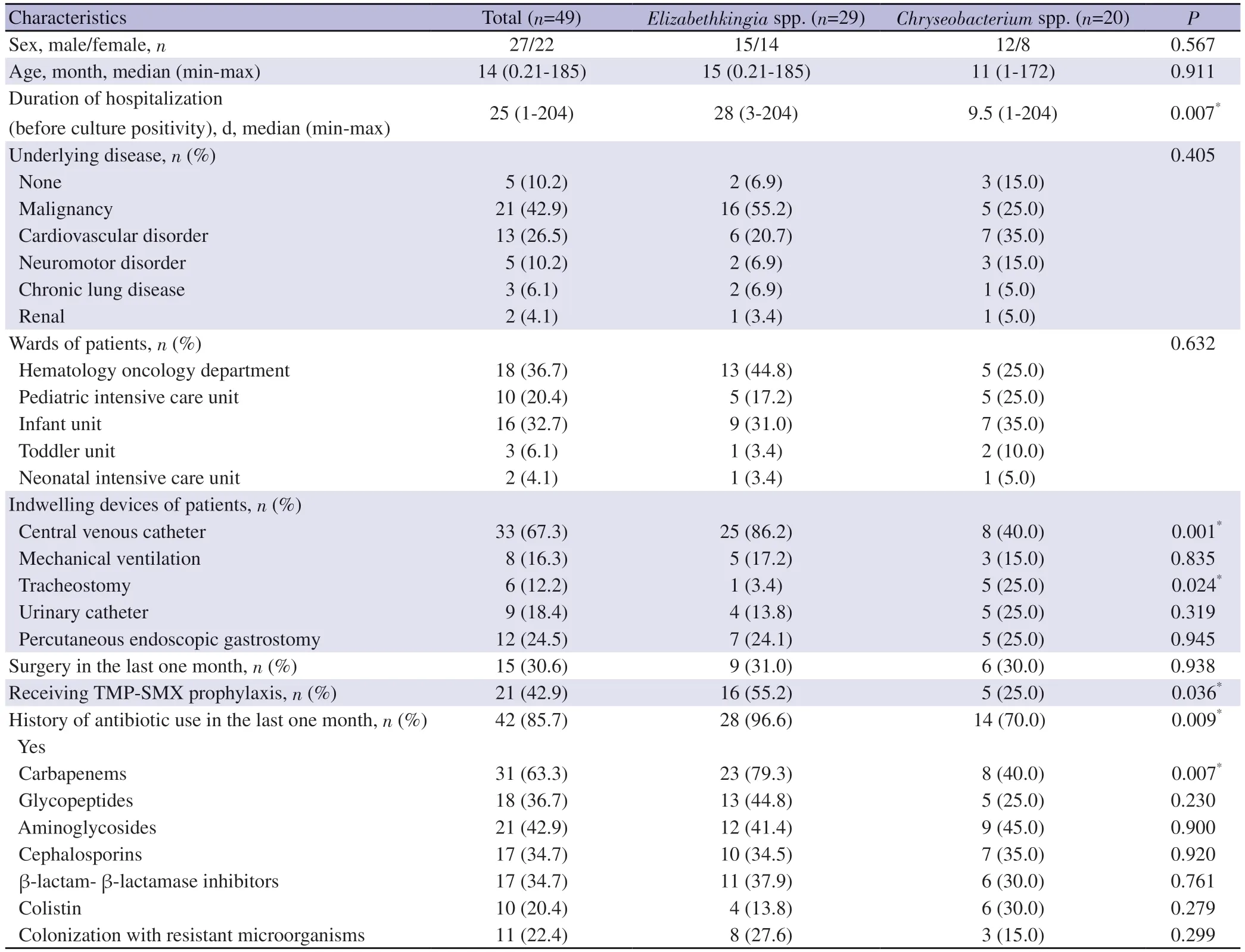
Table 1.Patients characteristics, underlying diseases and risk factors with Chryseobacterium and Elizabethkingia infections.

Table 2.Clinical and laboratory findings, treatment and outcome of patients with Chryseobacterium and Elizabethkingia infection.
The body fluid specimens of the patients where Elizabethkingia and Chryseobacterium were isolated consisted of blood (n=37),endotracheal aspirate (n=5), urine (n=3), and pus (n=4).There was no CSF, sputum, or swab cultures among the isolates included in the study.Eleven patients (22.4%) had colonization with resistant microorganisms.Eight patients were colonized with carbapenem resistant Klebsiella pneumoniae, two with carbapenem resistant Acinetobacter baumannii, and one patient with carbapenem resistant Pseudomonas aeruginosa.Patient characteristics, underlying diseases and risk factors, which vary according to the microorganisms, are shown in Table 1.
3.2.Clinical manifestation, treatment and outcome
Fever was present in 38 (77.5%) patients, and 15 of these patients had febrile neutropenia.A nosocomial infection was found in 44(89.7%) of the 49 patients.The clinical presentation was bacteremia and sepsis in 23 patients, central line-associated bloodstream infection (CLABSI) in 14 patients, ventilator-associated pneumonia in 5 patients, skin and soft tissue infection in 4 patients, and urinary tract infection in 3 patients.Two patients who had sepsis also needed circulatory support.Elizabethkingia was the causative agent in most (85.7%) CLABSI.The median central venous catheter duration was 28 (3-180) days.The catheter had to be removed during the infection in 16 patients, and this event was found to be significantly more common in Elizabethkingia infections (81.2%,44.8% vs.15.0%, P=0.029).
Median treatment duration was 12 (4-24) days.The duration of treatment varied according to the agent and the type of infection.The duration of treatment was 10 (4-15) days in bacteremia and sepsis, 14 (10-21) days in CLABSI, 14.5 (10-18) days in pneumonia, 16 (10-24) days in skin and soft tissue infection, and 10 days in urinary tract infection.Median time to clinical improvement was 3 (1-12) days and this was higher in Chryseobacterium infections(P=0.044).While median time to clinical improvement with treatment was longer in pneumonia (9 days) and skin and soft tissue infections (8 days), it was 3 days in the other infections.Median time to culture negativity was 4 (2-9) days.The median C-reactive protein level was 32.4 (3-52) mg/L and this value was significantly higher in Elizabethkingia infections (P=0.004).Median C-reactive protein levels were significantly higher in Elizabethkingia infections compared to Chryseobacterium infections (38.8 mg/L vs.18.6 mg/L, P=0.004).The median leukocyte count was 4 280/mm³ (350-19 550).The median neutrophil count was 2 050/mm³ (40-16 000).Table 2 presents the clinical and laboratory findings, treatment,and outcome of patients with Chryseobacterium and Elizabethkingia infection.
Four patients could not receive treatment.Bacteremia and sepsis were most commonly treated with ciprofloxacin (14/23, 60.9%).TMP-SMX monotherapy (5/14, 35.7%) or combined (1/14,7.1%) treatment was frequently used in the treatment of CLABSI.Ciprofloxacin was the second most frequently used treatment for CLABSI (4/14, 28.6%).TMP-SMX therapy (single and combined therapy) was the most commonly used treatment in pneumonia(3/5, 60.0%).Ciprofloxacin monotherapy was used for all urinary tract infections.In skin and soft tissue infections, vancomycin and ciprofloxacin monotherapy or combination treatment was administered.Considering the treatment of all patients, the most commonly used antibiotic was ciprofloxacin (25/49, 51.0%) (22/49,44.9% ciprofloxacin alone and 3/49, 6.1% in combination with vancomycin).TMP-SMX was given by itself at a rate of 14.3%(7/49) and in combination with ciprofloxacin in 6.1% (3/49).The other antibiotics used were vancomycin (5/49, 10.2%), cefepime(2/49, 4.1%), teicoplanin (2/49, 4.1%), and piperacillin-tazobactam(1/49, 2.0%).Combination therapy was used when there was no clinical improvement after 48-72 hours of monotherapy.
The 30-day mortality rate was 12.2% (n=6).Infection-related death occurred in 4 Elizabethkingia infections and 2 Chryseobacterium infections.Four patients died before effective treatment for Elizabethkingia and Chryseobacterium could be started although they had already received broad-spectrum empirical treatment.The underlying disease was a malignancy in 4 patients and cardiovascular disease in 2 patients.Four patients had bacteremia,sepsis, or catheter-related bloodstream infection, while one patient had ventilator-associated pneumonia and one patient had skin infection of the surgical site.The infection site, underlying diseases,antibiotic treatments in the last month, and colonization with resistant microorganisms were not related to mortality.Four patients were on mechanical ventilation, 5 patients had urinary catheters,and 4 patients had undergone surgery in the last month.
3.3.Bacterial isolates, antimicrobial susceptibility
Of the 49 cases, 29 (59.2%) were identified as Elizabethkingia and 20 (40.8%) as Chryseobacterium.While 3 (15.0%) of the Chryseobacterium species were C.gleum, 17 (85.0%) were C.indologenes.Antibiotic susceptibility of the Elizabethkingia and Chryseobacterium species is shown in Table 3.According to these findings, Elizabethkingia and Chryseobacterium spp.were found to be susceptible to ciprofloxacin in 91.8% (45/49), TMP-SMX in 91.6% (44/48), and levofloxacin in 87.5% (21/24).When the evaluations of antibiotic susceptibility were performed separately for Chryseobacterium and Elizabethkingia, Chryseobacterium isolates seemed to be more susceptible than Elizabethkingia isolates to cefepime, ceftazidime, gentamicin, imipenem and piperacillintazobactam and tetracycline.Elizabethkingia isolates were resistant to amikacin, netilmicin, gentamicin, and piperacillin-tazobactam,and weakly sensitive to cephalosporins and carbapenems.
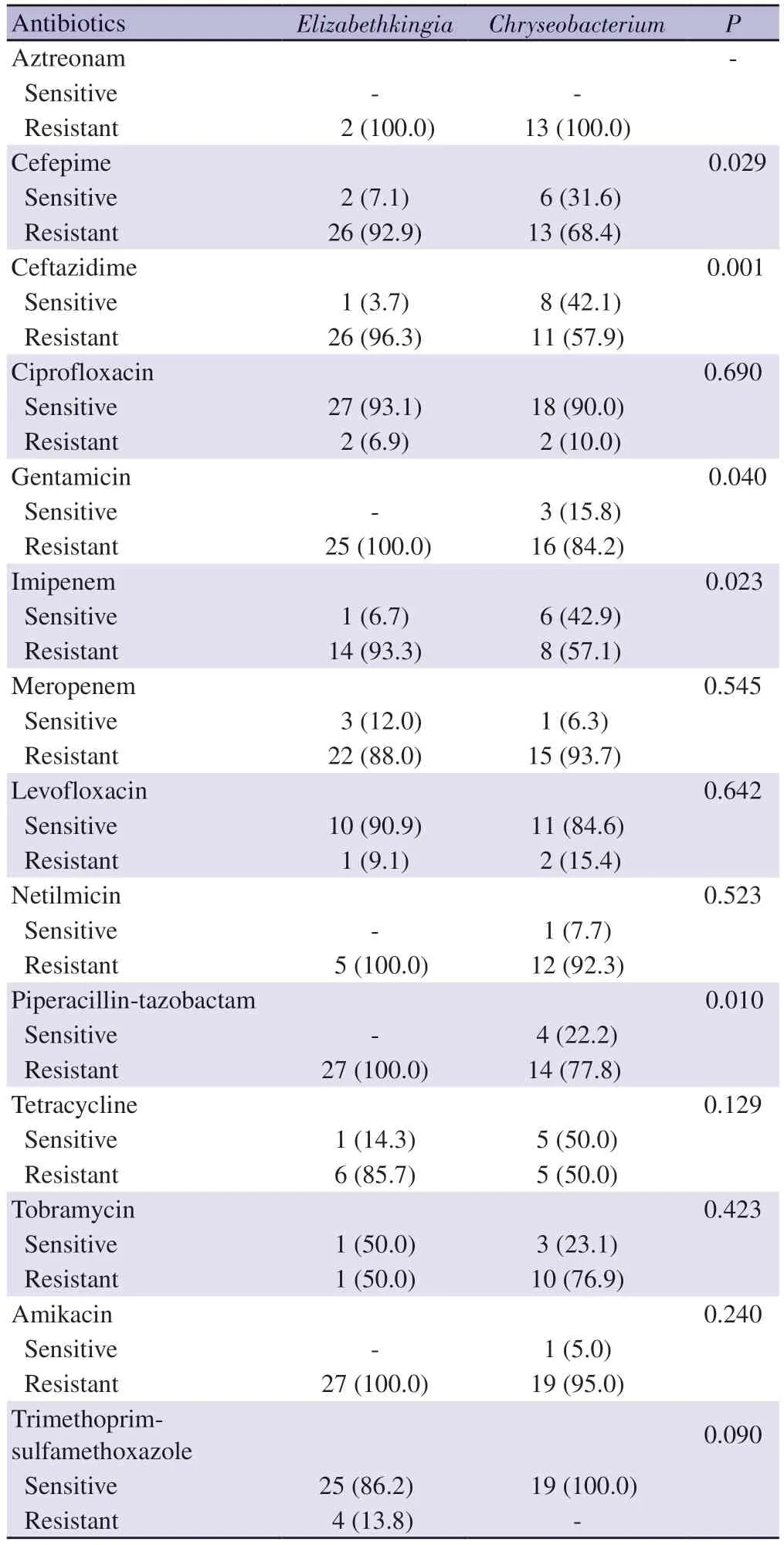
Table 3.Antimicrobial susceptibility rates of Chryseobacterium and Elizabethkingia isolates [n (%), N=49)].
4.Discussion
Elizabethkingia and Chryseobacterium species are rare, emerging,aerobic, Gram-negative bacteria that cause nosocomial infections,and data on these species in the pediatric population is scarce.To our knowledge, this is the largest pediatric data reported, with 49 patients[3-6,11-21].During the 5-year period of the SENTRY Surveillance Program (1997-2001), which showed the results of 50 cultures with Elizabethkingia and Chryseobacterium spp.,only 0.03% (50/155 811) of all bacterial isolates in children and adolescents were found to be Chryseobacterium and Elizabethkingia spp[11].Our hospital is the biggest tertiary pediatric hospital in Turkey, and we identified 49 patients with symptomatic disease due to Elizabethkingia and Chryseobacterium spp.in eight years.Elizabethkingia and Chryseobacterium infections were also uncommon in our study.
In the 1944-2017 review of Dziuban et al.on pediatric patients with E.meningoseptica infections, 76% were neonates and 34.6%of the cases were part of an outbreak cluster[2].Similarly, 30%of the patients were neonates in the study of Chan et al.with 13 pediatric invasive E.meningoseptica infections between 2010 and 2017[20].Contrary to most previous data, our study reported only two neonatal patients and there were no outbreaks or clusters.The median age was 14 months in the current study, and was similar to the study showing bacteremias in children due to C.indologenes and E.meningoseptica[4].In the study of Alyami et al., in which Chryseobacterium/Elizabethkingia spp.infections were reviewed recently, the rate of newborns among the pediatric patients was similar to our study (1/11), although the number of patients was limited[17].
In the SENTRY study, 52% of the isolates were from the respiratory tract, 46% from the bloodstream isolates, and only one from skin and soft tissue[11].In our study, only 10.2% of the samples were from the respiratory tract, 75.5% from the bloodstream, 8.2%from skin and soft tissue, and 6.1% from the urinary tract.Some studies have shown that Chryseobacterium and Elizabethkingia spp.can be rapidly cleared by the immune system when introduced into the bloodstream or respiratory tract of a healthy human host[11,17].This difference in results may be related to the fact that our study was generally conducted with pediatric patients who had an underlying disease.
Consistent with previous studies, most (89.8%) patients had an underlying disease, an indwelling device, history of recent surgery,and previous treatment with broad-spectrum antibiotics in the last month[3,4,6,17].Our carbapenem resistance rate was high, similar to previous data[3,10].In support of previous data, our results show that the use of carbapenem group antibiotics was significantly higher in the last month and the TMP-SMX prophylaxis rate was significantly higher in Elizabethkingia infections.
Chryseobacterium and Elizabethkingia spp.infections were more common in long-term hospitalized patients[3,16].The majority of the patients in the current study were hospitalized in the hematology-oncology services with a diagnosis of malignancy, and infections were frequently nosocomial with a median duration of hospitalization before infection of 25 days.These characteristics were more prominent in Elizabethkingia infections.
The treatment of Elizabethkingia and Chryseobacterium infections is challenging and there has been no consensus on the optimal antimicrobial therapy.They are resistant to most antibiotics used to treat Gram-negative bacteria, such as carbapenems,cephalosporins, and aminoglycosides[2-5].However, they have shown susceptibility to agents used to treat Gram-positive infections.Rifampin has shown activity in vitro against Elizabethkingia and Chryseobacterium spp.and is usually used in combination with vancomycin[11, 22].Vancomycin and rifampicin were not tested in our results.According to the SENTRY Antimicrobial Surveillance Program between 1997 and 2001, antibiotic susceptibility varies according to regions but susceptibility to the new quinolones,rifampin, TMP-SMX, ciprofloxacin and piperacillin-tazobactam was high while vancomycin showed poor potency[11].Güngör et al.have reported that all their isolates in the outbreak were susceptible to ciprofloxacin in vitro but 3 patients did not respond to ciprofloxacin therapy.These patients survived when they switched the therapy to vancomycin and rifampin[21].Vancomycin and ciprofloxacin monotherapy or combined treatments were used for skin and soft tissue infections in the current therapy.Chen et al.have demonstrated that all the Elizabethkingia spp.isolates were susceptible to piperacillin tazobactam and showed variable susceptibility to trimethoprim/sulfamethoxazole (78.6%),ciprofloxacin (33.3%), and levofloxacin (87.5%)[5].In the study of Cooper et al.on children with C.indologenes and E.meningoseptica bacteremia, the bacteria were found to be highly sensitive to piperacillin tazobactam, TMP-SMX, and ciprofloxacin[4].On the contrary, all Elizabethkingia isolates were resistant to piperacillin/tazobactam in our study while Chryseobacterium had low sensitivity,and ciprofloxacin had the highest potency.The majority of our patients were treated with ciprofloxacin, TMP-SMX, or a combination thereof, and most isolates were susceptible to those antibiotics.Our study suggests that ciprofloxacin and TMP-SMX use is adequate.
In previous studies, the mortality rates of C.indologenes and E.meningoseptica infections were high and variable.Such variable mortality rates have been reported (6.3%-31.4%), especially in studies where newborns were in the majority and had higher mortality rates[3,4,6,12-15,17,20,23].Although most of our patients had a favorable outcome, the mortality rate in our study was significant.Only two patients were newborns and most of the deaths occurred before the culture was concluded and before effective antibiotic treatment could be started.The decreased mortality in more recent cases may be due to newer antibiotic options, increasing use of antimicrobial susceptibility testing, or differences in delay to diagnosis over time.Our relatively low mortality rates may be due to early and appropriate antimicrobial therapy, low number of newborn patients, and taking more cultures, as the majority were patients with malignancies.
This study has several limitations.Although the number of patients was higher than in the previous studies, the study is limited by its retrospective nature.
In summary, Elizabethkingia and Chryseobacterium infections are rare and nosocomial, and not necessarily part of outbreaks.The surveillance of Chryseobacterium and Elizabethkingia infections is still very important, and the clinicians should be aware of the changing epidemiology and increasing resistance of these microorganisms.To our knowledge, our study has the largest number of cases in pediatric patients.In addition, although Elizabethkingia and Chryseobacterium are different genera, we chose to include both in this study since they are in the same family and share common features, and also aimed to reveal any differences by comparing their clinical and laboratory features and antimicrobial susceptibilities with each other.Our study is one of the rare studies comparing these two agents.These infections are associated with younger age, but the majority of our cases were older than the neonatal period.They are mainly seen in patients with long hospital stays and indwelling devices (especially a central venous catheter)and in patients who have received antibiotics within the last month,especially carbapenems.They are resistant to most empirical antibiotics, and the best antibiotics are ciprofloxacin and TMP-SMX according to the resistance patterns.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Funding
The authors received no extramural funding for the study.
Authors’contributions
AY contributed to the study conception and design.AY, GİB implemented the study.AY, GİB, AYG, BG, SKY, SÖ, TE, KK and AÖP analyzed and interpreted the data.AY and GİB revised the work critically for intellectual content and granted final approval for publishing.All authors have reviewed the manuscript and consent was given to publish.
 Asian Pacific Journal of Tropical Medicine2023年6期
Asian Pacific Journal of Tropical Medicine2023年6期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Knowledge and awareness of human mpox infection among healthcare workers: A cross-sectional study in southwestern Nigeria
- Behaviour and perception of parents on irrational use of antibiotics in children at primary care level: A cross-sectional study from Turkey
- Excess mortality in Northeast Iran caused by COVID-19: Neglect of offset community transformations of health
- Magnetic resonance imaging evaluation of head and neck involvement in IgG4-related disease
- Disseminated tuberculosis presenting as meningitis and spondylodiscitis in an immunocompetent adult
- Association between the extent of public health measures and other respiratory infectious diseases cases amidst the COVID-19 pandemic in Thailand
