Altered Expression of COMP, Collagen 1, and MMP9 in Pelvic Organ Prolapse*
CHEN Yi Fei, XU Hai Nan, DUAN Yi Nan, and XIA Zhi Jun
Female pelvic organ prolapse (POP) is a common disorder that affects the quality of life of women considerably.Studies in China have reported a 9.6%prevalence of symptomatic POP in adult women[1],with a higher prevalence among older women.Approximately 25% of Chinese women over 60 years old experience POP to varying degrees, even though some patients may be asymptomatic[2].
Connective tissue and extracellular matrix (ECM)components play a vital role in maintaining the normal position of pelvic floor organs.Cartilage oligomeric matrix protein (COMP), or thrombospondin-5 (TSP-5),is a matricellular protein that promotes collagen secretion and assembly, ensuring ECM stability[3].Collagen, particularly collagen 1, is the primary structural protein in the vaginal wall.Matrix metalloproteinases (MMPs), especially MMP9, are crucial for regulating ECM degradation and collagen metabolism, and MMP9 can cleave various proteins,including collagen 1 and elastin[4].
However, the expression and role of the three key proteins in POP are still unknown, which is the motivation behind this study to examine changes in COMP, collagen 1, and MMP9 expression in POP.
In total, 30 patients with pelvic organ prolapse(POP) and 30 non-POP patients (control) were enrolled in this study.All the patients in the POP group were III or IV degrees according to the POP-Q scale.No significant difference between the POP group and control group in basic characteristics was found (Supplementary Table S1, available in www.besjournal.com).However, microscopic examination of hematoxylin-eosin and Masson trichrome stained samples revealed notable differences in anterior vaginal wall tissue between the control and POP groups.Hematoxylin-eosin staining and Masson trichrome staining were performed to confirm the full layer structure and the collagen fibers of the anterior vaginal wall.Specifically, the anterior vaginal wall tissue of individuals in the control group exhibited a considerable amount of fibrous connective tissue and was characterized by a wellorganized arrangement of collagen fibers with dense connections.In contrast, the POP group showed a significant reduction in fibrous connective tissue content, with irregular arrangement and loose connections of collagen fibers, indicating disordered structure (Figure 1).

Figure 1.Hematoxylin-eosin staining (A and B)and Masson trichrome staining (C and D) of the anterior vaginal wall.The collagen fibers were stained pink by HE staining and blue by Masson trichrome staining.Control group: the connective tissues were arranged orderly, and the collagen fibers were arranged regularly and densely connected (A and C).POP group:the content of fibrous connective tissue was reduced, and the structure of connective tissue was disordered, with irregular arrangement and loose connection of collagen fibers (B and D).
Immunohistochemical assay revealed the expression of COMP, collagen 1, and MMP9 in the anterior vaginal tissue of subjects in the POP and control groups.COMP (median = 0.1096vs.0.1621,P< 0.01) and collagen 1 (median = 0.1228vs.0.1646,P< 0.01) expression levels were significantly lower in the POP group than in the control group.In contrast,MMP9 expression was significantly higher in the POP group than in the control group (median = 0.2230vs.0.1123,P< 0.01), indicating an opposite trend in COMP and collagen 1 expression (Figure 2).Consistent with the results of the immunohistochemical staining, there was a significant decrease in the mRNA expression of COMP (median = 0.01957vs.0.2106,P< 0.01) and collagen 1 (median = 0.02469vs.0.1417,P< 0.01)and an increase in MMP9 mRNA expression(median = 1.683vs.0.3679,P< 0.01) in the POP group compared with that in the control group.Moreover, western blot analysis confirmed the lower expression of COMP (0.5118 ± 0.0595vs.0.1686 ±0.0250,P< 0.01) and collagen 1 (0.9015 ± 0.0500vs.0.6440 ± 0.0855,P< 0.05) and the higher expression of MMP9 (0.5330 ± 0.0130vs.0.7943 ± 0.1177,P< 0.05) in the POP group (Figure 3).
COMP is a pentameric particle present in various ECMs and is involved in the regulation of collagen turnover through multiple pathways.COMP interacts with various proteins, including ECM components,such as collagen 1, cell surface receptors,complement proteins, growth factors, and intracellular and extracellular signaling molecules.COMP has been associated with numerous diseases,including cancer, cardiovascular diseases, and fibrosis disease[5].Studies have demonstrated that COMP defects can lead to the formation of larger diameter collagen fibers, reduced inter-fiber space,irregular shapes, decreased total collagen,particularly collagen 1, and weakened fibrosis[6].Overall, these findings suggest that decreased COMP expression in the vaginal wall tissue of POP patients may contribute to the weakening and disruption of the vaginal wall support system.
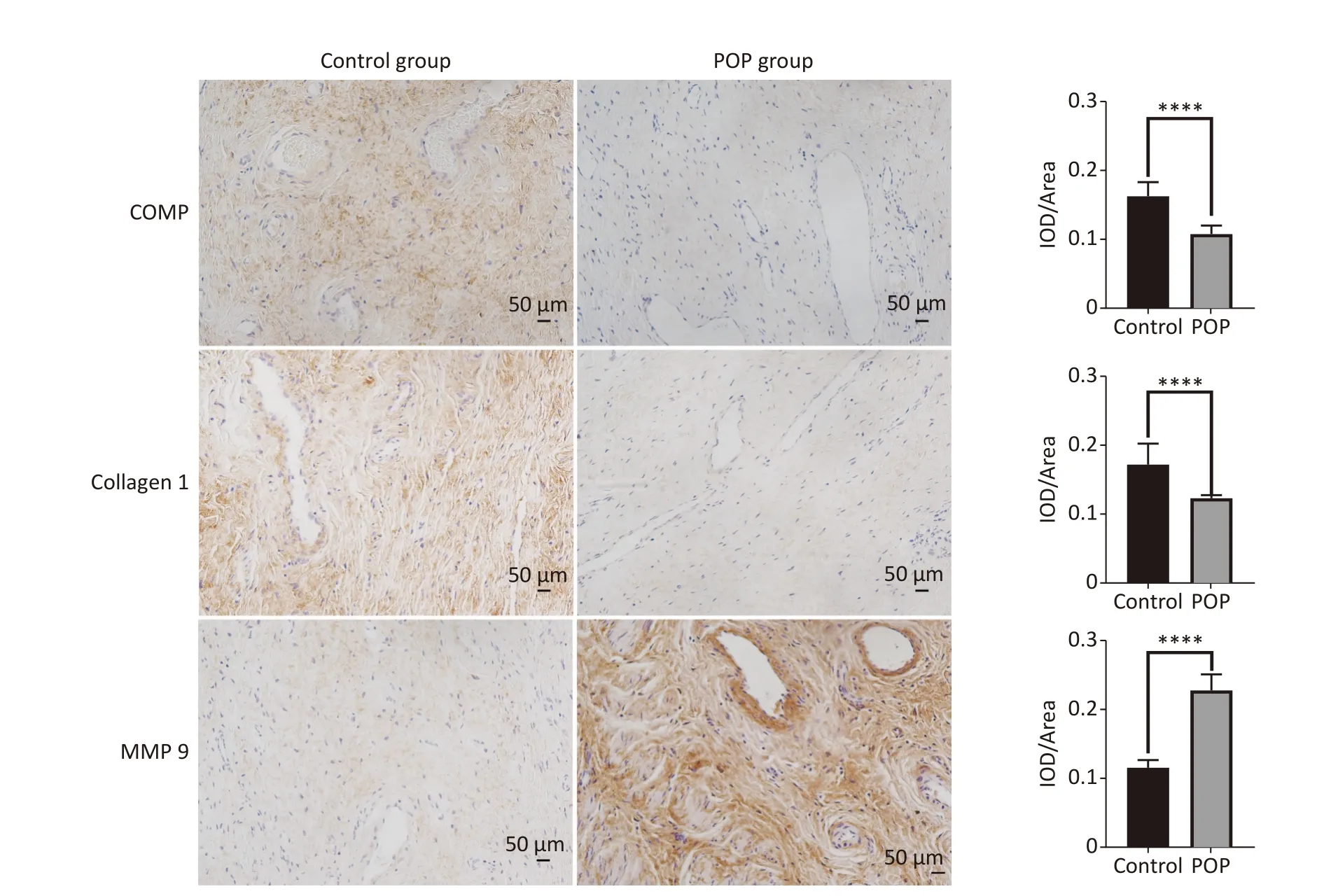
Figure 2.Immunohistochemical analysis of COMP, collagen 1 and MMP9 expression in the anterior vaginal wall of women with and without pelvic organ prolapse (×200).Sections of the anterior vaginal wall were stained with antibodies against COMP, collagen 1 and MMP9, and the percentages of positively stained areas were quantified using Image J software IOD/Area for quantitative analysis (Mann-Whitney U test).****P < 0.0001.

Figure 3.Relative mRNA expression of COMP (A), collagen 1 (B), and MMP9 (C) in the anterior vaginal wall of women with and without pelvic organ prolapse (Mann-Whitney U test).Protein expression levels(D) and relative protein expression (E) of COMP, collagen 1, and MMP9 in the anterior vaginal wall of women with and without pelvic organ prolapse (unpaired t test).*P < 0.05,**P < 0.01, ***P < 0.001,****P < 0.0001.
ECM plays a crucial role in maintaining the stability of pelvic floor tissues.A decrease in collagen content and an increase in immature collagen crosslinks have been reported in patients with POP,leading to decreased mechanical strength of the tissues.Previous studies have consistently shown lower levels of collagen 1 in the pelvic floor of women with POP[7].Additionally, research has indicated an association between pelvic organ prolapse and single nucleotide polymorphisms(SNPs) of the COL1A1 gene, specifically the rs1800012 variant, which is involved in the regulation of type 1 collagen formation[8].Consistent with previous findings, a significant decrease in collagen 1 expression was observed in the POP group compared with that in the control group in the present study.
MMPs are enzymes responsible for the degradation of ECM components, including collagen,gelatin, fibrin, and laminin, among which MMP9 is considered a key enzyme involved in the weakening and loss of integrity of collagen tissues[9].Moreover,the balance between collagen synthesis and degradation, which is closely related to MMPs, has been implicated in the development of POP.Although there is ongoing debate regarding the role of MMPs in POP, research evidence suggests that MMP9 upregulation can contribute to prolapse by affecting collagen metabolism[10].
Conclusively, this study provides evidence of differential gene expression levels of COMP, collagen 1, and MMP9 between patients with pelvic organ prolapse (POP) and control individuals (non-POP group).Further studies are necessary to elucidate the precise mechanisms and extent of ECM protein involvement in the pathogenesis of POP.
No potential conflicts of interest were disclosed.
CHEN Yi Fei conceived the design, XIA Zhi Jun supervised the study.CHEN Yi Fei performed the experiments and wrote the manuscript.DUAN Yi Nan analyzed the data.XU Hai Nan reviewed the manuscript.
#Correspondence should be addressed to XIA Zhi Jun,E-mail: xiazj@sj-hospital.org, Tel: 86-18940251266.
Biographical note of the first author: CHEN Yi Fei:female, born in 1998, MA, majoring in clinical and basic research of female pelvic floor dysfunction diseases.
Received: March 30, 2023;
Accepted: August 15, 2023
 Biomedical and Environmental Sciences2023年10期
Biomedical and Environmental Sciences2023年10期
- Biomedical and Environmental Sciences的其它文章
- Treatment Outcomes in COVlD-19 Patients with Brucellosis:Case Series in Heilongjiang and Systematic Review of Literature*
- Value of Pretreatment lnflammation-nutrition Score to Predict Non-response to Neoadjuvant Chemotherapy in Locally Advanced Rectal Cancer*
- A Comprehensive View on the Progress of Organoid Research with an Emphasis on its Relevance to Disease Characterization
- Synthesis of TiO2 Nanospikes for Dual Antibacterial Activity*
- Aurora A Kinase Plays a Key Role in Mitosis Skip during Senescence lnduced by lonizing Radiation*
- Ambient Temperature and Risk of Outpatient Chronic Pharyngitis Visits: A Time-series Analysis in Xinxiang, China*
