铁皮石斛DcNAC1基因克隆、表达及转录自激活活性分析
陈彧 邢文婷 李雨欣 张婷婷 饶丹丹 周扬


DOI:10.3969/j.issn.2095-1191.2023.06.003
摘要:【目的】克隆铁皮石斛NAC转录因子基因(DcNAC1),并进行表达模式及转录自激活活性分析,为铁皮石斛抗逆相关基因鉴定及其分子机制研究提供参考。【方法】以鐵皮石斛cDNA为模板,PCR扩增DcNAC1基因,运用生物信息学软件分析DcNAC1蛋白的理化性质、保守结构域、信号肽、跨膜结构域及亚细胞定位,通过实时荧光定量PCR检测DcNAC1基因在不同组织和不同逆境胁迫下的表达模式。同时构建该基因的酵母表达载体,分析其转录自激活活性。【结果】从铁皮石斛中PCR扩增获得DcNAC1基因的开放阅读框(ORF),全长为945 bp,与参考序列(LOC110104882)的核苷酸序列相似性为100%。该基因编码314个氨基酸残基,蛋白分子量为35.40 kD,理论等电点(pI)为8.16,为不稳定的亲水性蛋白,定位于细胞核,不含信号肽和跨膜结构域,含有特征性的NAC保守结构域。DcNAC1基因的启动子序列含茉莉酸甲酯响应元件(CGTCA-motif和TGACG-motif)、胁迫响应元件(TC-rich repeats)、光响应元件(G-box)、干旱诱导MYB结合位点(MBS)和低温响应元件(LTR)。根中DcNAC1基因的相对表达量在高温胁迫和低温胁迫处理6 h分别达最高,显著高于处理0 h(P<0.05,下同);茎中DcNAC1基因在盐胁迫处理48 h的相对表达量达最高,显著高于处理0 h。将构建的重组质粒pGBKT7-DcNAC1转化酵母菌株Y2HGold,结果发现该重组质粒无毒性,DcNAC1蛋白具有自激活活性。【结论】DcNAC1基因表达受到茉莉酸、低温、干旱、光信号和逆境胁迫等多种信号的调控。DcNAC1蛋白具有自激活活性,通过激活下游基因的表达,参与到植物生长发育和逆境胁迫响应的转录调控过程中。
关键词:铁皮石斛;NAC转录因子;基因克隆;转录自激活活性;逆境胁迫
中图分类号:S567.239 文献标志码:A 文章编号:2095-1191(2023)06-1612-10
Cloning,expression and trans-activation activity analysis of
DcNAC1 gene from Dendrobium catenatum
CHEN Yu1, XING Wen-ting1, 2, LI Yu-xin3, ZHANG Ting-ting3, RAO Dan-dan1, ZHOU Yang3*
(1Hainan Academy of Forestry (Hainan Academy of Mangrove),Haikou,Hainan 571100,China;2Tropical Crops Genetic Resources Institue,Chinese Academy of Tropical Agricultural Sciences,Haikou,Hainan 571101,China; 3School of Tropical Agriculture and Forestry (School of Agricultural and Rural Affairs,School of Rural Revitalization),Hainan University/Key Laboratory for Quality Regulation of Tropical Horticultural Crops of Hainan
Province,Haikou,Hainan 570228,China)
Abstract:【Objective】This study was to dissect the expression pattern and trans-activation activity of the NAC gene(DcNAC1) of Dendrobium catenatum,thus providing a foundation for stress-related genes identification and elucidating the molecular mechanism of D. catenatum stress-resistance. 【Method】DcNAC1 gene was amplified by PCR using D. catenatum cDNA as template. The physical and chemical properties, conserved domain, signal peptide, transmembrane domain and subcellular location of DcNAC1 protein were analyzed by bioinformatics softwares. The expression patterns of DcNAC1 gene in different tissues and under different stresses were detected by real-time fluorescence quantitative PCR. At the same time, yeast expression vector of this gene was constructed and its transcriptional self-activation activity was analyzed. 【Result】The open reading frame (ORF) of DcNAC1 gene was obtained by PCR amplification from D. catenatum. The total length was 945 bp, and the nucleotide sequence similarity to the reference sequence (LOC110104882) was 100%. This gene encoded 314 amino acid residues, had a molecular weight of 35.40 kD and a theoretical isoelectric point (pI) of 8.16. It was an unstable hydrophilic protein, localized in the nucleus, free of signal peptides and transmembrane domains, and contained a characteristic NAC conserved domain. The promoter sequences of DcNAC1 gene included jalapic acid response elements (CGTCA-motif and TGACG-motif), stress response elements (TC-rich repeats), light response elements (G-box), drought-induced MYB binding sites (MBS) and low temperature response elements (LTR). The relative expression of DcNAC1 gene in root reached the highest level at 6 h under high temperature stress and low temperature stress, respectively, and was significantly higher than that at 0 h under high temperature stress (P<0.05, the same below). The relative expression of DcNAC1 gene in stems was the highest at 48 h after salt stress treatment, which was significantly higher than that at 0 h. The recombinant plasmid pGBKT7-DcNAC1 was transformed into yeast strain Y2HGold. The results showed that the recombinant plasmid was not toxic, but DcNAC1 protein had certain self-activation activity. 【Conclusion】DcNAC1 gene expression is regulated by jasmonic acid, low temperature, drought, light signal and stress. DcNAC1 protein has self-activating activity, which is involved in transcriptional regulation of plant growth and development and response to stress by activating the expression of downstream genes.
Key words: Dendrobium catenatum; NAC transcription factor; gene cloning; transcriptional activation activity; adversity
Foundation items: Hainan Natural Science Foundation (320QN368,319MS009); Project of Hainan Key Laboratory for Biotechnology of Salt Tolerant Crops(HD-SYSZX-202107)
0 引言
【研究意義】铁皮石斛(Dendrobium catenatum)为多年生草本药用植物(李以格等,2019),具有养胃、增强免疫力和抗肿瘤等多种功能(Sun et al.,2015;Tang et al.,2017)。由于铁皮石斛生长常受到非生物胁迫影响,且长期过度采挖和栖息地遭受破坏,导致野生铁皮石斛资源逐渐枯竭、濒临灭绝(Ng et al.,2012)。NAC(NAM、ATAF和CUC)是植物特有的转录因子,参与植物生长发育和非生物胁迫应答(Gao et al.,2021),如促进侧根的生长,增强耐旱性(Tran et al.,2004);靶向调控气孔闭合和活性氧(ROS)稳态来调节非生物胁迫和氧化应激耐受性(You et al.,2013);增强渗透、盐和低温胁迫的耐受性(Hénanff et al.,2013)。因此,克隆铁皮石斛NAC1基因(DcNAC1),分析其在非生物胁迫下的表达情况,并探究DcNAC1蛋白转录自激活活性,以期了解DcNAC1基因的非生物胁迫响应机制,对铁皮石斛抗逆育种具有重要的意义。【前人研究进展】在植物生长发育过程中,高温、低温、干旱、盐和重金属等非生物胁迫会改变植物生物合成和养分获取的能力,并成为制约植物生长、影响作物产量和品质的因素(Bechtold and Field,2018)。在漫长的进化过程中,植物形成了一系列生理生化和分子机制来应对非生物胁迫(王计平等,2006)。基因通过转录和表达调控着植物细胞内许多重要的生命活动,如细胞形态发生、信号转导和环境胁迫响应(荣欢等,2020)。转录因子(TF)是一种调节蛋白,通过与特定的顺式作用元件结合来刺激或抑制其目标基因的表达,从而调控植物生长发育及非生物和生物胁迫响应(Singh et al.,2002;Chen and Tong,2004;Huang et al.,2012)。因此,转录因子对植物生长发育及抗逆响应起重要作用(Jin et al.,2014)。根据靶基因启动子中DNA结合结构域(DNA binding domain,DBDs)的不同,转录因子可分为NAC、WRKY、MYB、HB、bZIP和AP2/ERF等多个不同的家族(Mun et al.,2017;Baillo et al.,2019)。NAC转录因子为植物特有的转录因子家族,NAC蛋白的N末端包含1个将目标基因和顺式作用元件结合起来的高度保守NAM结构域,由约160个氨基酸残基组成,而C末端包含可变的转录激活区(Souer et al.,1996;Aida et al.,1997;Olsen et al.,2005)。研究表明,拟南芥中过表达AtNAC1基因可促进侧根的生长,并增强其耐旱性(Tran et al.,2004);水稻中过表达SNAC1基因可增强水稻植株的抗旱性和耐盐性,并增强对脱落酸的敏感性,其主要是通过关闭气孔减少水分流失来提高转基因植株的抗旱性和耐盐性(Hu et al.,2006),过表达SNAC3基因可显著增强水稻植株的抗高温能力,沉默该基因则可显著增强水稻植株对高温的敏感性(Fang et al.,2015);普通小麦中TaNAC69基因过表达可提高胁迫诱导基因的转录水平,从而增强普通小麦的耐旱性(Xue et al.,2011),TaNAC29基因过表达可减少H2O2积累和膜损伤,以提高耐盐性(Xu et al.,2015);葡萄VvNAC1是植物信号防御级联的重要调节成分,将VvNAC1基因转入拟南芥中过表达可增强拟南芥对渗透、盐和低温胁迫的耐受性(Hénanff et al.,2013);在盐和渗透胁迫下,白桦BpNAC012基因过表达会导致木质素生物合成基因表达水平升高,促进根系中木质素的积累(Hu et al.,2019);沉默辣椒CaNAC035基因后辣椒幼苗在低温、盐害和干旱胁迫下受损程度比对照辣椒幼苗(未胁迫处理)严重,电解质渗漏相应增加,且丙二醛含量增加,过氧化氢和超氧自由基含量升高,表明CaNAC035基因在非生物胁迫下起正向调节作用(Zhang et al.,2020)。【本研究切入点】NAC蛋白在响应生物和非生物胁迫的基因表达信号转导和调节中起着重要作用,但目前尚无铁皮石斛NAC转录因子基因克隆及表达分析的相关研究报道。【拟解决的关键问题】以铁皮石斛cDNA为模板PCR扩增DcNAC1基因,运用生物信息学软件分析DcNAC1蛋白的理化性质、保守结构域、信号肽、跨膜结构域及亚细胞定位,通过实时荧光定量PCR检测DcNAC1基因在不同组织和不同逆境胁迫下的表达模式,同时构建该基因的酵母表达载体,分析转录自激活活性,为铁皮石斛抗逆相关基因鉴定及抗逆分子机制研究提供参考。
1 材料与方法
1. 1 试验材料
供试材料为铁皮石斛的云南广南种。供试引物由生工生物工程(上海)股份有限公司合成。植物总RNA提取试剂盒购自天根生化科技(北京)有限公司,反转录试剂盒PrimeScript RT reagent Kit with gDNA Eraser采购自宝生物工程(大连)有限公司;2×Taq Plus Master Mix II(Dye Plus)购自南京诺唯赞生物科技有限公司;质粒DNA提取试剂盒、PCR产物回收和酶切产物纯化试剂盒购自生工生物工程(上海)股份有限公司。大肠杆菌DH5α和农杆菌GV3101感受态细胞购自上海唯地生物技术有限公司。ChamQTM Universal SYBR qPCR Master Mix购自诺唯赞生物科技有限公司。DNA Ligation Mix购自宝生物工程(大连)有限公司。Y2HGold酵母菌株由海南省林业科学研究院实验室保存。主要仪器设备:低温连接仪(珠海黑马医学仪器有限公司)、PCR仪(珠海黑马医学仪器有限公司)、超净工作台(无锡一净净化设备有限公司)和电泳槽(北京六一生物科技有限公司)。
1. 2 胁迫处理及样品采集
将3月龄长势一致的铁皮石斛组培苗分成4组,分别对其进行高温(42 ℃)、低温(4 ℃)、盐(200 mmol/L NaCl)和干旱(20% PEG8000)胁迫处理,处理3、6、9、12、24和48 h后每组随机取5株组培苗的根、茎和叶进行混合,放入液氮中速冻后置于-80 ℃保存,以未处理的组培苗为对照。培养条件为光照和黑暗时长各12 h,置于25 ℃恒温培养箱中培养(张婷婷等,2021)。
1. 3 基因克隆
从NCBI数据库下载铁皮石斛基因组相关信息,从拟南芥数据库(https://www.arabidopsis.org/)下载AtNAC1蛋白序列(AT3G12977.1),将其与铁皮石斛基因组进行BLAST比对,筛选获得核苷酸序列相似性最高的序列(LOC110104882),并命名为DcNAC1。利用植物总RNA提取试剂盒提取RNA,反转录试剂盒合成cDNA第一链。利用Primer Premier 5.0 设计DcNAC1基因的开放阅读框(ORF)克隆引物(表1),同时在基因的上游和下游分别引入EcoR I和BamH I酶切位点。以反轉录得到的cDNA为模板进行PCR扩增,反应体系和扩增程序参考尚金梦等(2021)的报道。利用1.2%琼脂糖凝胶电泳检测PCR扩增产物。
1. 4 重组质粒构建
PCR产物经纯化、双酶切后,利用DNA Ligation Mix连接至pGBKT7上,将连接产物转化大肠杆菌DH5α感受态细胞,挑取单菌落接种于含卡那霉素的LB液体培养基中,于37 ℃恒温振荡培养至饱和状态,经菌液PCR鉴定后,将阳性克隆菌液送至生工生物工程(上海)股份有限公司进行测序。
1. 5 生物信息学分析
利用ExPASy预测DcNAC1基因编码蛋白的理化性质;使用NCBI数据库的CDD预测蛋白的保守结构域;采用Plant-mPLoc预测蛋白的亚细胞定位情况;采用SOPMA预测蛋白的二级和三级结构;利用SignalP 5.0预测蛋白的信号肽;以TMHMM Server v. 2.0预测蛋白的跨膜结构域。从NCBI数据库下载拟南芥AtNAC1(AEE75627.1)、水稻OsNAC1(XP_015651404.1)和葡萄VvNAC1(XP_002282566)蛋白序列,利用DNAMAN 6.0对蛋白序列进行多重比对分析。利用PlantCARE分析DcNAC1基因起始密码子(ATG)上游2000 bp启动子序列的顺式作用元件。
1. 6 实时荧光定量PCR检测
以正常条件下生长的铁皮石斛组培苗作为对照,利用实时荧光定量PCR对DcNAC1基因在干旱、高温、低温和盐胁迫下的表达模式进行分析。利用Primer Premier 5.0设计定量引物(表1)。采用ChamQTM Universal SYBR qPCR Master Mix进行实时荧光定量PCR检测,反应体系和扩增程序参考尚金梦等(2021)的报道。以Actin作为内参基因(付亚娟等,2020),采用2-ΔΔCt方法计算基因的相对表达量。试验设3次生物学重复。
1. 7 pGBKT7-DcNAC1重组质粒毒性及自激活活性检测
利用PEG/LiAc法将pGBKT7-DcNAC1和pGBKT7空载体转化Y2HGold酵母菌株中,在SDO培养基上分别涂布转化产物,在28 ℃培养箱培养3~5 d,观察记录酵母菌斑的生长状况,分析pGBKT7-DcNAC1是否对Y2HGold酵母菌产生毒性。通过菌落PCR筛选阳性克隆,挑取阳性克隆菌落于SDO液体培养基中,于28 ℃恒温振荡培养至饱和状态,将菌液用无菌水稀释10倍,再分别接种于SDO和SDO/X/A培养基上培养,同时设置阴性对照(pGBKT7空载体)和阳性对照(pGBKT7-Gal4质粒),28 ℃恒温培养3~5 d,观察菌落的生长情况,分析pGBKT7-DcNAC1重组质粒是否具有自激活活性。
2. 结果与分析
2. 1 DcNAC1基因克隆及测序结果
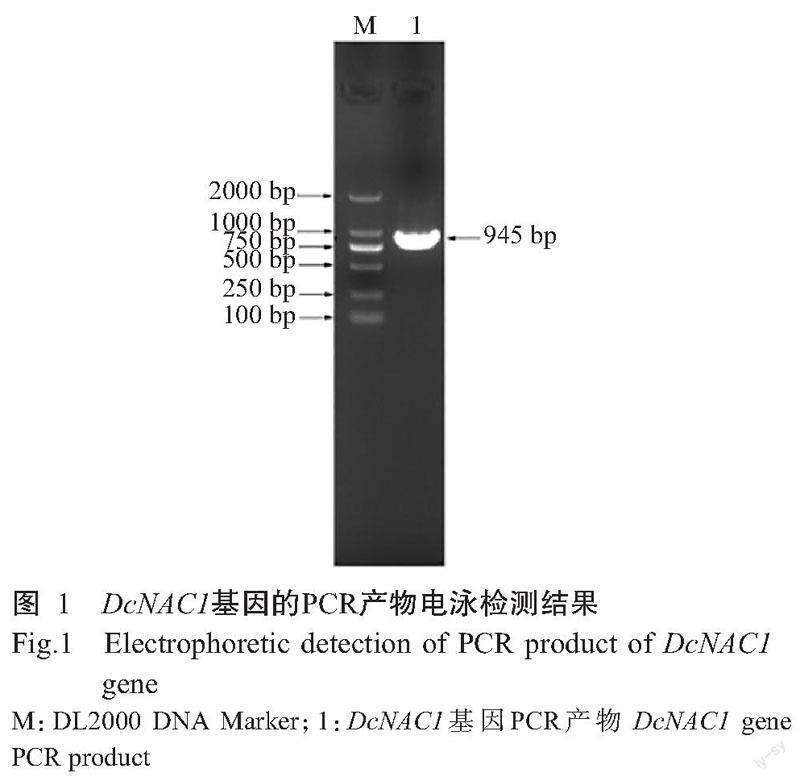
以铁皮石斛cDNA为模板,DcNAC1-F和DcNAC1-R为引物对DcNAC1基因进行扩增,结果发现扩增条带单一清晰明亮,大小约900 bp,与预期结果相符(图1)。测序结果显示,扩增条带与参考序列(LOC11 0104882)的核苷酸序列相似性为100%,表明成功获得DcNAC1基因。
2. 2 DcNAC1蛋白的生物信息学分析结果
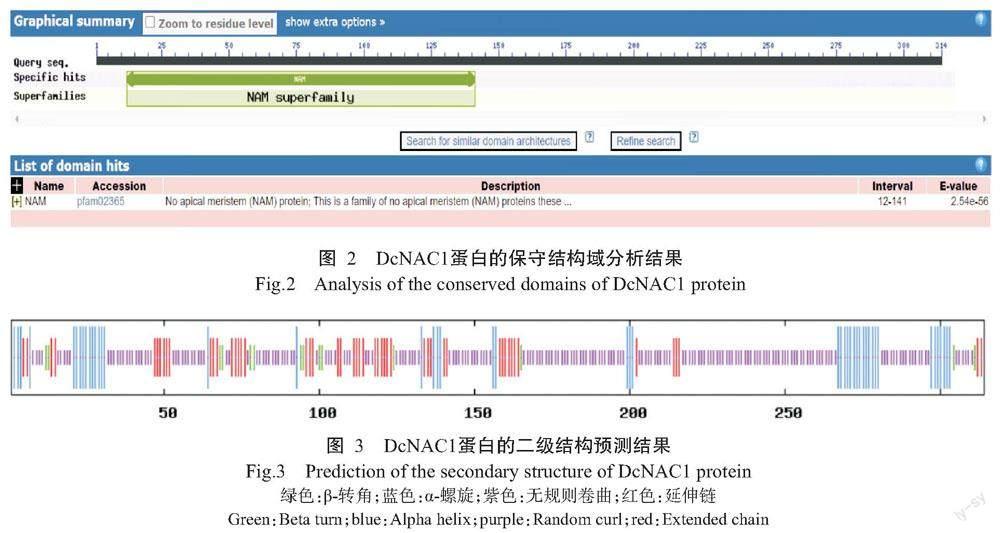
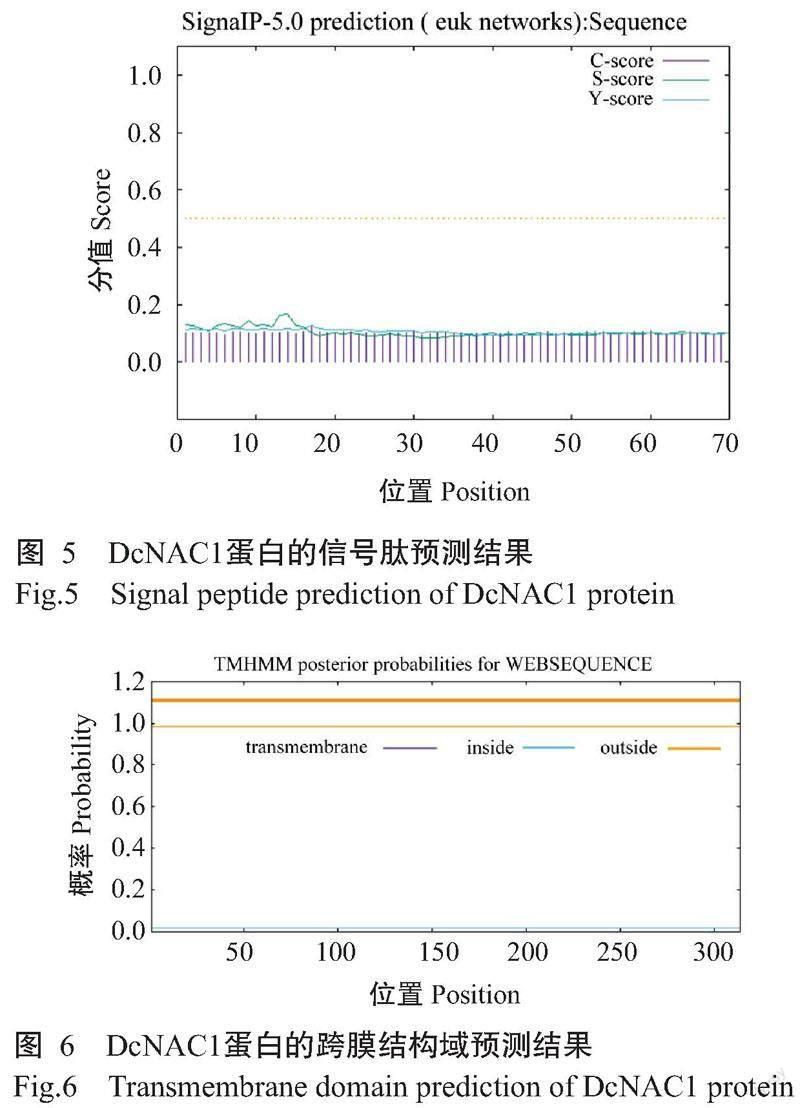
DcNAC1基因编码的蛋白由314个氨基酸残基组成,化学式为C1555H2407N437O469S21,理论分子量35.40 kD,理论等电点(pI)为8.16,不稳定系数为45.80,亲水性指数平均值(GRAVY)为-0.579,推测该蛋白为不稳定的亲水性蛋白。从图2可知,DcNAC1蛋白的N末端含有NAM保守结构域,说明该蛋白属于NAC基因家族。由图3可知,DcNAC1蛋白的二级结构由无规则卷曲(占63.69%)、α-螺旋(占15.61%)、延伸链(占16.24%)和β-转角(占4.46%)组成,与DcNAC1蛋白的三级结构预测结果(图4)基本一致。亚细胞定位结果显示,DcNAC1蛋白定位于细胞核。由图5和图6可知,DcNAC1蛋白不含信号肽和跨膜结构域,属于非分泌型蛋白或膜蛋白。
2. 3 DcNAC1蛋白的多序列比对结果
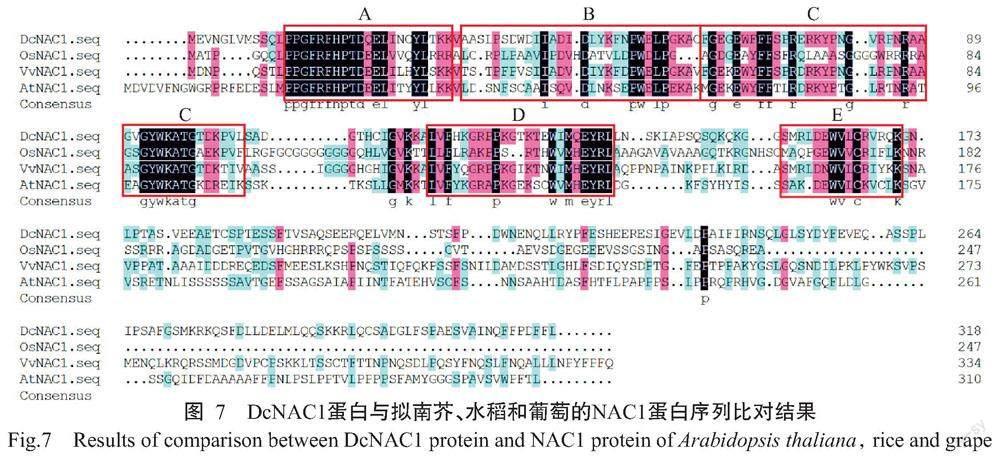
采用DNAMAN 10.0将获得的DcNAC1蛋白序列与拟南芥AtNAC1、水稻OsNAC1和葡萄VvNAC1进行序列比对分析,结果发现DcNAC1蛋白与AtNAC1的相似性最高,达51.50%,与VvNAC1和OsNAC1的相似性分别为45.02%和39.78%。进一步通过SMART数据库对DcNAC1、AtNAC1、OsNAC1和VvNAC1蛋白保守结构域进行比对分析,结果发现4个物种的NAC蛋白序列中均含有特征性的NAC保守结构域,其中DcNAC1蛋白的第13~162个氨基酸为NAC保守结构域,约由150个氨基酸残基组成,根据其序列差异性,进一步划分为5个亚结构(A~E)(图7)。
2. 4 DcNAC1基因上游启动子顺式作用元件预测结果
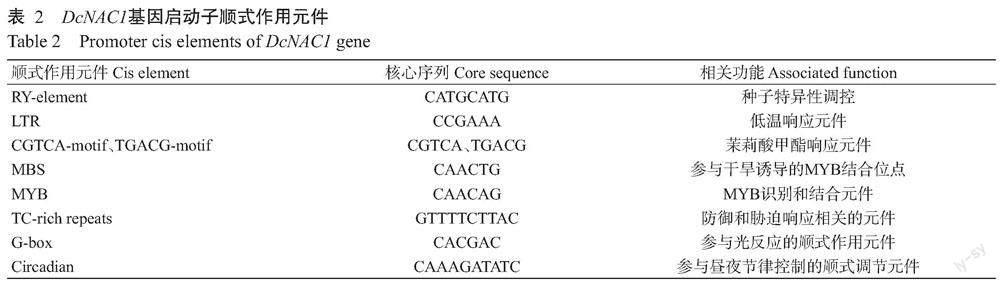
利用PlantCARE对DcNAC1基因起始密码子上游2000 bp的启动子序列进行顺式作用元件分析,结果发现,该基因启动子除包含植物启动子的基本元件CAAT-box和TATA-box外,还包含茉莉酸甲酯响应元件(CGTCA-motif和TGACG-motif)、胁迫响应元件(TC-rich repeats)、光响应元件(G-box)、干旱诱导MYB结合位点(MBS)和低温响应元件(LTR)(表2),推测DcNAC1基因的表达受茉莉酸、低温、干旱、光信号和逆境胁迫等多种信号的调控。
2. 5 DcNAC1基因在非生物胁迫下表达分析结果
采用实时荧光定量PCR对DcNAC1基因在高温、低温、盐和干旱胁迫处理下的表达模式进行分析,结果如图8所示。在高温胁迫下,根中DcNAC1基因除处理6 h的相对表达量与处理0 h的差异达显著水平(P<0.05,下同)外,其余处理天数均与处理0 h无显著差异;茎和叶中DcNAC1基因在处理3~48 h的相对表达量与处理0 h无显著变化(P>0.05,下同)(图8-A)。在低温胁迫下,根中DcNAC1基因在处理3~48 h的相对表达量均较处理0 h明显升高,尤其是在处理6、12和24 h的相对表达量显著高于0 h;茎和叶中DcNAC1基因在处理3~48 h的相对表达量与处理0 h相比无显著变化(图8-B)。在盐胁迫下,茎中DcNAC1基因在处理48 h达最大值,显著高于处理0 h;根和叶中DcNAC1基因在处理3~48 h的相对表达量均与处理0 h无显著差异(图8-C)。在干旱胁迫下,根、茎和叶中DcNAC1基因在处理3~48 h的相对表达量较处理0 h降低,但未达显著水平(图8-D)。综上所述,根中DcNAC1基因受高温和低温胁迫的诱导,茎中DcNAC1基因受盐胁迫的诱导,叶中DcNAC1基因在4种胁迫下的相对表达量变化不明显,说明不同逆境胁迫下不同组织中DcNAC1基因的表达模式不同。
2. 6 重组酵母菌株鉴定及毒性检测结果
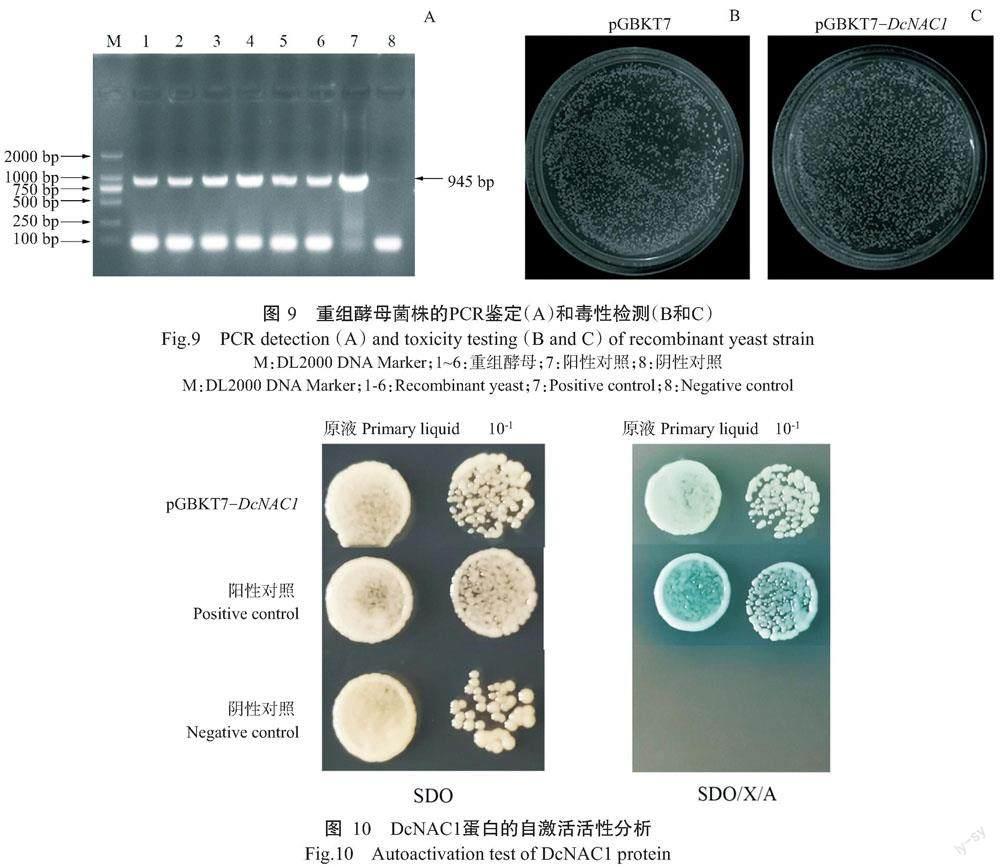
为了检测重组质粒pGBKT7-DcNAC1是否会对酵母生长产生毒性,将重组质粒pGBKT7-DcNAC1、pGBKT7-Gal4质粒(阳性对照)和pGBKT7空载体(阴性对照)分别转化酵母菌株Y2HGold,待生长2~3 d后挑取单菌落进行PCR扩增,结果如图9-A所示。菌落PCR扩增条带大小与目标基因大小一致,说明重组质粒pGBKT7-DcNAC1成功转化酵母菌株Y2HGold。由图9-B和图9-C可知,转化重组质粒pGBKT7-DcNAC1的酵母菌斑与转化pGBKT7空载体的酵母菌斑大小基本一致,说明重组质粒pGBKT7-DcNAC1不会抑制酵母菌株Y2HGold的生长。
2. 7 DcNAC1蛋白自激活活性检测结果
为了解DcNAC1蛋白是否具有自激活活性,将pGBKT7-DcNAC1、pGBKT7(阴性对照)和pGBKT7-Gal4(阳性对照)转化酵母菌株Y2HGold,分别涂布于SDO平板上,取单菌落进行PCR扩增,结果显示重组载体pGBKT7-DcNAC1成功转化酵母菌株Y2HGold。由图10可知,转化pGBKT7-DcNAC1、pGBKT7和pGBKT7-Gal4的酵母菌株均能在SDO平板上正常生长,且菌落为白色;转化pGBKT7-Gal4和pGBKT7-DcNAC1的酵母菌均能正常生长在SDO/X/A平板上,菌落呈蓝色,但转化pGBKT7载体的酵母菌在SDO/X/A平板上不能生长,说明DcNAC1蛋白具有自激活活性。
3 讨论
NAC是一种能调节植物纤维发育、次生壁合成、细胞扩增、叶片衰老及果实成熟等发育过程的转录因子(Pei et al.,2013;Hao et al.,2014;Huang et al.,2015;Gao et al.,2021)。但目前针对铁皮石斛NAC家族基因的研究报道较少。铁皮石斛是中国传统名贵珍稀药材,市场需求和市场价值高(杨豪男等,2020)。不利的生长环境条件,如高温、寒冷、干旱和高盐,会对铁皮石斛的生长造成不可逆转的损害。本研究克隆获得DcNAC1基因,其编码区(CDS)序列长度为945 bp,编码314个氨基酸残基,含有NAC蛋白家族的保守结构域,亚细胞定位于细胞核中,与柳树SlNAC1定位结果(田雪瑶等,2020)一致,推测二者在功能上具有一定的相似性。转录因子通过结合特定的顺式作用元件来调节基因的表达,从而影响目标基因的转录水平,进而提高植物对非生物胁迫的适应能力(吉璐,2013),如水稻Os08PTS基因在茎和种子胚中的表达受该基因启动子调控(颜静宛等,2021);谷子NAC家族基因(SiNAC)启动子参与茉莉酸甲酯、生长素和氧化胁迫应答(Puranik et al.,2011)。本研究发现,DcNAC1基因启动子区域内的顺式作用元件中以茉莉酸甲酯响应元件(CGTCA-motif和TGACG-motif)数目最多,还含有诱发干旱的MYB结合位点(MBS)和低温响应元件(LTR)等逆境胁迫响应元件。前人相关研究也表明,AtNAC019和AtNAC055通过茉莉酸甲酯信号途径参与拟南芥逆境胁迫的调控机制(Tran et al.,2004);ATAF1可诱导茉莉酸甲酯信号途径中相关防御信号标记基因的表达(Jensen et al.,2008);SiNAC1通过茉莉酸甲酯途径来抵御非生物胁迫(Puranik et al.,2011)。因此,推测DcNAC1基因通过茉莉酸甲酯信号通路参与植物生长发育和逆境胁迫的响应。
挖掘植物的耐鹽基因,改良其耐盐能力,对植物生长发育和生产活动具有重大意义。研究表明,水稻OsNAC5和OsNAC6基因表达受干旱、盐和低温等非生物胁迫的诱导(Ohnishi et al.,2005;Takasaki et al.,2010);拟南芥AtNAC019、AtNAC055和AtNAC072基因表达均受盐胁迫和干旱胁迫诱导(Jiang et al.,2009);小麦TaNAC69基因表达受多种非生物胁迫诱导(Xue et al.,2011);转基因拟南芥中过表达桉树EgrNAC1基因可显著提高拟南芥的抗低温能力(从青等,2021)。本研究对不同逆境胁迫下DcNAC1基因的表达模式进行分析,结果发现该基因受高温、低温和盐胁迫诱导,与水稻OsNAC6基因在高温、低温和盐胁迫下的表达模式相似(Singh et al.,2021),推测DcNAC1基因响应逆境胁迫,从而提高植株的抗逆能力。此外,本研究通过酵母自激活活性检测发现,DcNAC1蛋白具有自激活活性,说明其作为转录因子可通过激活下游基因的表达,参与到相应的转录调控过程中。
4 结论
DcNAC1基因表达受到茉莉酸、光信号、高温、低温和盐胁迫等多种信号的调控。DcNAC1蛋白具有自激活活性,通过激活下游基因的表达,参与到植物生长发育和逆境胁迫响应的转录调控过程中。
参考文献:
从青,倪晓祥,程龙军. 2021. 异源表达EgrNAC1提高拟南芥抗寒性和对干旱、高盐的敏感性[J]. 核农学报,35(3):567-575. [Cong Q,Ni X X,Cheng L J. 2021. Ectopic express of EgrNAC1 enhances cold tolerance and sensitivity to drought and salt in Arabidopsis thaliana[J]. Journal of Nuclear Agricultural Aciences,35(3):567-575.] doi:10.11869/j.issn.100-8551.2021.03.0567.
付亚娟,陈霞婷,乔洁,王晶,李文静,侯晓强. 2020. 铁皮石斛亲环蛋白基因DoCyP的克隆及表达分析[J]. 园艺学报,47(3):581-589. [Fu Y J,Chen X T,Qiao J,Wang J,Li W J,Hou X Q. 2020. Molecular cloning and expression characterization of Cyclophilin gene (DoCyP) in Dendrobium officinale[J]. Acta Horticulturae Sinica,47(3):581-589.] doi:10.16420/j.issn.0513-353x.2019-0357.
吉璐. 2013. 南荻抗逆相关NAC转录因子的克隆及功能鉴定[D]. 长沙:湖南农业大学. [Ji L. 2013. Cloning and function identification of stress resistance-related NAC transcription factors from Miscanthus lutarioriparius(Poaceae)[D]. Changsha:Hunan Agricultural University. ]
李以格,杨杭,姜琪梦,陈研硕,王晓锋,陈勇. 2019. 珍稀药用植物铁皮石斛的组学及功能基因研究进展[J]. 生命科学, 31(9):959-967. [Li Y G,Yang H,Jiang M Q,Chen Y S,Wang X F,Chen Y. 2019. Investigation on omics and functional genes of Dendrobium officinale (Orchidaceae),a precious medicinal herb[J]. Bulletin of Life Scien-ces,31(9):959-967.] doi:10.13376/j.cbls/2019118.
荣欢,任师杰,汪梓坪,王飞,周勇. 2020. 植物NAC转录因子的结构及功能研究进展[J]. 江苏农业科学,48(18):44-53. [Rong H,Ren S J,Wang Z P,Wang F,Zhou Y. 2020. Research progress on structure and function of plant NAC transcription factors[J]. Jiangsu Agricultural Sciences,48(18):44-53.] doi:10.15889/j.issn.1002-1302. 2020.18.008.
尚金梦,王汝颖,轩淑欣,江丹,费得清,王彦华,冯大领,申书兴. 2021. 大白菜—结球甘蓝易位系外源NAC086基因的鉴定与表达分析[J]. 农业生物技术学报,29(9):1678-1687. [Shang J M,Wang R Y,Xuan S X,Jiang D,Fei D Q,Wang Y H,Feng D L,Shen S X. 2021. Identification and expression analysis of foreign NAC086 gene in Chinese cabbage(Brassica campestris ssp. Pekinensis)-cabbage(B. oleracea var. Capitata) translocation line[J]. Journal of Agricultural Biotechnology,29(9):1678-1687.] doi:10.3969/j.issn.1674-7968.2021.09.003.
田雪瑶,周洁,王保松,何开跃,何旭东. 2020. 柳树NAC基因的克隆与表达模式分析[J]. 南京林业大学学报(自然科学版),44(1):119-124. [Tian X Y,Zhou J,Wang B S,He K Y,He X D. 2020. Cloning and expression pattern analysis of NAC gene in Salix[J]. Journal of Nanjing Forestry University(Natural Sciences Edition),44(1):119-124.] doi:10.3969/j.issn.1000-2006.201905031.
王計平,史华平,毛雪,李润植. 2006. 转录因子网络与植物对环境胁迫的响应[J]. 应用生态学报,17(9):1740-1746. [Wang J P,Shi H P,Mao X,Li R Z. 2006. Transcription factors networks and their roles in plant responses to environmental stress[J]. Chinese Journal of Applied Ecology,17(9):1740-1746.]
顏静宛,林智敏,周淑芬,陈子强. 2021. 水稻胚特异表达基因Os08PTS启动子的克隆及分析[J]. 福建农业科技,52(3):6-10. [Yan J W,Lin Z M,Zhou S F,Chen Z Q. 2021. Cloning and analysis of the promoter of rice Embryo-specific expression gene Os08PTS[J]. Fujian Agricultural Science and Technology,52(3):6-10.] doi:10.13651/j.cnki.fjnykj.2021.03.002.
杨豪男,张帮磊,张宁,沈晓静,盛军,王宣军,字成庭. 2020. 铁皮石斛的化学组成及其活性研究概述[J]. 广东化工,47(11):87-88. [Yang H N,Zhang B L,Zhang N,Shen X J,Sheng J,Wang X J,Zi C T. 2020. Study on chemical structure and biological activity of Dendrobium candidum[J]. Guangdong Chemical Industry,47(11):87-88.] doi: 10.3969/j.issn.1007-1865.2020.11.035.
张婷婷,罗琴,傅思毅,王健,宋希强,周扬. 2021. 铁皮石斛CIPK24与CBL1的互作及盐胁迫下的表达分析[J]. 分子植物育种,19(16):5326-5334. [Zhang T T,Luo Q,Fu S Y,Wang J,Song X Q,Zhou Y. 2021. Protein interaction and gene expression analysis under salt stress of CIPK24 and CBL1 from Dendrobium catenatum[J]. Molecular Plant Breeding,19(16):5326-5334.] doi:10.13271/j.mpb.019. 005326.
Aida M,Ishida T,Fukaki H,Fujisawa H,Tasaka M. 1997. Genes involved in organ separation in Arabidopsis:An analysis of the cup-shaped cotyledon mutant[J]. The Plant Cell,9(6):841-857. doi:10.1105/tpc.9.6.841.
Baillo E H,Kimotho R N,Zhang Z,Xu P. 2019. Transcription factors associated with abiotic and biotic stress tolerance and their potential for crops improvement[J]. Genes(Basel),10(10):771. doi:10.3390/genes10100771.
Bechtold U,Field B. 2018. Molecular mechaniss controlling plant growth during abiotic stress[J]. Journal of Experimental Botany,69(11):2753-2758. doi:10.1093/jxb/ery157.
Chen W J,Tong Z. 2004. Networks of transcription factors with roles in environmental stress response[J]. Trends in Plant Science,9(12):591-596. doi:10.1016/j.tplants.2004. 10.007.
Fang Y J,Liao K F,Du H,Xu Y,Song H Z,Li X H,Xiong L Z. 2015. A stress-responsive NAC transcription factor SNAC3 confers heat and drought tolerance through modulation of reactive oxygen species in rice[J]. Journal of Experimental Botany,66(21):6803-6817. doi:10.1093/jxb/erv386.
Gao Y J,An K X,Guo W W,Chen Y M,Zhang R J,Zhang X,Chang S Y,Rossi V,Jin F M,Cao X Y,Xin M M,Peng H R,Hu Z R,Guo W L,Du J K,Ni Z F,Sun Q X,Yao Y Y. 2021. The endosperm-specific transcription factor TaNAC019 regulates glutenin and starch accumulation and its elite allele improves wheat grain quality[J]. The Plant Cell,33(3):603-622. doi:10.1093/plcell/koaa040.
Hao Y J,Sun J Y,Xu P,Zhang R,Li L G. 2014. Intron-media-ted alternative splicing of WOOD-ASSOCIATED NAC TRANSCRIPTION FACTOR1B regulates cell wall thic-kening during fiber development in Populus species[J]. Plant Physiology,164(2):765-776. doi:10.1104/pp.113. 231134.
Hu H H,Dai M Q,Yao J L,Xiao B,Li X,Zhang Q,Xiong L. 2006. Overexpressing a NAM,ATAF,and CUC (NAC) transcription factor enhances drought resistance and salt tolerance in rice[J]. Proceedings of the National Academy of Sciences of the United States of America,103(35):12987-12992. doi:10.1073/pnas.0604882103.
Hu P,Zhang K M,Yang C P. 2019. BpNAC012 positively regu-lates abiotic stress responses and secondary wall biosynthesis[J]. Plant Physiology,179(2):700-717. doi:10. 1104/pp.18.01167.
Huang D B,Wang S G,Zhang B C,Shang-Guan K,Shi Y Y,Zhang D M,Liu X L,Wu K,Xu Z P,Fu X D,Zhou Y H. 2015. A gibberellin-mediated DELLA-NAC signaling cascade regulates cellulose synthesis in rice[J]. The Plant Cell,27(6):1681-1696. doi:10.1105/tpc.15.00015.
Huang G T,Ma S L,Bai L P,Zhang L,Ma H,Jia P,Liu J,Zhong M,Guo Z F. 2012. Signal transduction during cold,salt,and drought stresses in plants[J]. Molecular Bio-logy Reports,39(2):969-978. doi:10.1007/s11033-011-0823-1.
Hénanff G L,Profizi C,Courteaux B,Rabenoelina F,Gérard C,Clément C,Baillieul F,Cordelier S,Dhondt-Cordelier S. 2013. Grapevine NAC1 transcription factor as a convergent node in developmental processes,abiotic stresses,and necrotrophic/biotrophic pathogen tolerance[J]. Journal of Experimental Botany,64(16):4877-4893. doi:10.1093/jxb/ert277.
Jensen M K,Hagedorn P H,de Torres-Zabala M,Grant M R,Rung J H,Collinge D B,Lyngkjaer M F. 2008. Transcriptional regulation by an NAC(NAM-ATAF1,2-CUC2) transcription factor attenuates ABA signalling for efficient basal defence towards Blumeria graminis f. sp. hordei in Arabidopsis[J]. The Plant Journal,56(6):867-880. doi:10.1111/j.1365-313X.2008.03646.x.
Jiang H L,Li H M,Bu Q Y,Li C Y. 2009. The RHA2a-intera-cting proteins ANAC019 and ANAC055 may play a dual role in regulating ABA response and jasmonate response[J]. Plant Signaling Behavior,4(5):464-466. doi:10. 1104/pp.109.135269.
Jin J P,Zhang H,Kong L,Gao G,Luo J C. 2014. PlantTFDB 3.0:A portal for the functional and evolutionary study of plant transcription factors[J]. Nucleic Acids Research,42:D1182-D1189. doi:10.1093/nar/gkt1016.
Mun B G,Lee S U,Park E J,Kim H H,Hussain A,Imran Q M,Lee I J,Yun B W. 2017. Analysis of transcription factors among differentially expressed genes induced by drought stress in Populus davidiana[J]. 3 Biotech,7(3):209. doi:10.1007/s13205-017-0858-7.
Ng T B,Liu J,Wong J H,Ye X,Wing S S C,Tong Y,Zhang K Y. 2012. Review of research on Dendrobium,a prized folk medicine[J]. Applied Microbiology and Biotechnology,93(5):1795-1803. doi:10.1007/s00253-011-3829-7.
Ohnishi T,Sugahara S,Yamada T,Kikuchi K,Yoshiba Y,Hirano H Y,Tsutsumi N. 2005. OsNAC6,a member of the NAC gene family,is induced by various stresses in rice[J]. Genes and Genetic Systems,80(2):135-139. doi:10.1266/ggs.80.135.
Olsen A N,Ernst H A,Leggio L L,Skriver K. 2005. NAC transcription factors:Structurally distinct,functionally diverse[J]. Trends in Plant Science,10(2):79-87. doi:10. 1016/j.tplants.2004.
Pei H X,Ma N,Tian J,Luo J,Chen J,Li J W,Zheng Y,Chen X,Fei Z J,Gao J P. 2013. An NAC transcription factor controls ethylene-regulated cell expansion in flower petals[J]. Plant Physiology,163(2):775-791. doi:10.1104/pp. 113.223388.
Puranik S,Bahadur R P,Srivastava P S,Prasad M. 2011. Molecular cloning and characterization of a membrane associated NAC family gene,SiNAC from foxtail millet[Setaria italica (L.) P. Beauv][J]. Molecular Biotechnology,49(2):138-150. doi:10.1007/s12033-011-9385-7.
Singh K,Foley R C,Oñate-Sánchez L. 2002. Transcription factors in plant defense and stress responses[J]. Current Opinion in Plant Biology,5(5):430-436. doi:10.1016/s1369-5266(02)00289-3.
Singh S,Koyama H,Bhati K K,Alok A. 2021. The biotechnological importance of the plant-specific NAC transcription factor family in crop improvement[J]. Journal of Plant Research,134(3):475-495. doi:10.1007/s10265-021-01270-y.
Souer E,van Houwelingen A,Kloos D,Mol J,Koes R. 1996. The no apical meristem gene of Petunia is required for pattern formation in embryos and flowers and is expressed at meristem and primordia boundaries[J]. Cell,85(2):159-170. doi:10.1016/s0092-8674(00)81093-4.
Sun J,Guo Y D,Fu X Q,Wang Y S,Liu Y,Huo B,Sheng J,Hu X. 2015. Dendrobium candidum inhibits MCF-7 cells proliferation by inducing cell cycle arrest at G2/M phase and regulating key biomarkers[J]. Onco Targets and Thera-py,9:21-30. doi:10.2147/OTT.S93305.
Takasaki H,Maruyama K,Kidokoro S,Ito Y,Fujita Y,Shinozaki K,Yamaguchi-Shinozaki K,Nakashima K. 2010. The abiotic stress-responsive NAC-type transcription factor OsNAC5 regulates stress-inducible genes and stress tolerance in rice[J]. Molecular Genetics and Genomics,284(3):173-183. doi:10.1007/s00438-010-0557-0.
Tang H X,Zhao T W,Sheng Y J,Zheng T,Fu L,Zhang Y. 2017. Dendrobium officinale Kimura et Migo:A review on its ethnopharmacology,phytochemistry,pharmacology,and industrialization[J]. Evidence-based Complementary and Alternative Medicine,2017:7436259. doi:10.1155/2017/7436259.
Tran L S,Nakashima K,Sakuma Y,Simpson S D,Fujita Y,Maruyama K,Fujita M,Seki M,Shinozaki K,Yamaguchi-Shinozaki K. 2004. Isolation and functional analysis of Arabidopsis stress-inducible NAC transcription factors that bind to a drought-responsive cis-element in the early responsive to dehydration stress 1 promoter[J]. The Plant Cell,16(9):2481-2498. doi:10.1105/tpc.104.022699.
Xu Z Y,Gongbu Z X, Wang C Y,Xue F,Zhang H,Ji W G. 2015. Wheat NAC transcription factor TaNAC29 is involved in response to salt stress[J]. Plant Physiology and Biochemistry,96:356-363. doi:10.1016/j.plaphy.2015. 08.013.
Xue G P,Way H M,Richardson T,Drenth J,Joyce P A,McIntyre C L. 2011. Overexpression of TaNAC69 leads to enhanced transcript levels of stress up-regulated genes and dehydration tolerance in bread wheat[J]. Molecular Plant,4(4):697-712. doi:10.1093/mp/ssr013.
You J,Zong W,Li X K,Ning J,Hu H H,Li X H,Xiao J H,Xiong L Z. 2013. The SNAC1-targeted gene OsSRO1c modulates stomatal closure and oxidative stress tolerance by regulating hydrogen peroxide in rice[J]. Journal of Experimental Botany,64(2):569-583. doi:10.1093/jxb/ers349.
Zhang H F,Ma F,Wang X K,Liu S Y,Saeed U H,Hou X M,Zhang Y M,Luo D,Meng Y C,Zhang W,Abid K,Chen R G. 2020. Molecular and functional characterization of CaNAC035,an NAC transcription factor from pepper (Capsicum annuum L.)[J]. Frontiers in Plant Scien-ce,11:14. doi:10.3389/fpls.2020.00014.
(責任编辑 陈 燕)
收稿日期:2022-08-07
基金项目:海南省自然科学基金项目(320QN368,319MS009);海南省耐盐作物生物技术重点实验室项目(HD-SYSZX-202107)
通讯作者:周扬(1988-),https://orcid.org/0000-0002-7501-4154,副教授,主要从事植物抗逆分子生物学研究工作,E-mail:zhouyang@ hainanu.edu.cn
第一作者:陈彧(1984-),https://orcid.org/0000-0002-5593-4623,林业高级工程师,主要从事珍贵树种培育及药用植物育种研究工作,E-mail:cfstuchen@126.com

