MOFs-based functional materials for aqueous micro/nanoplastics elimination
The massive distribution of microplastics (MPs) and even nanoplastics (NPs), which resulted from the wide utilization and mismanagement of plastics, exerted serious risk and threat to ecosystem and human health due to their physical damages and chemical toxicity.Especially, both MPs and NPs can interact with toxic emerging pollutants and heavy metals to induce synergetic toxicity [1].Up to now, capture (adsorption and filtration) and destruction (converting into value-added chemicalsviachemical or biological technologies) were two major routines to effectively remove the MPs and NPs from the water bodies.Recently, adsorption was deemed as effective approach to remove MPs and NPs due to that it was feasible to select suitable adsorbents with strong affinity.It was deemed that electrostatic attraction, hydrogen bonding interaction,π-πstacking interaction, hydrophobic interaction, complexation, as well as physical adhesion might be formed between adsorbents and MPs/NPs.From this point, metalorganic frameworks (MOFs) might be optimal adsorbent candidates as they demonstrated outstanding adsorption performances toward different aqueous pollutantsviathe different interactions.Besides adsorption, MOFs and their composites demonstrated excellent photocatalysis activity under visible/UV light, which might be potentially used to convert MPs or NPs into some value-added or environment-friendly materials.
As a proof-of-concept, the zeolitic imidazolate framework-67(ZIF-67), as one of typical MOF, was adopted to accomplish adsorptive removal of polystyrene microplastics (PS MPs,<10 μm) from water sample [2].Considering the zeta potential and stability of ZIF-67, the optimal adsorption condition was set as pH 8.0 at the temperature of 298 K.The findings revealed that ZIF-67 displayed good adsorption activity toward PS MPs with adsorption capacity of 34.5 mg/g.The interactions between ZIF-67 and PS MPs were affirmed by the scanning electron microscope (SEM) and fluorescence of individual ZIF-67, PS MPs, and the composite of PS MPs adsorbed onto ZIF-67, in which the individual PS MPs and ZIF-67 displayed spherical shapes with significant fluorescence and irregular shapes with negligible fluorescence, respectively.After interaction with PS MPs, the formed ZIF-67/PS MPs composite demonstrated noticeable fluorescence originated from PS MPs.
To overcome the limitations of powder adsorbents, ZIF-8 was immobilized on wood-derived aerogel to fabricate ZIF-8@Aerogel, which can achieve effective removal toward poly(1,1-difluoroethylene) (PVDF, 60-110 nm) NPs and polystyrene (PS, 90-140 nm) NPs [3].The as-prepared ZIF-8@Aerogel was packed into the cylindrical filter to achieve 91.4% and 85.8% removal efficiencies toward PVDF NPs and PS NPs (initial concentration of 0.5 g/L)as the simulated NPs suspension flowed through the filter at a flow velocity of 1.4 L/h.It was explored that electrostatic interaction between negatively NPs and positively charged ZIF-8, hydrophobic interaction (both ZIF-8 and selected NPs are all hydrophobic), hydrogen bonding interaction between C-F in PVDF NPs and the -OH groups in ZIF-8@Aerogel, as well as van der Waals interactions contributed to the superior NPs removal performance over ZIF-8@Aerogel.
To investigate interaction between MOF with the functional groups and the composition of MPs/NPs, UiO-66(Zr)-X MOFs with different groups like X=H, NH2, OH, Br and NO2were immobilized on melamine foam (MF) to remove different nanoplastics like poly(vinylidenefluoride) (PVDF, zeta potential of -18.9±2.3 mV,particle size ofca.260 nm), polystyrene (PS, zeta potential of -7.5±1.2 mV, particle size ofca.183 nm) and polymethylmethacrylate (PMMA, zeta potential of -10.5±2.7 mV, particle size ofca.325 nm) [4].The UiO-66-OH@MF-3 exhibited superior removal efficiencies toward PVDF, PS and PMMA NPs, due to that the zeta potential of UiO-66-OH (58.6±5.1 mV) was higher than those of UiO-66 (23.4±3.7 mV), UiO-66-NH2(35.6±2.3 mV), UiO-66-Br (42.8±4.7 mV) and UiO-66-NO2(31.7±3.6 mV).The strong interactions like electrostatic attraction, hydrogen bond, along with the Van der Waals force between UiO-66-OH@MF-3 and various NPs/MPs, the interpenetration of the MF pore structure in UiO-66-OH@MF-3 enhanced the quick passage of the MPs/NPs solution to be treated (Fig.1a).Considering that some inorganic ions and emerging organics were either co-existed with MPs/ NPs or adsorbed onto the surface of MPs/NPs, the as-prepared UiO-66-OH@MF-3 was tested under various conditions like organic solvents, acidic solution, basic solution and even seawater.The findings revealed that UiO-66-OH@MF-3 can maintain both stability and NPs removal performances under above-mentioned conditions.To affirm the potential application of the as-prepared UiO-66-OH@MF-3 in the actual scenarios, an automatic filtration setup was constructed to pump the simulated seawater containing MPs through the three units of filtration setups powered by the solar panel under solar light.
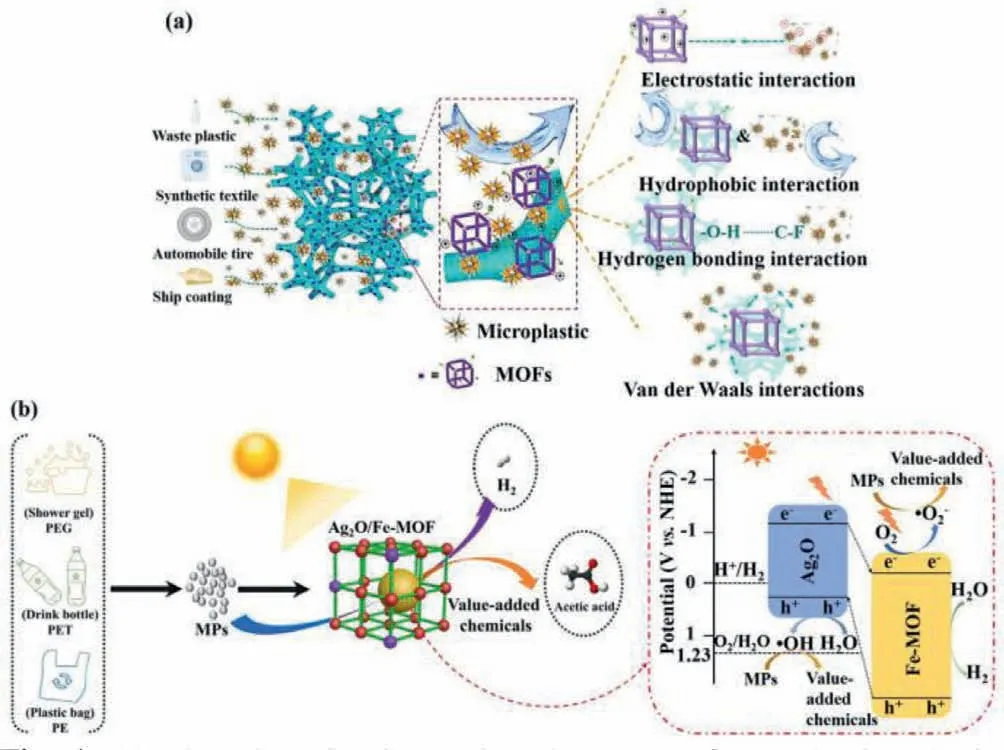
Fig.1.(a) The adsorptive interactions between MOFs and microplastics Adapted from [4], Copyright 2020, Royal Society of Chemistry.(b) The photocatalytic microplastics conversion mechanism over the Ag2O/Fe-MOF photocatalyst.Adapted from [5], Copyright 2022, Elsevier.
Besides adsorptive removal of MOFs toward MPs, a defective Ag2O/Fe-MOF photocatalyst was fabricated for MPs conversion and H2production under visible light [5], in which the type-II heterojunction was constructed at the interface between Fe-MOF and Ag2O.The polyethylene glycol terephthalate (PET), polyethylene glycol (PEG) and polyethylene (PE) microplastics were selected to assess the photocatalytic MPs conversion activity.Under the irradiation of simulated solar light, the electrons (e-) on the conduction band (CB) moved to the CB of Fe-MOF to yield superoxide radical(·O2-) and H2, and the holes (h+) over the valence band (VB) of Fe-MOF transferred to the VB Ag2O to produce hydroxyl radicals(·OH), in which both the formed·O2-and·OH could react with MPs like PEG into value-added chemical like acetic acid (Fig.1b).
Up to now, the investigations on the utilization of MOFs for removal of MPs could be counted on one hand due to that MOFs as organic-inorganic hybrid materials were easily to perform modification and regulation in the composition, structure and morphology.However, some clues concerning selection principle of MOFs for microplastics removal could be proposed.As to the qualified adsorbents for microplastics, MOFs should demonstrate strong interactions with the targeted MPs including but not limited to electrostatic attraction, hydrogen bond, hydrophobic interaction and van der Waals force, in which the MOFs’merits of ultrahigh surface areas and regular channels were not involved in the interactions with MPs.
To accomplish the large-scale application of MOFs for MPs adsorption removal, some efforts should be concerned in the future:formation of appropriate interactions to achieve easy capture and easy release for facile recycling, construction of sufficient porosity with the aid some substrates like sponge foam to guarantee the high flux of the influent and high stability to be tolerant of harsh conditions in the real scenarios.Especially, immobilizing highly stable and readily available MOFs onto macro-porous sponge foams with or without the aid of adhesives can achieve two purposes like forming macro-porosity for the rapid passage of influent wastewater and facilely constructing the corresponding setups for MPs or NPs elimination in the real scenarios.
The photocatalytic conversion of microplastics over MOFs or their composites is in its infancy, which might open a new window for the MPs removal or conversion with the aid of the adsorptive interactions.It was essential to develop other advanced oxidation processes (AOPs) including but not limited to photocatalysis, Fenton-like AOPs and ozone oxidation adopting MOFs or their composites as catalysts for MPs degradation and conversion.Also,the defect engineering can be considered to create defective and hierarchical porous MOFs for exposing more active sites, which can further enhance both the adsorption and AOPs activity toward MPs.
As expected, MOFs-based functional materials might make significant contribution to the MPs elimination, due to that some MOFs and their composites can achieve both adsorption and AOPs processes for the MPs removal.Especially, the adsorptive interactions with the MPs can facilitate the AOPs reaction as more MPs were accumulated near the active sites in the MOFs-based materials.However, some tremendous efforts should be devoted to considering potential issues to further clarify the possible adsorption and AOP mechanisms for MPs removal over MOFs-based materials.As well, some attentions should be paid to the large-scale setups constructed from the MOFs-based materials, in which the influences from the environmental factors, the operation parameters and various targeted MPs should be widely explored.
Chong-Chen Wang∗
Zi-Chen Zhang
Xiao-Hong YiBeijing Key Laboratory of Functional Materials for Building Structure and Environment Remediation,School of Environment and Energy Engineering,Beijing University of Civil Engineering and Architecture,Beijing 100044,China
∗Corresponding author.E-mail address: wangchongchen@bucea.edu.cn (C.-C.Wang)
Received 5 December 2022
Revised 16 January 2023
Accepted 29 January 2023
Available online 1 February 2023
 Chinese Chemical Letters2023年9期
Chinese Chemical Letters2023年9期
- Chinese Chemical Letters的其它文章
- Nanoplatform-based cellular reactive oxygen species regulation for enhanced oncotherapy and tumor resistance alleviation
- Recent advances in the synthesis and applications of furocoumarin derivatives
- High-performance and multifunctional organic field-effect transistors
- NHC-gold(I)-alkyne complexes induced hepatocellular carcinoma cell death through bioorthogonal activation by palladium complex in living system
- New insight into the synergy of nitrogen-related sites on biochar surface for sulfamethoxazole adsorption from water
- HDAC6 inhibitor loaded bimetallene nanosheets with antagonizing thermoresistance for augmented mild photothermal therapy
