Radiological parameters to predict pancreatic texture: Current evidence and future perspectives
Raja Kalayarasan, Mandalapu Himaja, Ananthakrishnan Ramesh, Kathirvel Kokila
Abstract
Preoperative prediction of the postoperative pancreatic fistula risk is critical in the current era of minimally invasive pancreatic surgeries to tailor perioperative management, thereby minimizing postoperative morbidity. Pancreatic duct diameter can be readily measured by any routine imaging used to diagnose pancreatic disease. However, radiological evaluation of pancreatic texture, an important determinant of pancreatic fistula, has not been widely used to predict the risk of postoperative pancreatic fistula. Qualitative and quantitative assessment of pancreatic fibrosis and fat fraction provides the basis for predicting pancreatic texture. Traditionally computed tomography has been utilized in identifying and characterizing pancreatic lesions and background parenchymal pathologies. With the increasing utilisation of endoscopic ultrasound and magnetic resonance imaging for evaluating pancreatic pathologies, elastography is emerging as a promising tool for predicting pancreatic texture. Also, recent studies have shown that early surgery for chronic pancreatitis is associated with better pain relief and preservation of pancreatic function. Pancreatic texture assessment can allow early diagnosis of chronic pancreatitis, facilitating early intervention. The present review outlines the current evidence in utilizing various imaging modalities for determining the pancreatic texture based on different parameters and image sequences. However, multidisciplinary investigations using strong radiologic-pathologic correlation are needed to standardize and establish the role of these non-invasive diagnostic tools in predicting pancreatic texture.
Key Words: Pancreatic fistula; Minimally invasive; Pancreaticoduodenectomy; Pancreatic cancer; Neoplasms; Computed tomography; Endoscopic ultrasound; Ultrasonography;Magnetic resonance imaging
INTRODUCTION
Pancreatoduodenectomy (PD) is the primary treatment for periampullary and pancreatic malignancies. Advancements in surgical techniques and perioperative management reduced the mortality after PD to less than 5% in high-volume centers[1]. However, PD-related morbidity remains high at 30%-50%[2,3]. Postoperative pancreatic fistula (POPF) is the primary determinant of morbidity and mortality after PD. Hence it is crucial to identify patients at high risk of POPF. Previous studies have identified pancreatic texture, pancreatic duct diameter, pancreatic stump ischemia, and operative blood loss as significant risk factors for POPF[4-8].
Pancreatic texture has been reported as an important predictor of POPF[6,7]. Soft pancreas is associated with increased risk of POPF and a firm pancreas is protective against POPF. The higher the fat fraction in the pancreas, the softer the pancreas is, however it becomes harder with the increasing grade of fibrosis. Traditionally assessment of the pancreatic texture is done by intraoperative palpation or histological evaluation of the operative specimen[7]. However, these assessment techniques cannot be used for the preoperative prediction of POPF. Also, intraoperative assessment of pancreatic texture during minimally invasive surgeries, especially the robotic approach, is challenging. With advancements in surgical techniques and instrumentation, a minimally invasive approach has been increasingly used to perform PD. However, multicenter randomized controlled trials (RCTs), including the recent Chinese trial, have failed to show short-term clinical benefits with minimally invasive PD compared to the open approach[9,10]. The results of these RCTs underscore that in PD, morbidity related to the procedure rather than access determines the short-term outcomes. As most of the morbidity in PD is related to POPF, preoperative prediction of patients with high risk of POPF can guide in adopting the intraoperative and postoperative management to reduce the POPF-related morbidity and thereby reducing the overall morbidity of PD. It also helps select patients at low risk of POPF who will benefit from minimally invasive PD. Hence, attempts have been made to correlate preoperative radiological parameters with pancreatic texture[11-16].
Another application of pancreatic texture evaluation is in patients with chronic pancreatitis. Recent studies have shown that early intervention for patients with chronic pancreatitis is associated with better outcomes than delayed intervention[17,18]. Pancreatic texture evaluation could facilitate the early identification of pancreatic fibrosis. The commonly employed diagnostic modalities for the assessment of pancreatic lesions are ultrasonography (USG), computed tomography (CT), magnetic resonance imaging (MRI), and endoscopic US (EUS). The present review aims to provide an overview of various parameters that can be assessed with each radiological investigation to detect the presence of fatty or fibrotic pancreas and predict the pancreatic texture.
PANCREATIC TEXTURE AND POSTOPERATIVE PANCREATIC FISTULA
The association between pancreatic texture and POPF risk has been documented in multiple retrospective and prospective studies. Kawaiet al[4] in a multicenter study analyzed the risk factors for POPF in 1239 patients who underwent pancreatoduodenectomy. The authors concluded that soft pancreas was one of the significant risk factors for clinical pancreatic fistula. Patients with soft pancreatic texture are at 2.7 times more risk of developing POPF. Ansorgeet al[5], in a single-center prospective study of 164 patients reported that softer pancreatic texture is associated with a significantly higher incidence of POPF (P< 0.001) and a higher incidence of symptomatic postoperative peripancreatic collections (P= 0.071) compared to those with firm pancreatic texture. Ridolfiet al[6] evaluated the morpho histological features of pancreatic stump after pancreatoduodenectomy in 143 patients and found them to be the primary determinant of pancreatic fistula after pancreatoduodenectomy. A soft pancreas was strongly associated with POPF development and with high-grade POPF. In their study 42% of patients with soft pancreas developed a high-grade fistula, compared to 4% of patients with firm pancreatic texture (P< 0.001). In their study pancreatic texture was confirmed with histological correlation using fibrosis and inflammation scores. Huet al[7] retrospectively analysed 539 patients who underwent pancreatoduodenectomy and found a significant correlation between pancreatic texture and POPF by univariate and multivariate analysis.
However similar correlation could not be established between pancreatic texture and POPF after distal pancreatectomy. This could be because of a different mechanism for leak and fistula formation from the pancreatic remnant after distal pancreatectomy compared to pancreaticoduodenectomy, which includes pancreatoenteric anastomosis. Chonget al[8], in a meta-analysis that included 43 studies with 8864 patients, found no difference in clinically relevant POPF rate between soft pancreas (25.3%, 373/1477) and hard pancreas (13.5%, 72/535) (P= 0.46). Pancreatic gland texture and duct size are not associated with the development of pancreatic fistula following distal pancreatectomy, unlike that of pancreatoduodenectomy. Hence, assessment of pancreatic texture is more useful in patients undergoing pancreatoduodenenctomy compared to those undergoing distal pancreatectomy.
ULTRASONOGRAPHY ABDOMEN
Transabdominal USG is the commonly used initial investigation to evaluate pancreatic pathology. The grayscale B- mode USG can evaluate the echotexture of the pancreas. The echotexture of the normal pancreas is isoechoic or slightly hyperechoic compared to the normal liver and shows a granular appearance with a smooth or minimally lobulated outline (Figure 1). With age, the echogenicity of the pancreas increases due to atrophy with fatty replacement. A fatty pancreas often occurs at the same time as fatty liver, which makes diagnosis more challenging. The most common limitations in scanning the pancreas by transabdominal approach are abdominal fat in obese patients and bowel air. As predicting pancreatic texture by routine B-mode USG is challenging, USG elastography has recently been used to measure the elasticity of different tissues[19,20].

Figure 1 Transabdominal ultrasonography. A: Grayscale appearance of the normal pancreas (LL- Left lobe of liver, H- Head of pancreas, N- neck of pancreas, B-Body of pancreas). The pancreas is isoechoic compared to normal liver and shows a granular appearance and smooth outline; B: 2D Shear wave elastography measurement from the normal pancreas-neck region (3.84 ± 0.45 kPa).
Elastography
Elastography measures the stiffness of various organs and has been used to evaluate liver fibrosis and breast lesions[19,20]. Elastography of the pancreas can be performed using transabdominal USG, EUS, or MRI[21]. The techniques of USG elastography include strain elastography and shear wave elastography (SWE)[21]. The stiffness of tissue in the strain elastography is estimated by measuring the grade of strain generated by external pressure: the greater the strain, the softer the stiffness of the target tissue. The SWE relies on the principle of acoustic radiation force impulse (ARFI) using a USG probe which propagates through the tissue, and stiffness is estimated by measuring the propagation speed of the shear wave (Figure 1). The shear wave velocity (SWV) depends on the stiffness of the tissue: the higher the SWV, the harder the target tissue[22,23]. As SWE is less operator-dependent, it is preferred over strain elastography. Strain elastography is challenging to measure when the ultrasound probe, the pancreas, and the aorta are not in line. Hence it is easy to get a fine elastogram in the pancreatic body but not in the pancreatic head and tail regions. However, SWE can be easily performed anywhere in the pancreas because ARFI can be emitted wherever desired. Over the last few years, there has been increasing interest in assessing the role of elastography in evaluating pancreatic texture, differentiating benign and malignant pancreatic lesions, and diagnosing chronic pancreatitis[24-28].
Yashimaet al[27] used ARFI elastography of the pancreas and reported high elasticity in patients with chronic pancreatitis compared to normal patients. SWV in patients with chronic pancreatitis was significantly higher than that in healthy volunteers in each part of the pancreas (Figure 2). However, the measurement was difficult in the tail of the pancreas (Table 1). Haradaet al[29] reported a good correlation between SWV and the histological grade of fibrosis. Pancreatic SWV, measured by preoperative ARFI imaging, was shown to have significant correlations with the grade of pathologic fibrosis, influencing the risk of POPF.
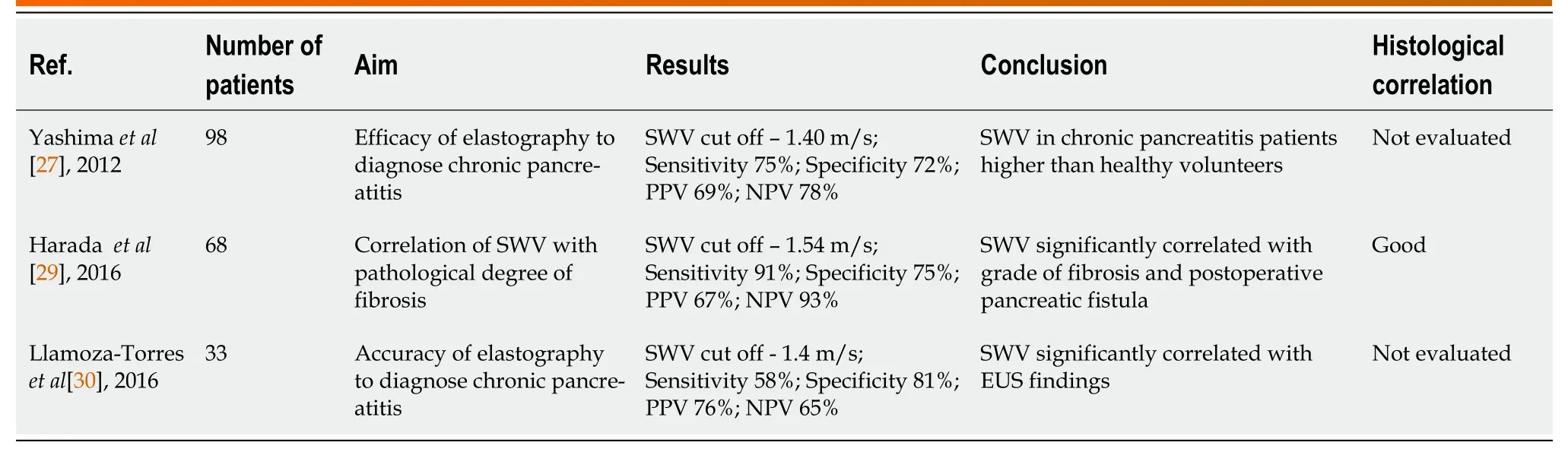
Table 1 Summary of studies evaluating the role of transabdominal ultrasonography in assessing pancreatic texture
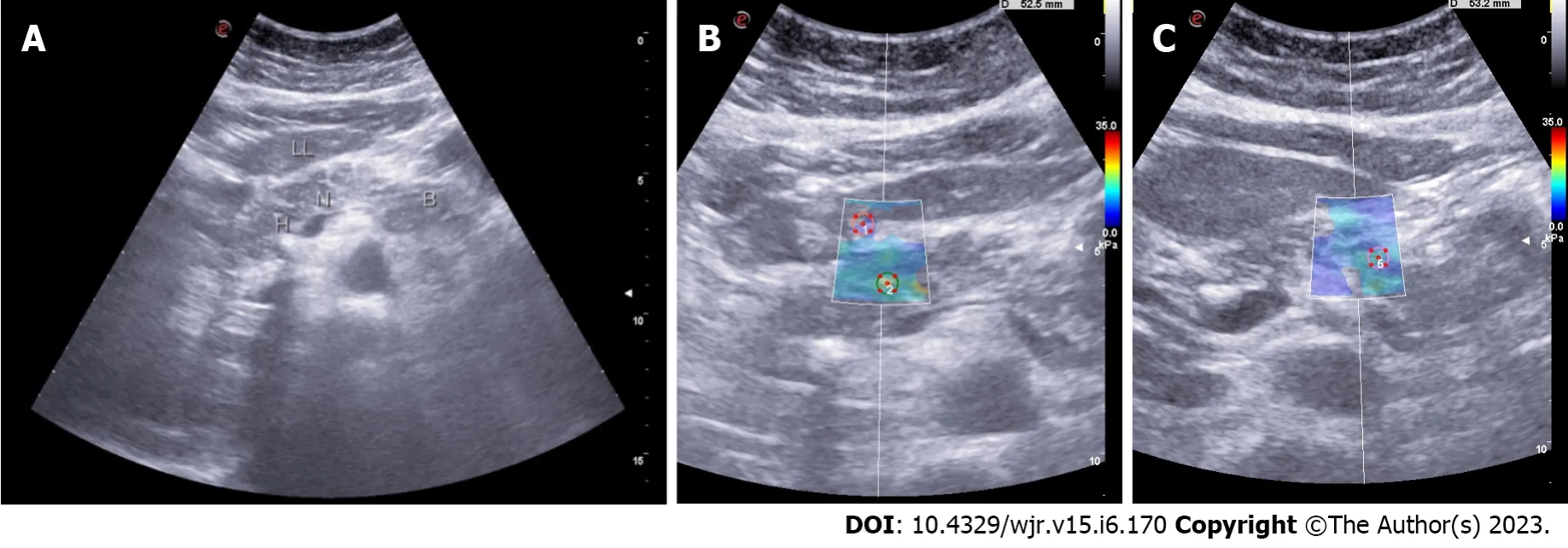
Figure 2 Shear wave velocity in patients with chronic pancreatitis was significantly higher than that in healthy volunteers in each part of the pancreas. A: Grayscale appearance of the pancreas in chronic calcific pancreatitis. (LL- Left lobe of liver, H- Head of pancreas, N- neck of pancreas, B-Body of pancreas). It shows a focal hyperechoic signal compared to normal liver and a mildly lobulated outline, a focus of calcification in the head region with posterior acoustic shadowing; B: 2D Shear wave elastography measurement of the pancreas in chronic calcific pancreatitis - head region (15.55 ± 2.64 kPa); C: 2D Shear wave elastography measurement of the pancreas in chronic calcific pancreatitis - region (11 ± 2.07 kPa).
Llamoza-Torreset al[30], in their study of 33 patients, established the diagnostic accuracy of transabdominal USG-guided elastography in evaluating patients with suspected chronic pancreatitis. Patients included in the study were initially evaluated by EUS and/or MRI to establish their chronic pancreatitis status. Also, none of the included patients were found to have advanced-stage pancreatitis. The study results underscore the role of trans-abdominal USG elastography in assessing patients with early-stage chronic pancreatitis. However, the correlation with histological fibrosis was not evaluated. Further multicenter trials would be crucial to establish the role of transabdominal USG elastography in evaluating pancreatic texture and diagnosing early-stage chronic pancreatitis.
COMPUTED TOMOGRAPHY ABDOMEN
Pancreatic texture on CT abdomen can be predicted based on the patterns of attenuation and enhancement of its parenchyma on various phases. They were evaluated as preoperative predictors of POPF in several studies[11-15]. While pancreatic attenuation index (PAI) like Liver Attenuation Index can measure pancreatic fat, pancreatic enhancement ratio (PER) can be measured to grade the pancreatic fibrosis (Figure 3). The higher the PER, the firmer the gland is. The presence of a higher PER and lower PAI can be considered to be associated with the low risk of development of POPF after PD.

Figure 3 Calculation of pancreatic attenuation index and pancreatic enhancement ratio. A: Hounsfield unit (HU) of the pancreatic neck in the plain phase; B: HU of the spleen in plain phase; C: HU of the pancreatic neck in the arterial phase; D: HU of the pancreatic neck in the equilibrium phase. ROI: Region of interest.
Pancreatic attenuation index
PAI has been proposed as a simple tool by Yardimciet al[31] to assess pancreatic fat fraction by evaluating 76 patients who underwent PD. PAI was calculated with non-enhanced computed tomography by dividing the pancreas density measured in Household Units by the spleen density. They reported that higher PAI was associated with a high POPF rate and determined the value of 0.67 as an optimum cut-off value for predicting POPF. PAI has been reported to be useful in assessing pancreatic fat fraction by few other studies as well[11,12]. However, Gnanasekaranet al[32] reported that PAI was not helpful in predicting CR-POPF. Also, in their study, PAI did not correlate with histological estimation of pancreatic fat fraction. It might be due to the use of a region of interest-based assessment. In future studies, area-based assessment for the pancreatic fat fraction should be correlated with histopathological fat fraction.
Pancreatic enhancement ratio
An increase in the fibrosis of the pancreas makes the pancreatic texture hard. A fibrotic pancreas shows delayed enhancement in the pancreatic phase and nearly normal enhancement in the hepatic phase on dual-phase CT[33]. In contrast, the normal pancreas shows maximum enhancement in the pancreatic phase and washout in the hepatic phase[33]. Thus, predicting the degree of pancreatic fibrosis may be possible on analysis of enhancement patterns on pancreatic protocol CT done routinely to evaluate pancreatic tumors.
宫颈成熟是能够顺利生产的重要因素之一[1],因此在孕产妇实际生产过程中,促进孕产妇宫颈成熟,能够提高孕产妇分娩的安全性[2]。常见的促进宫颈成熟的方法有乳房按摩方法[3],本篇文章对乳房按摩促进足月孕妇宫颈成熟与分娩进行研究,讨论如下:
Kanget al[15] determined a PER cut-off of 1.10 as a useful predictor for POPF based on their retrospective analysis of 146 patients. PER on the equilibrium phase was significantly higher in the patients without POPF compared to patients with POPF (2.26 ± 3.63vs1.04 ± 0.51,P= 0.001). In the logistic regression analyses, PER was an independent predictor for the development of POPF (odds ratio = 0.243,P= 0.002). Maehiraet al[13] retrospectively analysed 115 patients and concluded the pancreatic enhancement pattern as a reliable predictor for the development of POPF. Gnanasekaranet al[32] showed a positive correlation of PER with pancreatic fibrosis. Their study utilised a PER cut-off value of 0.661 which was 78% sensitive and 55 % specific in predicting POPF (Table 2). In the same study, PAI is reported to have negative correlation with PER, indicating that the pancreatic fat content and fibrosis are inversely related. However, the estimation of PER depends on the perfusion of organs with injected contrast which relies upon the hemodynamic status of the subjects. In comparison, PAI is independent of contrast injection.
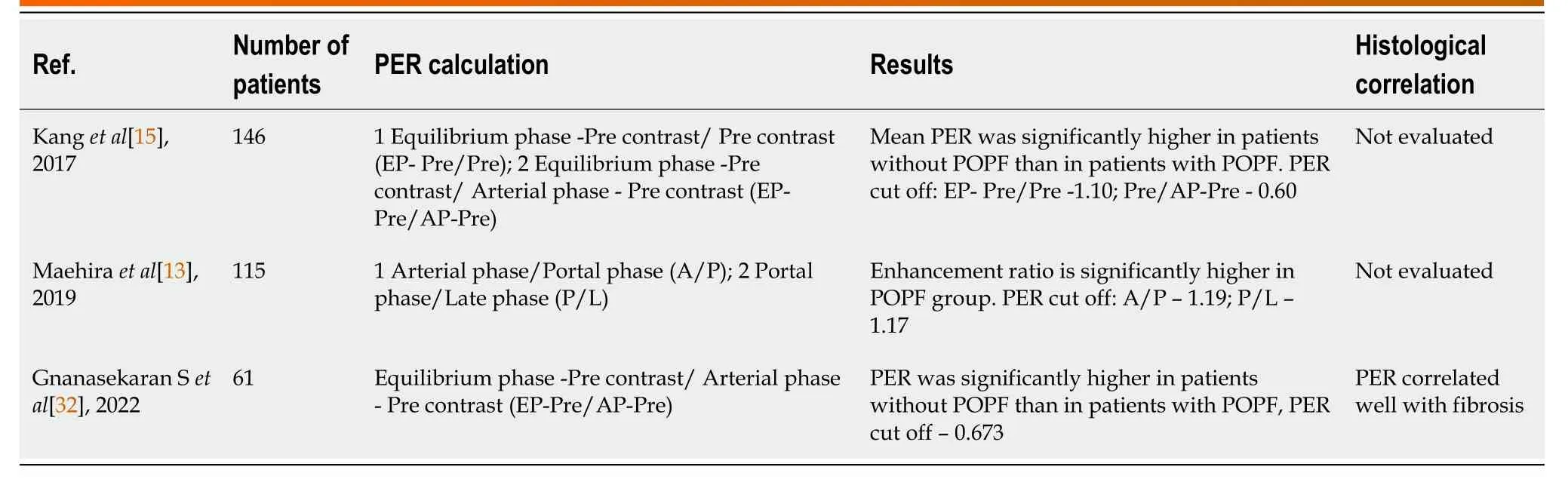
Table 2 Summary of studies evaluating the role of computed tomography abdomen in assessing pancreatic texture
MAGNETIC RESONANCE IMAGING
MRI allows the detection of fibrotic change of pancreatic parenchyma and hence can predict the risk of POPF. A normal pancreas shows hyperintensity on T1-weighted images irrespective of fat saturation. The fibrosis makes the pancreatic parenchyma to lose its high signal intensity (SI) owing to the replacement of the high protein content of the pancreas by fibrosis.
Winstonet al[34] reported that the SI of pancreatic parenchyma, compared to that of the liver, decreases on fat-saturated T1-weighted images in patients with type 2 diabetes. Nodaet al[35] found that the pancreatic fibrosis grade was negatively correlated with the SI ratio on in-phase T1-weighted images (r= -0.67,P= -0.0002). Another retrospective study by Watanabeet al[36] on 29 patients demonstrated that the SI ratio on T1-weighted images constantly decreased as the pancreatic fibrosis progressed. The higher risk of POPF is associated with a high SI ratio. Multiple regression analysis showed that pancreas-to-muscle SI ratios on T1-weighted images and apparent diffusion coefficient (ADC) values were independently associated with pancreatic fibrosis (r(2) = 0.66,P< 0.001) and with activated pancreatic stellate cell expression (r(2) = 0.67,P< 0.001). The mean pancreas-to-muscle SI ratio (± standard deviation) on T1-weighted images was higher (P= 0.0029) for patients with POPF (1.6 ± 0.2) than for those without (1.2 ± 0.2), and the odds ratio for POPF was 21.3 in patients with an SI ratio of 1.41 and higher[36].
Kimet al[16], in their pilot study, studied the correlation of pancreatic fibrosis with POPF after PD with the use of breath-hold unenhanced fat-suppressed T1 weighted images. The pancreas-to-liver SI ratio between the fistula and no fistula groups was -0.0009 ± 0.2 and -0.1297 ± 0.2, respectively (P= 0.0004). Each group's pancreas-to-spleen SI ratio was 0.423 ± 0.25 and 0.288 ± 0.32, respectively (P= 0.014). Using qualitative analysis where the pancreas SI was qualitatively assessed relative to liver and spleen SI using a five-point scale (-2, -1, 0, 1, 2), the SI ratios were 1.27 and 0.66 in each group (P= 0.013). The diagnostic performance for preoperative predictions of POPF was better with the qualitative analysis (Az = 0.653) than with the pancreas-to-liver SI ratio (Az = 0.640) and pancreas-spleen SI ratio (Az = 0.613); although, statistically significant difference was not found in each MRI parameter.
Yoonet al[37] reported that multiparametric MR imaging of the pancreas, including imaging with the T2*-corrected Dixon technique and intravoxel incoherent motion diffusion-weighted imaging (DWI), may yield quantitative information regarding pancreatic steatosis and fibrosis. The mean pancreas-tomuscle SI ratio on T1-weighted MRI values for F0, F1, and the cut-off value for predicting POPF was 1.51, 1.48, and 1.40, respectively (Table 3). Fukadaet al[38], in their single-center retrospective study comprising 117 patients, reported 1.37 as the cut-off value for the pancreas-to-muscle SI ratio for predicting POPF.

Table 3 Summary of studies evaluating the role of magnetic resonance imaging abdomen signal intensity in assessing pancreatic texture
Diffusion-weighted imaging
DWI is used to evaluate fibrosis using ADC values. In the fibrotic pancreas, diffusion is restricted because of the replacement of normal pancreatic parenchyma with fibrous tissue. ADC values can be used to identify the presence of fibrosis and to grade its extent. Studies have reported lower ADC values in chronic pancreatitis patients[39]. Bieliunieneet al[40] identified a significant negative correlation between ADC value and histologically determined pancreatic fibrosis (PF) (r= -0.752,P< 0.001). In addition, a significant negative correlation was observed between T1SI and histologically determined pancreatic fibrosis (r= -0.631,P< 0.001). Also, by combining the ADC and T1SI measurements, PF can be detected with greater sensitivity and specificity during the early stages of the disease when other clinical signs are absent.
Tirkeset al[41] conducted a multi institutional, prospective study to evaluate the diagnostic value of four quantitative MRI parameters in chronic pancreatitis: T1 relaxation time, extracellular fraction, fat signal fraction and ADC. Except ADC, all the parameters were reported to be significantly higher in the patients with chronic pancreatitis and also were showed to have moderately high diagnostic value after adjustment for covariates. A Q-MRI score has been proposed by combining these three MR parameters which was shown to have improved diagnostic performance. However, ADC values were reported to be not helpful for diagnosing chronic pancreatitis.
Magnetic resonance elastography
Magnetic resonance elastography (MRE) can also be used to estimate pancreatic stiffness. The technique of MRE involves three steps similar to transabdominal and EUS elastography: Generation of shear waves in the tissue, acquisition of MR images depicting the propagation of the induced shear waves, and generation of elastograms, the quantitative maps of tissue stiffness by processing the acquired images of the shear waves.
Patients with chronic pancreatitis were reported to have significantly higher stiffness values than normal people (1.53vs1.11 kPa)[42]. Wanget al[43] reported the usefulness of MRE for the assessment of the severity of chronic pancreatitis and showed that the pancreatic stiffness was significantly low in healthy controls (mean -1.21 kPa), when compared to patients with a mild degree of chronic pancreatitis (mean - 1.50 kPa), and also those with a moderate/severe degree of chronic pancreatitis (mean - 1.90 kPa).
ENDOSCOPIC ULTRASONOGRAPHY ELASTOGRAPHY
EUS elastography is a novel diagnostic tool to assess pancreatic fibrosis. Like transabdominal ultrasound, EUS elastography can be strain elastography or SWE.
Strain elastography
In strain elastography, the target tissue is compressed with a EUS probe to create a stain, which is reflected on ultrasound images. Softer tissue has a larger strain when compared to harder tissues. However, this gives only qualitative estimation of tissue elasticity. Second-generation EUS elastography has been developed, giving two semi-quantitative tissue stiffness measures[28]. The strain ratio (SR), one of two semi quantitative measure is based on comparing stiffness between specific regions of interest in two tissue areas and is expressed as a relative ratio. The strain histogram (SH) is another semi-quantitative parameter representing the selected area's mean strain value.
Six articles reported the diagnostic performance of EUS strain elastography for chronic pancreatitis; three reported SR, and three used SH (Table 4)[28,44-48]. Of them two SR articles reported that EUS elastography is helpful for differentiating the normal pancreas from chronic pancreatitis[28,47]. Two of the SH articles also reported the usefulness of EUS elastography in differentiating between normal pancreas and chronic pancreatitis[44,48]. One report showed that the SH elastography values significantly correlated with the degree of fibrosis assessed on histology of the surgical specimens[45].
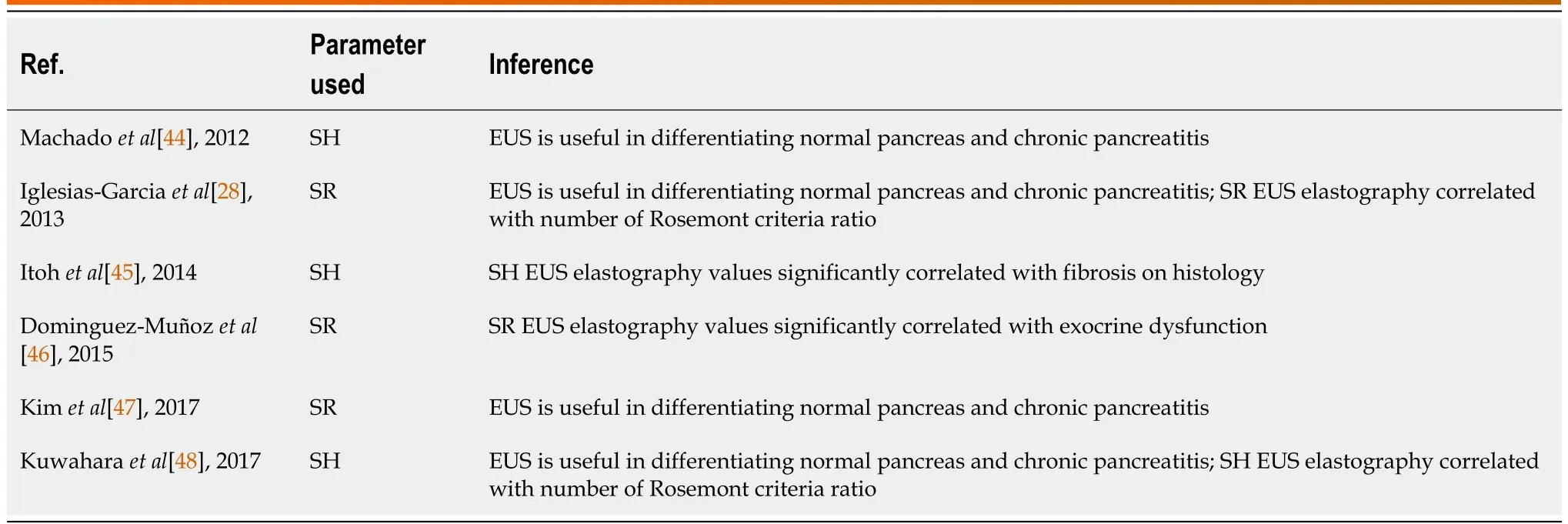
Table 4 Summary of studies evaluating the role of endoscopic ultrasonography strain elastography in assessing pancreatic texture
Shear wave elastography
Acoustic radiation force is sent to the region of interest, and this push pulse generates a shear wave at the edge. The shear wave propagates faster in harder tissues. EUS-SWE is has better diagnostic value in chronic pancreatitis than strain elastography by providing the absolute values of pancreatic hardness.
Since it is a novel investigation, only two articles report the utility of EUS-SWE[49,50]. For diagnosis of chronic pancreatitis, a SWV cut-off value of 2.19 (Rosemont criteria) and 1.96 (Japan Pancreatic Society criteria) had a sensitivity of 100% and 83%, respectively, and specificity of 94% and 100%. EUSSWE values correlate well with the stage of chronic pancreatitis and predicted exocrine dysfunction compared to transabdominal ultrasound. EUS-SWE data are better than those published using transabdominal ultrasound[30]. However, EUS is an invasive technique compared to trans-abdominal ultrasound.
FUTURE PERSPECTIVES
Identifying potential preoperative predictors for POPF is a critical step in our journey to improve perioperative outcomes after PD. Also, early diagnosis of chronic pancreatitis is essential to improve long-term outcomes of patients undergoing surgery for chronic pancreatitis. Recent studies have shown that pancreatic texture parameters like mean positive pixel before initiating neoadjuvant therapy, kurtosis and changes in kurtosis during neoadjuvant therapy can be used in predicting response to neoadjuvant therapy[51,52]. While the current evidence suggests the promising role of radiological parameters in predicting pancreatic texture, it is essential to understand the limitations of available evidence. Most of the studies had a smaller sample size. Hence, studies with larger sample sizes and multicentric studies are required for all the radiological modalities to determine the reference values for the normal and diseased pancreas. Also, socio-demographic variables need to be correlated with the pancreatic texture in all age groups to determine appropriate reference standards for all the available radiological modalities.
Most studies have assessed individual radiological parameter's role in predicting pancreatic texture. However, studies comparing different radiological parameters are not available. Hence future studies are required to study the efficacy of one imaging modality over the other and the effectiveness of combining several radiological modalities to devise a quantitative variable such as fistula risk score based on texture.
The accepted gold standard to find the pancreatic texture is histology. Since there can be an uneven distribution of pancreatic fatty infiltration or fibrosis, using the same focal area of the pancreas in imaging modalities and histology in future studies will provide a better correlation of pancreatic texture. Also, it is essential to understand that multiple factors influence POPF. Hence, a homogenous patient population and standardized surgical techniques are prerequisites for future studies. However, it isn't easy to achieve and reproduce that in complex procedures like PD. Nevertheless, identifying potential preoperative predictors for POPF is vital in decreasing the morbidity associated with PD.
CONCLUSION
Pancreatic texture can be assessed using radiological parameters derived from preoperative imaging modalities. With advancements in imaging techniques, the accuracy of preoperative prediction of the fatty or fibrotic pancreas has improved. However, more studies are required comparing different imaging modalities to standardize their measurement. Also, the correlation of radiological parameters with histological findings is required to improve predictive accuracy.
FOOTNOTES
Author contributions:Himaja M and Kokila K did the literature search and wrote the first draft of the review; Kalayarasan R and Ramesh A conceptualized the work, supervised the writing, gave intellectual inputs, and critically revised the manuscript.
Conflict-of-interest statement:All authors have no conflicts of interest to report.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:India
ORCID number:Raja Kalayarasan 0000-0003-4056-8672; Mandalapu Himaja 0009-0004-8267-2018; Ananthakrishnan Ramesh 0000-0002-1404-6400; Kathirvel Kokila 0000-0002-9157-9067.
S-Editor:Liu JH
L-Editor:A
P-Editor:Liu JH
 World Journal of Radiology2023年6期
World Journal of Radiology2023年6期
- World Journal of Radiology的其它文章
- Acute pancreatitis: Structured report template of magnetic resonance imaging
- Role of contrast-enhanced serial/spot abdominal X-rays in perioperative follow-up of patients undergoing abdominal surgery:An observational clinical study
- Interobserver reliability of computed tomography angiography in the assessment of ruptured intracranial aneurysm and impact on patient management
- Computed tomography angiographic study of surgical anatomy of thyroid arteries: Clinical implications in neck dissection
