The photosensory function of Zmphot1 differs from that of Atphot1 due to the C-terminus of Zmphot1 during phototropic response
Jindong Zhu,Fangyuan Zhou,Yuxi Wang,Yuping Liang,Qingping Zhao,Yuanji Han,Xiang Zhao
State Key Laboratory of Crop Stress Adaptation and Improvement,School of Life Sciences,Henan University,Kaifeng 475004,Henan,China
Keywords: Blue light Zmphot1 Atphot1 Hypocotyl phototropism
ABSTRACT The role of phot1 in triggering hypocotyl phototropism and optimizing growth orientation has been wellcharacterized in Arabidopsis,whereas the role of Zmphot1 in maize remains largely unclear.Here,we show that Zmphot1 is involved in blue light-induced phototropism.Compared with Atphot1,Zmphot1 exhibited a weaker phototropic response to very low-fluence rates of blue light (<0.01 μmol m-2 s-1),but stronger phototropic response to high-fluence rates of blue light (> 10 μmol m-2 s-1) than Atphot1.Notably,blue light exposure induced Zmphot1-green fluorescent protein (GFP),but not Atphot1-GFP,to form the aggregates in the cytoplasm of Nicotiana benthamiana cells.Furthermore,by generating the chimeric phot1 proteins,we found that the serine-threonine kinase (STK) domain at the C-terminus is responsible for a more volatile membrane association of Zmphot1.Consistently,the chimeric phot1 protein fusing the STK domain of Zmphot1 with other domains of Atphot1 responded similarly as Zmphot1 to both low and high fluence rates of blue light.Interestingly,although both Zmphot1 and Atphot1 interact with AtNPH3,Zmphot1 induced weaker dephosphorylation of NONPHOTOTROPIC HYPOCOTYL 3 (NPH3) than Atphot1.Together,our findings indicate that Zmphot1 and Atphot1 exhibit different photosensory function during phototropic response and that the STK domain may play a key role in determining their properties.
1.Introduction
Light plays an important role in plant development and plants have evolved sensitive phototropic regulatory mechanisms to adapt to various light conditions.Phototropins (phot1 and phot2)are the major photoreceptors involved in the regulation of phototropism [1-3].Phot1 was discovered in a non-phototropichypocotyl mutant under weak blue light irradiation[4],and phot2 was discovered in a chloroplast avoidance detection mutant under high blue light irradiation [5].Subsequent studies revealed that low-fluence blue light-induced phototropism is mainly mediated by phot1,whereas high-fluence blue light-induced phototropism is regulated redundantly by phot1 and phot2 [1].Phototropins are involved in various plant movement responses,including phototropic responses [1,6-8],stomatal aperture [9],chloroplast movement [5],and leaf positioning and flattening [10-12].
Phototropins consist of two light-oxygen-voltage (LOV)domains and a Ser/Thr protein kinase domain(STK)[7],and Aihara et al.[13,14] showed that the differences in photosensitivity between phot1 and phot2 depend mainly on the LOV domains.Under blue light,phot1 and phot2 are internalized from the plasma membrane and simultaneously undergo autophosphorylation[15-18].Unlike the phot2 response under blue light irradiation,which exhibits localized migration to Golgi vesicles[15],phot1 is dynamically distributed in cytoplasmic structures [16,17,19].These differences in phototropic sensitivity between phot1 and phot2 result from the different N-terminal moieties of the two molecules[13].
Downstream of phot1 and phot2,NON-PHOTOTROPIC HYPOCOTYL 3 (NPH3) plays a vital role in the regulation of blue lightinduced hypocotyl phototropism and has been shown to be necessary for phot1-and phot2-mediated phototropism,as well as phot1-mediated leaf localization,but not for stomatal opening and chloroplast movement [20,21].The phototropism of anph3mutant was impaired under both low and high (< 1 or >1 μmol m-2s-1) intensity blue light [22].The NPH3 protein consists of a BTB (Broad-complex,Tramtrack,and Bric-à-brac)/POZ (poxvirus and zinc finger) domain at the amino terminus and a coiled coil domain at the carboxyl terminus,which are considered recognition sites for interaction with phot1[23].Under blue light irradiation,the phosphorylation status and subcellular localization of NPH3 were regulated in a phot1-dependent manner[24,25].Recently,Reuter reported that a phospholipid affinity structure exists at the end of the carboxyl-terminus of NPH3[26].Additionally,Sullivan identified that NPH3 is the kinase substrate for phot1,whereas the S744 is phosphorylated both in vivo and in vitro by phot1 [27].
InArabidopsis,phot1,but not phot2,modulates hypocotyl phototropism in a ROOT PHOTOTROPISM 2 (RPT2) dependent manner with increasing blue light irradiation [20].RPT2 consists of an Nterminal BTB/POZ domain and a C-terminal coiled-coil functional domain,which is a homolog of NPH3 inArabidopsis[22,28,29].The transcriptional level of RPT2 shows an up regulated pattern with increasing blue light irradiation [24,30].The N-terminal BTB/POZ domain of RPT2 inhibits autophosphorylation of phot1 by binding to its LOV1 domain.Although the LOV1 domain of phot2 can also bind to the N-terminal BTB/POZ domain of RPT2,the latter does not inhibit its autophosphorylation activity[20,31].Under high blue light irradiation (HBL),rpt2-1andphot2-1 rpt2-1mutants both exhibit defects in hypocotyl phototropism,similar tophot1-5 phot2-1[20],whereas thephot1-5 rpt2-1double mutant exhibits normal phototropism under HBL,indicating that RPT2 is a key factor for phot1,but not for phot2[20].Phot1 exhibits an inhibitory function in phot2-mediated phototropism under HBL[2],suggesting that phot1-mediated inhibition may be involved in phot2-mediated phototropism and that RPT2 acts as a vital transducer in the inhibition process [24,32].In a previous study,we found that phot2-mediated phototropism was inhibited by JAC1 and RPT2 inArabidopsis[32].Although the phototropic mechanism has been widely explored,several issues remain unresolved.
The first and second positive phototropism in monocot coleoptiles has been studied systematically under low and high intensity blue light irradiation [3,33,34].Maize (Zea mays),an important grain crop domesticated from wild grasses (teosintes) in Mexico 9000 years ago [35],is essential for improving corn yield,and is a subject of ongoing research.Recently,researchers have focused on phototropism in maize,a typical monocotyledon [36].The mechanism of phototropism in monocots and dicots is highly conserved[37,38].Phototropic mechanisms may vary between monocotyledons and dicotyledons as both adapt to distinct organs in response to unilateral blue light irradiation.
Although it has previously been widely studied in the phototropism regulated by phototropins,many underlying mechanisms in the process still need to be elucidated.Furthermore,it is unclear whether the phototropic mechanisms vary between monocotyledons and dicotyledons.In this study,we identified an evolutionary ortholog of AtPHOT1 in maize,ZmPHOT1,which is involved in blue-light-induced phototropism.ZmPHOT1 showed a weaker phototropic sensitivity under low blue light irradiation(0.01 μmol m-2s-1) and a stronger phototropic sensitivity in regulating the phototropism under high blue light irradiation(10 μmol m-2s-1)than AtLOV-ZmSTK.In view of the different functions of AtPHOT1 and ZmPHOT1,the intrinsic mechanism by which phot1 mediates phototropism is expected to be elucidated by studying the effect of structural differences between ZmPHOT1 and AtPHOT1 on the phototropic response,as well as their different functions in mediating the dephosphorylation of NPH3 under blue light(BL).
2.Materials and methods
2.1.Plant materials and growth conditions
The Col-0 ecotype ofArabidopsiswas used as the wild-type,and thephot1-5mutants have been previously described [9].Seeds were surface-sterilized and sown in Murashige and Skoog (MS;Americ,Phytotech,M519) plates containing 1.0% agar,and placed in darkness at 4 °C for 3 d for vernalization.Subsequently,seeds were transferred to white light at 21-22 °C for 24 h to promote uniform germination.Seeds that were about to germinate were transferred to the dark again for vertical incubation for approximately 3 d at 21-22 °C.At this stage,the etiolated seedlings measured approximately 5-8 mm in length and were irradiated with blue light of different intensities for the indicated times in figures(3 h or 12 h).
2.2.Generation of phot1 transgenic plants
Using polymerase chain reaction(PCR),we amplified the entireZmPHOT1andAtPHOT1coding region and inserted it into a pCAMBIA-1300 green fluorescent protein(GFP)vector.Meanwhile,the fragments of AtLOV (1-622 amino acids),AtSTK (623-996 amino acids),ZmLOV (1-541 amino acids),and ZmSTK (542-911 amino acids) were also amplified and combined into AtLOVZmSTK and ZmLOV-AtSTK via homologous recombination.Seeds of thephot1-5null mutant were transformed with a construct encoding translationally fusedZmPHOT1orAtPHOT1gene to GFP(hereafter referred to as the35S::ZmPHOT1-GFPorAtPHOT1-GFPgenotype) by floral dip viaAgrobacterium-mediated transformation.The overexpression transgenic plant ofWT 35S::AtPHOT1,constructed in our previous study [2],was also used here.Subsequently,transgenic plants were selected using a medium containing 25 μg mL-1hygromycin,where the phenotypes of true transgenic plants were analyzed.
2.3.Measurement of hypocotyl curvature
The degree of hypocotyl curvature was quantified using previously reported methods [2,39].Accordingly,dark grown (for 3 d)seedlings were treated with 0.01,1,10,or 100 μmol m-2s-1unilateral blue light for 3,6,or 12 h.Photographs of their phenotypes were taken using a Canon camera (Japan,Canon,EOS 90D),and the degree of curvature was measured with E-ruler software [39].The experiments were replicated thrice,and the means and standard deviations (SDs) were calculated for each genotype.
2.4.Yeast two-hybrid assay
The cDNA-encoding sequences of AtPHOT1N,ZmPHOT1N,and ZmPHOT1mutwere separately cloned into the pGADT7-AD vector.The cDNAs encoding sequences of RPT2N and NPH3C were cloned separately into the pGBKT7-BD vector,and the sections of AtPHOT1,ZmPHOT1,AtNPH3,and AtRPT2 were selected using diagrammatic representations(Fig.6A).The Y2H assay was performed as described previously [32],and the bait and prey plasmids were co-transferred into theSaccharomyces cerevisiaestrain Y2H.The transferred Y2H strains were cultured on the SD medium of -Leu/-TRP (+His) and -Leu/-TRP/-His (-His),respectively,and the growth activity of the transferred Y2H strains was cultured on the SD medium of -Leu/-TRP/-His (-His),indicating an interaction between the two proteins.
2.5.Co-immunoprecipitation assay
The third to sixth leaves of 21-d-old tobacco plants were infected with eitherAgrobacteriumsuspension liquid (OD 600=0.8) or two types ofAgrobacteriumsuspension liquids in a 1:1 ratio.The plants were cultivated in an incubator with low light irradiation at 25°C for 2 d,followed by dark treatment for 24 h.The tobacco leaves were sampled,weighed,and frozen in liquid nitrogen.The samples were dissolved in a (Tris-HCl solution+tween 20) buffer solution (TBST) containing an inhibitor cocktail in liquid nitrogen,and were then centrifuged at 12,000×gfor 10 min.Ten microliters anti-Flag beads (Abmart)were mixed with the supernatant,and the mixture was incubated for 3 h at 4°C on a mute mixer(Qilinbeier,WH986).After washing thrice with TBST buffer solution,5 μL SDS loading buffer was mixed with the immunoprecipitate and boiled for 5 min.The mixture was subjected to western blotting with anti-FLAG (Abmart)and anti-GFP (Abmart) antibodies [40].
2.6.Luciferase complementary assay
Complementary DNAs encoding the Atphot1N,Zmphot1N,and Zmphot1Nmutcoding sequences were cloned separately into the pCAMBIA-1300cLUC vector (China,Bio-Transduction Lab Co.,Ltd.),which contained a sequence encoding the C-terminal half of luciferase under the control of the 35S promoter.The cDNAs encoding the RPT2N and NPH3C coding sequences were cloned separately into the pCAMBIA-1300nLUC vector,which contains a sequence encoding the N-terminal half of luciferase driven by the 35S promoter,the sections of Atphot1,Zmphot1,AtNPH3,and AtRPT2 were selected by the diagrammatic representations (Fig.6A).The procedure forAgrobacteriuminfection in tobacco plants was similar to that used in the Co-IP assay[32].Finally,a chemiluminescence immunoanalyzer (America,PerkinElmer,IVIS Lumina S5) was used to detect luciferase signals while the tobacco leaves were treated with the luciferase substrate solution.
2.7.Immunodetection
The total proteins extracted from the whole seedlings,cotyledons,hypocotyls,and roots of 3-d-old etiolatedArabidopsisseedlings were diluted to equal concentrations and subsequently subjected to SDS-PAGE.Western blotting was performed following a previously reported protocol [2].The anti-NPH3 polyclonal antibody was prepared by the Beijing Huada Protein Research and Development Center,and anti-rabbit IgG was used as the secondary antibody (Beyotime).After incubation with Lumi-Light Western Blotting Substrate(Roche)for 10 min,chemiluminescence was detected using a chemiluminescence imager (Fusion FX6-XT;Vilber).Ponceau staining and specific bands represent equal loading controls.
2.8.Confocal laser scanning microscopy
The subcellular localization of GFP was observed in the transformedN.benthamianaand etiolated hypocotyl seedlings harboring the GFP fusion protein using a Carl Zeiss LSM 710 laser scanning confocal microscope at an excitation wavelength of 488 nm.Green fluorescent protein fluorescence was observed between 505 and 530 nm.The dark-grown etiolated seedlings and leaves of dark-adapted (for 12 h)-transformedN.benthamianawere transferred to a glass-bottom silica gel dish,a drop of MS solution was added,and a coverslip was immediately placed over the seedling [8].
2.9.Statistical analyses
Each experiment was replicated thrice,using at least 10 samples per genotype.Student’st-test was used for statistical analysis(P<0.05).
3.Results
3.1.Zmphot1 exhibited different phototropic sensitivity from Atphot1
By comparing the protein homology between Zmphot1 and Atphot1,we found that the homology of the full-length protein was only 63.86% (Fig.S1).We also analyzed the homology of the corresponding functional domain,and found that AtLOV1 and ZmLOV1,AtLOV2 &ZmLOV2,and AtSTK and ZmSTK showed up to 80% homology (Fig.S1A).These results suggest that Zmphot1 may possess a highly conserved function similar to Atphot1.To explore evolutionary conservation and differences between Atphot1 and Zmphot1 in regulating hypocotyl phototropism,we transformed35S::ZmPHOT1-GFPand35S::AtPHOT1-GFPintophot1mutant,the transcriptional level ofZmPHOT1-GFPandAtPHOT1-GFPinphot1 35S::ZmPHOT1-GFP,andphot1 35S::AtPHOT1-GFPtransgenic plants was analyzed by qRT-PCR assay.The transcriptional level ofGFPin the transgenic plant was significantly upregulated compared with wild type andphot1mutant (Fig.1B).Meanwhile,the abundance of Zmphot1-GFP and Atphot1-GFP were detected by western-blot assay,and the Atphot1-GFP exhibited a band of larger components than Zmphot1-GFP (Fig.1C).To distinguish the effect of overexpression of Zmphot1,a relatively low expression line ofZmPHOT1(phot1 35S::ZmPHOT1-GFP#1)and a relatively high expression line ofZmPHOT1(phot1 35S::ZmPHOT1-GFP#5) were selected in this study.We analyzed the hypocotyl phototropism of etiolated seedlings under low blue light irradiation(0.01&0.1 μmol m-2s-1)in transgenic linesphot1 35S::ZmPHOT1-GFP#1,phot1 35S::ZmPHOT1-GFP#5andphot1 35S::AtPHOT1-GFP#1.Results showed thatphot1 35S::ZmPHOT1-GFP#1andphot1 35S::ZmPHOT1-GFP#5exhibited a much weaker phototropic response under a fluence of 0.01 μmol m-2s-1blue light irradiation for 3 h,than the wild type,which presented a curvature of approximately 60° under similar conditions (Fig.1A,D).When irradiation was prolonged to 12 h,the phototropic curvature of the wild type andphot1 35S::AtPHOT1-GFP#1reached 80°,which was larger than that ofphot1 35S::ZmPHOT1-GFP#1andphot1 35S::ZmPHOT1-GFP#5transgenic lines (Fig.1A,D).These results suggested that Zmphot1 exhibited a weaker sensitivity than Atphot1 in regulating phototropism under a fluence of 0.01 μmol m-2s-1blue light irradiation.In contrast,the transgenic lines ofZmPHOT1andAtPHOT1exhibited a similar phototropic sensitivity under a fluence of 0.1 μmol m-2s-1blue light irradiation(Fig.1A,E).Additionally,western blotting confirmed the expression of the Zmphot1-GFP fusion protein in the transgenic lines,and different expression levels ofZmPHOT1did not affect the phototropic responses ofphot1 35S::ZmPHOT1-GFP#1andphot1 35S::ZmPHOT1-GFP#5transgenic lines (Fig.1).
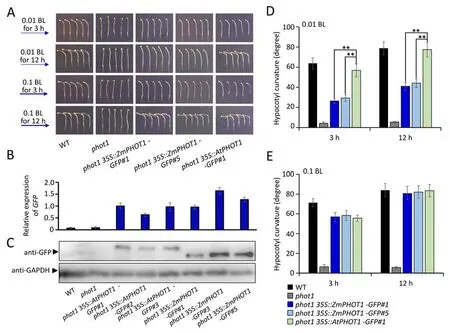
Fig.1.Detection of phototropism in dark grown etiolated seedlings of WT, phot1, phot1 35S::ZmPHOT1-GFP#1, phot1 35S::ZmPHOT1-GFP#5,and phot1 35S::AtPHOT1-GFP#1 under low blue light irradiation.(A)Dark grown etiolated seedlings of WT and transgenic lines were irradiated with continuous blue light(0.01 or 0.1 μmol m-2 s-1)for 3 h and 12 h respectively,and the curvature of the hypocotyls were analyzed.(B) The transcriptional level of ZmPHOT1-GFP and AtPHOT1-GFP in phot1 35S::ZmPHOT1-GFP,and phot1 35S::AtPHOT1-GFP transgenic plants was analyzed by qRT-PCR assay.(C) The abundance of Zmphot1-GFP and Atphot1-GFP were detected by subjecting to anti-GFP immunoprecipitation using anti-GFP antibodies,and the anti-GAPDH immunoprecipitation indicates the loading control.(D,E)The phototropic curvature was measured in 3-day-old etiolated seedling irradiated with unilateral blue light.Error bars represent±SD of 30 seedlings.The asterisks indicate statistically significant differences between the two groups. **, P <0.01 (Student’s t-test).
Because phot1 was also involved in the high blue light-induced phototropism,the hypocotyl phototropism of the etiolated seedlings were analyzed under high blue light irradiation (10 &50 μmol m-2s-1) for the above materials.Interestingly,thephot1 35S::ZmPHOT1-GFP#1andphot1 35S::ZmPHOT1-GFP#5transgenic lines exhibited a much higher phototropic response thanphot1 35S::AtPHOT1-GFP#1,and the phototropic curvature was evenly >100°while irradiated with a fluence of 10 or 50 μmol m-2s-1blue light irradiation for 3 h (Fig.2).Nevertheless,with prolonged processing time,the phototropic curvatures ofphot1 35S::ZmPHOT1-GFP#1andphot1 35S::ZmPHOT1-GFP#5transgenic lines gradually decreased,and the transgenic lines of Zmphot1 and Atphot1 exhibited similar phototropic curvatures when exposed to HBL for 12 h.A previous report showed that phot1 exhibits an inhibitory function in phot2-mediated phototropism under HBL [2],which suggest that the higher phototropism ofZmPHOT1transgenic line may be a consequence of the lower inhibition in phot2-mediated phototropism under a fluence of 10 and 50 μmol m-2s-1blue light irradiation,which was also associated with the lower phototropic sensitivity of Zmphot1 than that of Atphot1.

Fig.2.Detection of phototropism in dark grown etiolated seedlings of WT, phot1, phot1 35S::ZmPHOT1-GFP#1, phot1 35S::ZmPHOT1-GFP#5,and phot1 35S::AtPHOT1-GFP#1 under HBL irradiation.(A) Dark grown etiolated seedlings of WT and transgenic lines were irradiated with continuous blue light (10 or 50 μmol m-2 s-1) for 3 h and 12 h respectively.(B,C)The phototropic curvature was measured in 3-day-old etiolated seedling irradiated with unilateral blue light.Error bars represent±SD of 30 seedlings.The asterisks indicate statistically significant differences between the two groups.**, P <0.01 (Student’s t-test).
3.2.Zmphot1 exhibited a different internalized response under BL compared to Atphot1
Although it has been previously reported that phot1 exhibits internalization from the plasma membrane and that phot2 locally migrates to the Golgi vesicles in response to blue light irradiation[15],recent studies have reported contrasting results in which phot1 is distributed in a dynamic cytoplasmic structure[16,17,19].To assess the different processes of internalization of Zmphot1 and Atphot1 induced by blue light irradiation,we observed the subcellular localization of the fusion proteins Zmphot1 and Atphot1,byAgrobacterium-mediated transient expression assay in tobacco plants.Under dark conditions,Zmphot1 and Atphot1 fusion proteins were both located on the plasma membrane (Fig.3A),and repeated scanning with 488 nm laser for 10 min was used to excite Zmphot1-GFP,inducing its relocation into aggregates,while the Atphot1-GFP showed dynamic cytoplasmic structures under similar conditions,instead of GFP particles,such as that of Zmphot1-GFP (Fig.3A).In contrast,with the increase in processing time,the Zmphot1-GFP particles gradually disappeared,while the dynamic dispersion of the Atphot1-GFP signal consistently distributed in the cytoplasm (Fig.3A).These results suggest that Zmphot1 exhibits blue light-induced internalization similar to phot2 [15],and the different sensitivities of Zmphot1 and Atphot1 may be attributed to the different internalization patterns induced by blue light irradiation.
3.3.Different functional domain between Zmphot1 and Atphot1 determined their different phototropic sensitivity
Previous reports have shown that a small region at the Cterminus of phot1 and phot2 is important for membrane association [41].Given that distinct internalization patterns occurred between Zmphot1 and Atphot1 under blue light irradiation.Therefore,we propose that the different membrane associations of Zmphot1 and Atphot1 may be attributed to their different STK domains at the C-terminus.To investigate how each of these domains contributed to the functional specificity of Zmphot1 and Atphot1,they were exchanged between Zmphot1 and Atphot1 fused to GFP (AtLOV-ZmSTK-GFP and ZmLOV-AtSTK-GFP) (Fig.3J)and expressed in tobacco leaves.Full-length Zmphot1-GFP and Atphot1-GFP were expressed in tobacco leaves,andArabidopsis phot1-5was used as a control.The plants were repeatedly scanned with a 488 nm laser for 10 min to excite the GFP fusion protein to analyze the different membrane associations between Zmphot1 and Atphot1.We found that ZmLOV-AtSTK-GFP exhibited tight membrane association and there was almost no GFP aggregates in the cytoplasm similar to what occurs with Atphot1-GFP(Fig.3B,D).Interestingly,AtLOV-ZmSTK-GFP was located in the cytoplasm in the form of aggregates,similar to what occurs with Zmphot1-GFP (Fig.3C,E).These results suggested that the STK domain at the C-terminus of Zmphot1 results in a more volatile membrane association than that of Atphot1-GFP.Additionally,the subcellular localization of the Zmphot1-GFP and Atphot1-GFP transgenic lines were also analyzed,and the Zmphot1-GFP presented a more volatile membrane association compared to that of Atphot1-GFP (Fig.3F,G,H,I).We also analyzed the transgenic lines ofAtPHOT1,ZmPHOT1,ZmLOV-AtSTK,andAtLOV-ZmSTKby irradiating the dark grown etiolated seedlings of wild type and transgenic lines with continuous blue light (0.01,0.1,10 and 50 μmol m-2s-1)for 3 h,respectively.It was found that thephot1 35S::AtLOV-ZmSTK-GFPexhibited a higher phototropic response thanphot1 35S::ZmPHOT1-GFP#1under low blue light irradiation(0.01 and 0.1 μmol m-2s-1).Consistently,thephot1 35S::ZmLOVAtSTK-GFPexhibited a lower phototropic response thanphot1 35S::AtPHOT1-GFP#1under low blue light irradiation,indicating that the AtLOV exhibited a higher phototropic sensitivity than ZmLOV under low blue light.Thephot1 35S::AtLOV-ZmSTK-GFPexhibited a phototropic response similar tophot1 35S::ZmPHOT1-GFP#1under high blue light irradiation (10 and 50 μmol m-2s-1) (Fig.4A,B),which suggested that the enhanced phototropism mediated by Zmphot1 resulted from the ZmSTK domain.In contrast,phot1 35S::ZmLOV-AtSTK-GFPexhibited a higher phototropic response thanphot1 35S::AtPHOT1-GFP#1under high blue light irradiation,suggesting that the ZmLOV may have a higher sensitivity than AtLOV under high blue light irradiation.AtLOV exhibited a higher phototropic sensitivity than ZmLOV under low blue light,and the higher phototropism under high blue light may have resulted from the decrease in the inhibitory effect on phot2-mediated phototropism by ZmSTK compared to that ofArabidopsis.

Fig.4.Detection of phototropism in dark grown etiolated seedlings of WT, phot1, phot1 35S::AtLOV-ZmSTK-GFP, phot1 35S::ZmLOV-AtSTK-GFP, phot1 35S::ZmPHOT1-GFP #1,and phot1 35S::AtPHOT1-GFP #1 under different fluence of blue light irradiation.(A) Dark grown etiolated seedlings of WT and transgenic lines were irradiated with continuous blue light (0.01,0.1,10 or 50 μmol m-2 s-1) for 3 h respectively.(B) The phototropic curvature was measured in 3-day-old etiolated seedling irradiated with unilateral blue light.Error bars represent±SD of 30 seedlings.The asterisks indicate statistically significant differences between the two groups.**,P <0.01(Student’s t-test).
3.4.Zmphot1 showed a weaker potential for mediating the dephosphorylation of NPH3 than Atphot1
The phosphorylation status of NPH3 is regulated in a phot1-dependent manner in response to blue light irradiation [24,25].To explore the differences in phosphorylation of Zmphot1 and Atphot1 induced by blue light irradiation,as well as in that of the phosphorylation of NPH3 regulated by Zmphot1 and Atphot1 under blue light,the dark grown etiolated seedlings ofphot1 35S::AtPHOT1-GFP#1andphot1 35S::ZmPHOT1-GFP#5were irradiated with the fluence of 0.01 and 10 μmol m-2s-1blue light,respectively for 0.5 or 1 h and then were subjected to anti-GFP immunoprecipitation using anti-GFP antibodies.The group exposed to dark conditions was set as the control,and the Atphot1 and Zmphot1 exhibited a phosphorylation response under the fluence of 10 μmol m-2s-1blue light,while the dephosphorylation response under the fluence of 0.01 μmol m-2s-1blue light(Fig.5A) was barely noticeable.Additionally,the dark grown etiolated seedlings of wild type andphot1 35S::ZmPHOT1-GFP#5were irradiated with 0.01 and 10 μmol m-2s-1,respectively for 0.5 or 1 h,and the group exposed to dark conditions was set as the control.Interestingly,the NPH3 in wild type exhibited a significant dephosphorylation (Fig.5B).In contrast,the NPH3 inphot1 35S::ZmPHOT1-GFP#5transgenic lines showed a partial dephosphorylation under blue light irradiation of 0.01 and 10 μmol m-2s-1(Fig.5B).These results indicate that Zmphot1 possessed a weaker ability in mediating the dephosphorylation of NPH3 than Atphot1,which explains the phot1-mediated dephosphorylation of NPH3 in mediating the phototropism under low blue light irradiation [42],and the inhibitory effect of dephosphorylated NPH3 on phot2-mediated phototropism [43].

Fig.5.Phosphorylation analysis of Zmphot1,Atphot1 and NPH3 protein under blue light irradiation.(A) The Phosphorylation status analysis of Atphot1 and Zmphot1.The phot1 35S::AtPHOT1-GFP#1 and phot1 35S::ZmPHOT1-GFP#5 etiolated seedlings were irradiated with blue light of 0.01 and 10 μmol m-2 s-1 for 0,0.5 and 1 h respectively and subjected to anti-GFP immunoprecipitation using anti-GFP antibodies.(B) The Phosphorylation status analysis of NPH3.The wild type and phot1 35S::ZmPHOT1-GFP#5 etiolated seedlings were irradiated with blue light of 0.01 and 10 μmol m-2 s-1 for 0,0.5 and 1 h and subjected to anti-NPH3 immunoprecipitation using anti-NPH3 antibodies.
3.5.Zmphot1 physically interacted with AtRPT2 and AtNPH3
Phot1 has previously been reported to physically interact with RPT2 and NPH3[20,24].As the Zmphot1 could partially contribute to the Atphot1-mediated phototropism under blue light irradiation,it is suggested that there exists a highly conservative correlation between Zmphot1 and Atphot1 in regulating the hypocotyl phototropism.To verify whether Zmphot1 physically interacts with two important components-AtRPT2 and AtNPH3,we performed Y2H assays and found that Zmphot1 interacts with the N-terminus of RPT2 and the C-terminus of NPH3,similar to Atphot1 (Fig.6B).In addition,we used Co-IP and luciferase complementary assays to verify whether Zmphot1 physically interacts with AtRPT2 and AtNPH3 inArabidopsis,and the results were consistent with those of the Y2H assays(Fig.6C-F).As a previous report showed that three phosphorylation sites on Zmphot1(S269,S369,and S376) play an important role in regulating the first positive phototropism [36],the function of these three phosphorylation sites in regulating the interaction of Zmphot1 with AtRPT2 and AtNPH3 were also tested.Y2H,Co-IP,and luciferase complementary assays all showed that the Zmphot1Nmutcould interact with AtNPH3 and AtRPT2,similar to that of Zmphot1(Fig.6).The interaction of Zmphot1 with AtRPT2 and AtNPH3 indicates that Zmphot1 could synergistically cooperate with AtRPT2 and AtNPH3 to regulate hypocotyl phototropism inArabidopsis.
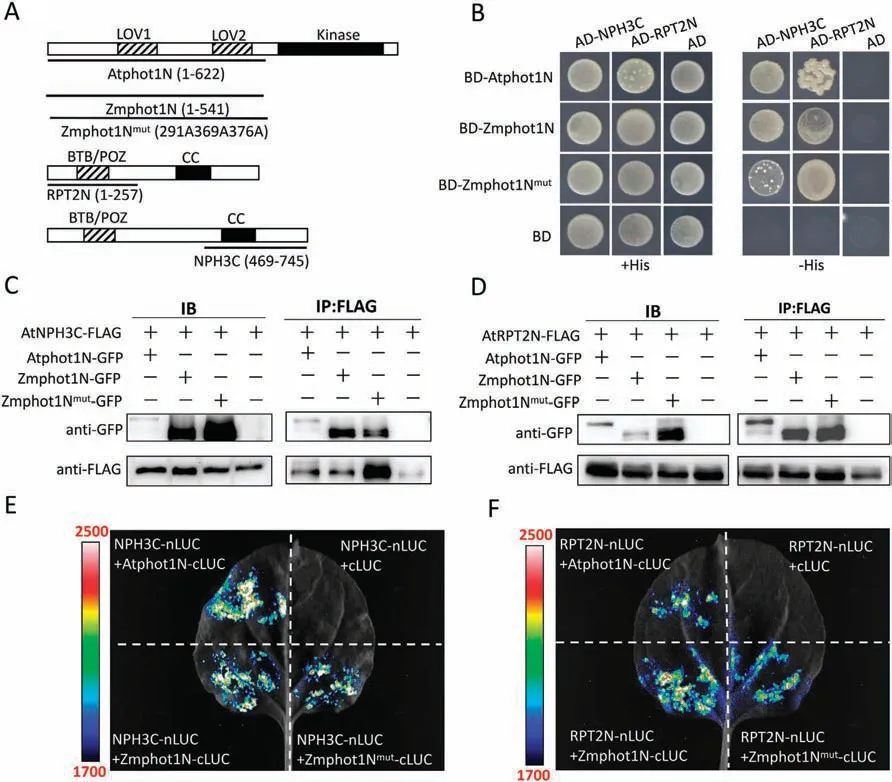
Fig.6.Verification of the interaction of Zmphot1 and Atphot1 with AtRPT2 and AtNPH3.(A)Diagrammatic representations of Atphot1N,Zmphot1N,Zmphot1Nmut,AtRPT2N and AtNPH3C proteins.(B)Interaction of Atphot1N,Zmphot1N,and Zmphot1Nmut with AtNPH3C and AtRPT2N was demonstrated by Y2H assay.(C)Interaction of Atphot1N,Zmphot1N,and Zmphot1Nmut with AtNPH3C demonstrated by Co-IP assay transiently expressed in leaves of N.benthamiana.(D) Interaction of Atphot1N,Zmphot1N,and Zmphot1Nmut with AtRPT2N demonstrated by Co-IP assay transiently expressed in leaves of N.benthamiana.(E)Interaction of Atphot1N,Zmphot1N,and Zmphot1Nmut with AtNPH3C demonstrated by Luciferase complementary assay transiently expressed in leaves of N.benthamiana.(F)Interaction of Atphot1N,Zmphot1N,and Zmphot1Nmut with AtRPT2N demonstrated by Luciferase complementary assay transiently expressed in leaves of N.benthamiana.
4.Discussion
In this study,we identified an evolutionary ortholog of Atphot1 in maize,Zmphot1,which mediates blue light-induced phototropism.Zmphot1 exhibited a weaker and stronger phototropic response to very low-and high-intensity blue light (0.01 and >10 μmol m-2s-1) irradiation,respectively,than Atphot1.A series of biological experiments proved that the weak phototropism under Low blue light (LBL) was attributed to the weak kinase activity and low photosensitivity of Zmphot1 in regulating the dephosphorylation of NPH3,and the strong phototropism under HBL was due to rapid internalization into aggregates which was mainly dependent on the STK domain of Zmphot1.The low dephosphorylation level of NPH3 inphot1 35S::ZmPHOT1GFPalso facilitated strong phototropism under HBL.
Given that Zmphot1 significantly enhanced phototropism compared with Atphot1 under strong blue light (10 μmol m-2s-1),as the lower negative gravitropism of hypocotyl could facilitate the phototropism[44,45],whether Zmphot1 could suppress the negative gravitropism of hypocotyl was unclear.Therefore,3-d-old etiolated seedlings were irradiated horizontally with strong blue light(10 μmol m-2s-1)for 3 h.We hypothesized that if Zmphot1 could inhibit negative gravitropism of the hypocotyl,it would not exhibit enhanced hypocotyl phototropism.The result revealed that Zmphot1 exhibited significantly enhanced phototropism compared with Atphot1(Fig.S2),suggesting that the enhanced phototropism mediated by Zmphot1 did not result from a decrease in negative gravitropism of the hypocotyl.In addition,the abundance of Zmphot1-GFP and Atphot1-GFP were detected by western-blot assay (Fig.1C).To distinguish the effect of overexpression ofZmPHOT1on phototropism,we selected a relatively low expression line ofZmPHOT1(phot1 35S::ZmPHOT1-GFP#1)and a relatively high expression line ofZmPHOT1(phot1 35S::ZmPHOT1-GFP#5) in this study,while these two transgenic lines both exhibited a similar phototropic response under weak or high intensity blue light illumination,suggesting that the function of Zmphot1 in regulating the hypocotyl phototropism did not rely strictly on its expression level.
A previous study showed that phot1 is involved in the chloroplast accumulation response under weak or strong blue light irradiation [5].To explore whether the different functions between Atphot1 and Zmphot1 in the regulation of hypocotyl phototropism would infiltrate the chloroplast movement response,we analyzed the chloroplast movement in rosette leaves under various blue light irradiations (1,5,10,and 100 μmol m-2s-1) forphot1 35S::ZmPHOT1-GFP#1,phot1 35S::ZmPHOT1-GFP#5,andWT 35S::AtPHOT1transgenic lines.The result showed that thephot1 35S::ZmPHOT1-GFP#1andphot1 35S::ZmPHOT1-GFP#5transgenic lines exhibited a weaker chloroplast accumulation response under a fluence of 1 μmol m-2s-1blue light irradiation than the wild type and WT35S::AtPHOT1(Fig.S3A,E).Under 5 μmol m-2s-1blue light irradiation,no significant difference was found in the chloroplast accumulation response among wild type,phot1 35S::ZmPHOT1-GFP#1,phot1 35S::ZmPHOT1-GFP#5,and WT35S::AtPHOT1transgenic lines (Fig.S3B,F).Interestingly,thephot1 35S::ZmPHOT1-GFP#1andphot1 35S::ZmPHOT1-GFP#5transgenic lines exhibited a stronger chloroplast accumulation response under a fluence of 10 μmol m-2s-1blue light irradiation compared to that of wild type andWT 35S::AtPHOT1(Fig.S3C,G).However,there was no significant difference among wild type,phot1 35S::ZmPHOT1-GFP#1,phot1 35S::ZmPHOT1-GFP#5,andWT 35S::AtPHOT1transgenic lines in chloroplast avoidance response under a fluence of 100 μmol m-2s-1blue light irradiation(Fig.S3D,H).These results indicate that the functional differences between Atphot1 and Zmphot1 in the regulation of hypocotyl phototropism were also suite to the chloroplast movement response.
Reportedly,the phototropic response under weak blue light is specifically mediated by phot1 [1].In response to weak blue light irradiation,NPH3 in the dephosphorylated state is more likely to mediate the phototropic response [42],and this process is specifically mediated by phot1.However,under strong blue light irradiation,dephosphorylated NPH3 inhibits the phot2-mediated phototropic response [43].In this study,the response of Atphot1 under both weak or strong blue light were compared with those of Zmphot1,and the ability of Zmphot1-mediated NPH3 dephosphorylation was shown to be significantly weakened(Fig.5),which further showed that the dephosphorylation of NPH3 was crucial mediating the phototropism under weak blue light(0.01 μmol m-2s-1) [42].Additionally,the enhanced phototropic response mediated by Zmphot1 under strong blue light was induced by the weakened dephosphorylation of NPH3 which was consistent with a study by Kimura [43].
Under blue light irradiation,both phot1 and phot2 could internalize from the plasma membrane and undergo autophosphorylation [15-18].Phot2 exhibits a localized migration to the Golgi vesicles under blue light irradiation [15],and in contrast,phot1 is dynamically distributed in cytoplasmic structures [16,17,19].Subcellular localization analysis of Atphot1-GFP and Zmphot1-GFP showed that Atphot1 and Zmphot1 exhibited different patterns of membrane migration,and Zmphot1 generated aggregates in response to blue irradiation,similar to Atphot2[15].As Zmphot1 showed high similarity to Atphot2,we also compared the homology among Zmphot1 proteins,and phylogenetic analysis was performed using the minimum-evolution method.Sequences of Atphot1 (AT3G45780),Atphot2 (AT5G58140),Zmphot1(Zm00001d044599),and Zmphot2 (Zm00001d032353) were retrieved from UniProt accessions based on the results of the PANTHER plant orthologs,and Zmphot1 was found to exhibit higher homology with Atphot1 than with Atphot2 and Zmphot2(Fig.S1B).Moreover,Zmphot1 also could mediate hypocotyl phototropism under weak blue light,which was not dependent on phot2 [1],indicating that Zmphot1 functions as a physiological phot1 regardless of its patterns of membranous migration.In contrast,the function of Zmphot1 in regulating phototropism was weakened under LBL and enhanced under HBL compared with Atphot1.
In summary,our findings indicate that low phototropic sensitivity under low blue light results from the weak dephosphorylation of NPH3 regulated by Zmphot1.Meanwhile,the weak dephosphorylation of NPH3 regulated by Zmphot1 could also contribute to high phototropic sensitivity under high-fluence blue light.However,further investigation is required to verify whether the internalization pattern of Zmphot1,which is similar to that of Atphot2,also enhances phototropic sensitivity.
CRediT authorship contribution statement
Jindong Zhu:Funding acquisition,Formal analysis,Data curation,Writing -original draft.Fangyuan Zhou:Data curation,Resources,Writing -review &editing.Yuxi Wang:Investigation,Writing -review &editing.Yuping Liang:Investigation.Yuanji Han:Investigation.Qingping Zhao:Funding acquisition,Writing-review &editing.Xiang Zhao:Funding acquisition,Writing -review &editing,Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank Dr.Ken-ichiro Shimazaki (Kyushu University,Japan)for providing thephot1(phot1-5) mutants.This work was supported by the National Natural Science Foundation of China(31871419,32200252,and 32100225),the Program for Innovative Research Team(in Science and Technology)at University of Henan Province(21IRTSTHN019),the Henan Overseas Expertise Introduction Center for Discipline Innovation (CXJD2020004),and the Natural Science Foundation of Henan Province (212300410214).
Appendix A.Supplementary data
Supplementary data for this article can be found online at https://doi.org/10.1016/j.cj.2023.04.007.
- The Crop Journal的其它文章
- Reversible protein phosphorylation,a central signaling hub to regulate carbohydrate metabolic networks
- Genetic and environmental control of rice tillering
- High-throughput phenotyping of plant leaf morphological,physiological,and biochemical traits on multiple scales using optical sensing
- The R2R3-MYB transcription factor GaPC controls petal coloration in cotton
- Disruption of LEAF LESION MIMIC 4 affects ABA synthesis and ROS accumulation in rice
- OsTHA8 encodes a pentatricopeptide repeat protein required for RNA editing and splicing during rice chloroplast development

