Protective effects of catalpol on cardio-cerebrovascular diseases: A comprehensive review
Zixi Zhang ,Yongguo Dai ,Yichao Xiao ,Qiming Liu
a Department of Cardiovascular Medicine, The Second Xiangya Hospital, Central South University, Changsha, Hunan, 410011, China
b Department of Clinical Pharmacology, College of Pharmacy, Dalian Medical University, Dalian, Liaoning,116044, China
c Department of Pharmacology, Wuhan University TaiKang Medical School (School of Basic Medical Sciences), Wuhan, 430071, China
Keywords:Catalpol Cardio-cerebrovascular diseases Anti-atherosclerosis Cerebrovascular protection Heart protection
ABSTRACT Catalpol,an iridoid glucoside isolated from Rehmannia glutinosa,has gained attention due to its potential use in treating cardio-cerebrovascular diseases (CVDs).This extensive review delves into recent studies on catalpol's protective properties in relation to various CVDs,such as atherosclerosis,myocardial ischemia,infarction,cardiac hypertrophy,and heart failure.The review also explores the compound's anti-oxidant,anti-inflammatory,and anti-apoptotic characteristics,emphasizing the role of vital signaling pathways,including PGC-1α/TERT,PI3K/Akt,AMPK,Nrf2/HO-1,estrogen receptor (ER),Nox4/NF-κB,and GRP78/PERK.The article discusses emerging findings on catalpol's ability to alleviate diabetic cardiovascular complications,thrombosis,and other cardiovascular-related conditions.Although clinical studies specifically addressing catalpol's impact on CVDs are scarce,the compound's established safety and well-tolerated nature suggest that it could be a valuable treatment alternative for CVD patients.Further investigation into catalpol and related iridoid derivatives may unveil new opportunities for devising natural and efficacious CVD therapies.
1.Introduction
Population aging is a significant global challenge facing humanity in the 21st century.Cardio-cerebrovascular diseases(CVDs)have emerged as the most prevalent diseases in the elderly population.CVDs refer to a broad range of disorders that impact the heart or blood vessels,including coronary heart disease,cerebrovascular disease,heart failure,peripheral vascular disease,and other conditions[1,2].According to the World Health Organization,CVDs are the leading cause of mortality worldwide,accounting for approximately 17.9 million deaths annually,which represents 32%of all deaths globally.In China,the number of CVD patients has exceeded 290 million,accounting for more than 40%of deaths and posing a significant economic burden to society and families [3].This highlights the urgent need to identify safe and effective novel therapeutic approaches to alleviate the burden of CVDs on the health and longevity of the global population.
The pathogenesis of CVDs is multifactorial,resulting from the prolonged interaction of various factors.Current research suggests that oxidative stress,mitochondrial dysfunction,and inflammation are the primary initiators or mediators of cardiac and vascular injury during the development of CVDs [4-9].Catalpol (PubChem CID: 91520),an iridoid glucoside compound extracted primarily from the roots ofRehmannia glutinosa,possesses potent biological properties such as anti-oxidant,anti-inflammatory,and antiapoptotic activities.Catalpol has been explored as a potential therapeutic agent for neurodegenerative diseases for many years[10].A substantial amount of evidence supports the beneficial biological effects of catalpol in preventing or treating CVDs.This review aims to provide a comprehensive overview of the physicochemical properties,biogenesis,sources,detection methods,pharmacokinetic properties,and safety of catalpol,as well as a detailed discussion of its protective effects on the cardiocerebrovascular system and related mechanisms.
2.Catalpol and its sources and biogenesis
Catalpol is an iridoid compound with a polar structure,as illustrated in Fig.1.Due to the presence of polar functional groups,catalpol is highly hydrophilic and soluble in both water and methanol.Catalpol's structure contains glycosidic bonds,making it unstable in acidic conditions and susceptible to hydrolysis [11].To date,the majority of studies have employed catalpol derived fromRehmannia glutinosain their experiments.However,catalpol is also widely distributed in various plant families,includingPlantaginaceae,Lamiaceae,andBignoniaceae[12-14].Catalpol is typically synthesized via the mevalonic acid or 2-C-methyl-D-erythritol-4-phosphate pathways (Fig.1 and Section 1 in the Supplementary data) [14-19].
3.Detection methods and pharmacokinetics of catalpol
Accurate and efficient detection methods are crucial for the research and development of catalpol.Currently,several analytical methods used for the determination of catalpol are mainly based on high-performance liquid chromatography (HPLC),ultraperformance liquid chromatography (UPLC),high-performance thin layer chromatography,micellar electrokinetic capillary chromatography (MECC),and liquid chromatography-tandem mass spectrometry (LC-MS/MS) [20-24].Catalpol is a highly polar molecule with limited ultraviolet (UV) absorption,making the initially developed HPLC-UV method [25-28] insufficiently sensitive for its detection.Mass spectrometry detection outperforms other detection technologies,including UV,fluorescence,and electrochemical detection,in terms of sensitivity and specificity.Subsequently,MECC-MS and gas chromatography-MS have also been developed for the determination of catalpol in plants or formulations [9,24-29].Additionally,multiple rapid,simple and sensitive LC-MS/MS methods have been continuously developed to detect catalpol and study its pharmacokinetic characteristics[21,23,29-33].A detailed description of these detection methods is provided in the Section 2 in the Supplementary data [20-39].
Understanding the pharmacokinetic characteristics of catalpol is essential for drug discovery and development.The pharmacokinetic characteristics of catalpol have been studied in normal physiological conditions and disease model animals[11,20,32,33,40,41],as summarized in Table 1[11,20,23,29,32,33,40,41]and the Section 3 in the Supplementary data[20,23,29-33,40-45].Studies have shown that catalpol can be rapidly absorbed by the intestine after oral administration [23,40,42],but its bioavailability can be affected by intestinal bacteria that convertit[14,30,43].Catalpol can be distributed to multiple tissues through blood circulation and can even penetrate the blood-brain barrier (BBB) [20,32].Catalpol is metabolized by hydrogenation after deglycosylation and eliminated quickly by the body [23,29,31,32,44,45].However,under pathological conditions such as diabetes and chronic kidney disease,absorption of catalpol is accelerated,and elimination is slowed[33,41].
4.Effects of catalpol on the cardio-cerebrovascular system
Numerous studies have utilized in vitro and in vivo models of CVDs to demonstrate the protective effect of catalpol on the cardiocerebrovascular system,indicating that catalpol exerts its anti-CVD effects by modulating various signaling pathways(Table 2)[46-71].
4.1.Atherosclerosis
Atherosclerosis is a chronic inflammatory disease that results from various injury stimuli acting on the arterial wall,and it is the main pathological basis of CVDs [72,73].The production of atherosclerotic plaques is initiated by the transport of low-density lipoprotein (LDL) to the arterial wall,which is followed by macrophage invasion and transformation to foam cells,infiltration of smooth muscle cells,and the production of fibrin and extracellular matrix [9,74].Endothelial dysfunction is a primary pathophysiological event in the development of atherosclerosis.The migration,proliferation,differentiation,and engulfment of lipids into foam cells by macrophages and smooth muscle cells are all important processes in the formation of atherosclerotic plaques.Numerous studies have shown that catalpol can attenuate these deleterious processes and exert an anti-atherosclerotic effect(Table 2)[46-52].
Several animal studies have demonstrated that catalpol can improve the blood lipid profile by reducing the levels of triglyceride(TG),total cholesterol (TC),and LDL-cholesterol (LDL-C) while increasing the level of high-density lipoprotein cholesterol(HDL-C)in multiple animal models,leading to a potential antiatherosclerotic effect [46,75-79].For instance,catalpol was shown to reduce atherosclerotic lesions in a rabbit model of atherosclerosis induced by a high cholesterol diet,as evidenced by a decrease in serum levels of TG,TC,and LDL-C and an increase in serum HDL-C levels[47].Similarly,our previous study using a highfat diet(HFD)-fed LDLr-/-mouse model of atherosclerosis showed that catalpol could improve lipid profiles and inhibit the development of atherosclerotic lesions[48].In a recent study,catalpol was found to significantly reduce serum levels of TG,TC,and LDL-C,upregulate HDL-C levels,and reduce atherosclerotic lesions and lipid accumulation in a HFD-ovariectomy-induced ApoE-/-mouse model of atherosclerosis[46].These findings suggest that the lipidlowering effects of catalpol may play a crucial role in preventing the development of atherosclerosis.
Atherosclerosis is initiated by dysfunction of vascular endothelial cells,which is caused by various pathophysiological stimuli[80].Catalpol has been shown in numerous studies to have significant anti-oxidant,anti-inflammatory,and anti-apoptotic effects on vascular endothelial cell injury induced by H2O2,ox-LDL,tumor necrosis factor-α (TNF-α),homocysteine (HCY),D-galactose,and high glucose [47,49,81-89].In animal studies,catalpol has been found to inhibit morphological damage to aortic vascular endothelial cells in D-galactose-induced aging rats,possibly by inhibiting Bax and Caspase-3 and upregulating Bcl-2 expression and the Bcl-2/Bax ratio in aortic endothelial cells [50].These results suggest that catalpol could protect vascular endothelial cells by inhibiting their apoptosis in D-galactose-induced aging rats.Studies have demonstrated that catalpol can inhibit oxidative stress,inflammation,and apoptosis induced by ox-LDL in EA.hy926 cells by activating the Nrf2/HO-1 pathway and inhibiting NF-κB transactivation[47,81].Moreover,pretreatment with catalpol can prevent H2O2-induced apoptosis of vascular endothelial cells by scavenging reactive oxygen species,activating the PI3K/Akt signaling pathway,and altering Bcl-2 and Bax levels [85].In a TNF-α-treated human aorta epithelial cell (HAEC) model,catalpol was found to inhibit oxidative stress,inflammation,and apoptosis while promoting autophagy,which may be linked to AMPK activation[88].Our study further confirmed that catalpol can attenuate TNF-α-induced injury in HAECs by activating the AMPK signaling pathway through enhancing AMPK activity rather than by changing AMPK promoter activity [86].Furthermore,Hu et al.[87] found that catalpol could reduce HCY-induced endoplasmic reticulum,oxidative stress,and inflammatory damage in HAECs by inhibiting the Nox4/NF-κB and GRP78/PERK signaling pathways.Additionally,catalpol was found to have a protective effect against vascular endothelial cell injury induced by high glucose stimulation (as detailed in Section 4.5).
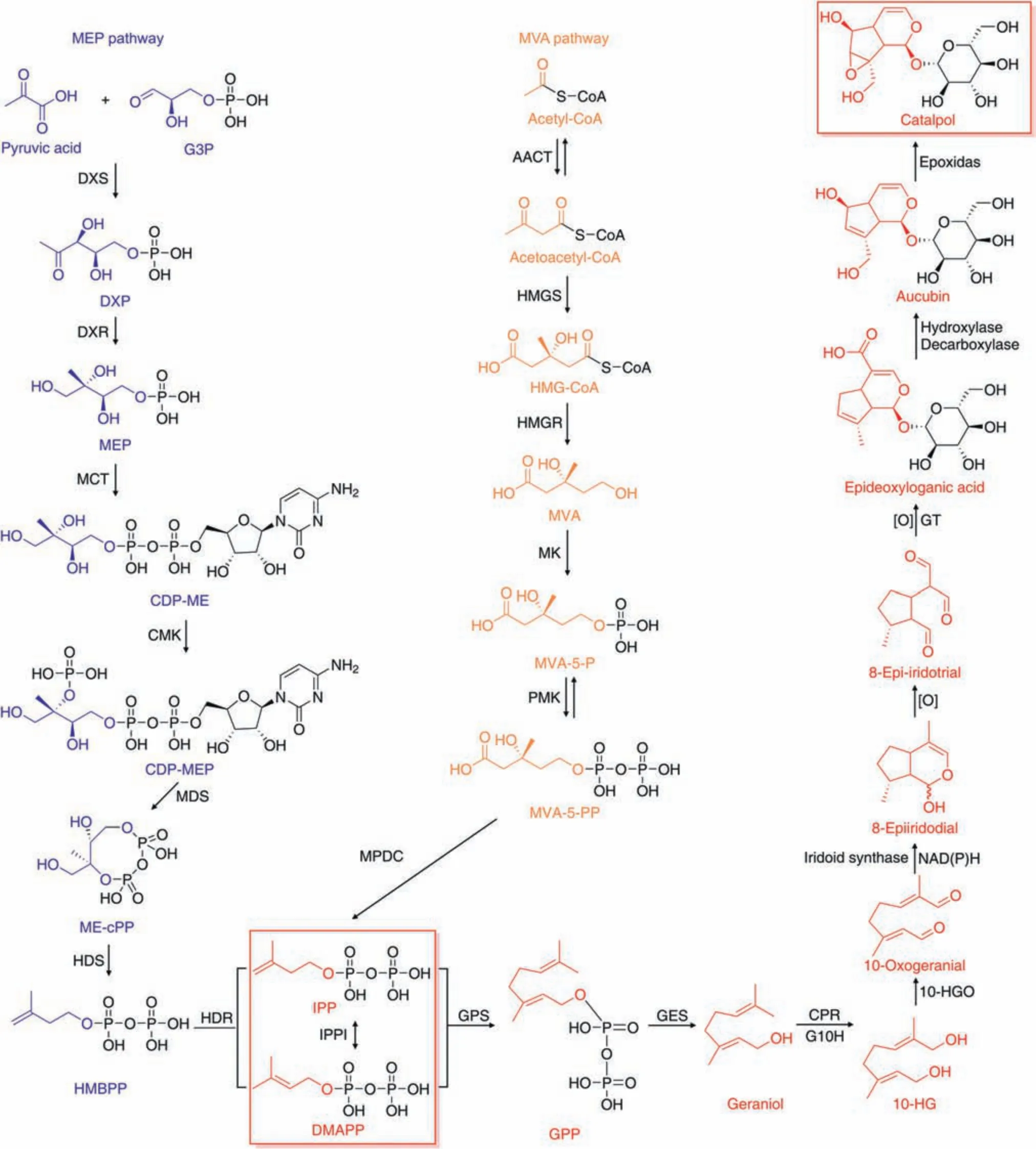
Fig.1.Overview of catalpol biogenesis.MEP:2-C-methyl-D-erythritol 4-phosphate;G3P:glyceraldehyde 3-phosphate;DXS:1-deoxy-d-xylulose 5-phosphate(DXP)synthase;DXR:DXP reductoisomerase;MCT: MEP cytidylyltransferase;CDP-ME: 4-(cytidine 5′-diphospho)-2-C-methyl-D-erythritol;CMK: 4-(cytidine 5′-diphospho)-2-c-methyl-D-erythritol kinase;MDS:2-C-methyl-D-erythritol-2,4-cyclodiphosphate synthase;cPP:2,4-cyclodiphosphate;HDS:4-hydroxy-3-methylbut-2-enyl-diphosphate synthase;HMBPP:4-hydroxy-3-methylbut-2-enyl diphosphate;MVA: mevalonate;AACT: acetyl-CoA C-acetyltransferase;HMGS: 3-hydroxy-3-methylglutaryl (HMG)-CoA synthase;HMGR: HMG-CoA reductase;MK: MVA kinase;MVA-5-P: MVA-5-phosphate;PMK: phospho-MVA kinase;MVA-5-PP: MVA-5-pyrophosphate;MPDC: diphospho-mevalonate decarboxylase;IPP: isopentenyl diphosphate;IPPI: IPP delta-isomerase;DMAPP: dimethylallyl diphosphate;GPS: geranyl diphosphate synthase;GPP: geranyl diphosphate;GES: geraniol synthase;CPR: cytochrome P450 reductase;G10H:geraniol 10-hydroxylase;10-HG:10-hydroxygeraniol;10-HGO:10-HG oxidoreductase;NADH:reduced nicotinamide adenine dinucleotide;NADPH:reduced nicotinamide adenine dinucleotide phosphate;GT: glycosyltransferase.

LDL is modified to form ox-LDL and is phagocytosed by macrophages,which are important processes in the development of atherosclerosis.The resulting foam cells trigger atherosclerotic lesions [47].Catalpol treatment was shown to significantly reduce serum ox-LDL concentrations and inhibit neointimal hyperplasia and macrophage infiltration in a rabbit model of atherosclerosis induced by a high-cholesterol diet [47].Additionally,catalpol significantly inhibited macrophage recruitment in the aortic wall of a diabetic rabbit model of atherosclerosis [49].In our previous study,catalpol was found to reduce foam cell production in the aortic walls of HFD-fed LDLr-/-mice with atherosclerosis [48].Recently,in a HFD-ovariectomy-induced ApoE-/-mouse model of atherosclerosis,catalpol treatment effectively decreased the expression of M1 macrophage markers and increased the expression of M2 macrophage markers in the aorta [46].In vitro studies have also shown that catalpol inhibited NLRP3 expression in the inflammasome of lipopolysaccharide(LPS)-treated THP-1 cells[90].Furthermore,catalpol was found to inhibit inflammation by inhibiting reactive oxygen species(ROS)overproduction and NF-κB activity in advanced glycation end product-treated monocytic series-1 (THP-1) cells [91].In our THP-1 cell models,catalpol effectively reversed oxidative stress,DNA oxidative damage,cell apoptosis,cell senescence,and telomere dysfunction induced by ox-LDL in macrophages [48].The PGC-1α/TERT pathway was identified as the underlying mechanism for these protective effects of catalpol [48].Moreover,recent studies have demonstrated that catalpol inhibits macrophage polarization,the inflammatory response,and oxidative stress induced by LPS and interferon-γ(IFN-γ)while enhancing the function of macrophages activated by classical pathways by increasing estrogen receptor α (ERα)expression [46].Overall,catalpol inhibits a series of adverse reactions produced by macrophages and plays a crucial role in preventing atherosclerosis.
In summary,catalpol exhibits a powerful anti-atherogenic effect by inhibiting oxidative stress and endoplasmic reticulum stress,reducing the inflammatory response,relieving cell senescence and telomere dysfunction,and inhibiting apoptosis.These effects are mediated by activation of the PGC-1α/TERT,PI3K/Akt,AMPK,Nrf2/HO-1,and ERα signaling pathways,as well as inhibition of the Nox4/NF-κB and GRP78/PERK signaling pathways.
4.2.Cerebral vascular diseases
Cerebral vascular disease,also known as“stroke,”encompasses a group of diseases resulting from various cerebral vascular lesions that cause damage to brain tissue.These conditions are typically categorized as ischemic or hemorrhagic cerebrovascular disease.Research has demonstrated that catalpol has a positive effect on brain protection in animal stroke models [92,93].While the majority of previous studies have focused on the anti-oxidant,antiinflammatory,and anti-apoptotic properties of catalpol in reducing brain tissue damage[92,94],recent studies have also highlighted its protective effects on cerebral vessels in the amelioration of stroke(Table 2) [53-60].
Vascular endothelial growth factor (VEGF) is a trophic factor secreted by nerve cells that plays a crucial role in angiogenesis,vascular permeability,and neuroprotection [95].Studies have shown that catalpol,a natural compound,can increase VEGF expression and promote angiogenesis through various pathways.For instance,catalpol has been found to stimulate VEGF expression and promote angiogenesis in a rat stroke model via the JAK2/STAT3 pathway and activate the HIF-1α/VEGF pathway to protect vascular structure and promote angiogenesis in rats with focal cerebral ischemia[53,54].Additionally,catalpol has been shown to regulate VEGF and its receptor VEGFR to activate the Notch signaling pathway,promoting angiogenesis and neural function remodeling[55].Recent research has also revealed that catalpol can increase VEGF expression by upregulating PI3K/AKT signaling,followed by increasing focal adhesion kinase (FAK) and paxillin,activating the PI3K/AKT and MEK1/2-ERK1/2 pathways,promoting angiogenesis and neurogenesis,and improving the impaired neurovascular unit(NVU) in the ischemic region [56].Studies have shown that erythropoietin (EPO) works synergistically with VEGF to promote angiogenesis and protect neurons,as well as reduce the increased vascular permeability and BBB edema caused by increased VEGF expression [96,97].Similarly,catalpol has been found to not only increase VEGF levels but also enhance the expression of EPO,promoting adhesion,proliferation,and migration of endothelial cells.This leads to a significant reduction in cerebral capillary edema and an increase in angiogenesis in infarct areas of cerebral ischemia model animals[53,98,99].In a study in 2022,catalpol was found to regulate the communication between mesenchymal stromal cells and macrophages through the paracrine pathway,thereby promoting angiogenesis[100].These findings suggest that catalpol has potential as a medicine for the treatment of cerebral vascular diseases by promoting angiogenesis.
In a study on the protective effects of catalpol on the NVU,it was found that catalpol increased the density and length of microvessels in the NVU [57],indicating a potential role in promoting angiogenesis.Moreover,catalpol increased the maturation and stability of blood vessels around the infarct in rats with cerebral ischemia by upregulating the expression of angiopoietin-1 and its receptor Tie-2[58],suggesting a potential protective effect on blood vessels.In addition,catalpol has been shown to inhibit apoptosis and autophagy induced by the Aβ1-42protein in brain microvascular endothelial cells(BMECs).Specifically,catalpol reduced the expression of Bax,cytochrome C,cleaved caspase-3,Beclin 1,and LC3-II and upregulated the expression of Bcl-2,which protected BMECs from damage [101].Furthermore,catalpol upregulated the expression of tight junction proteins such as claudin-5,occludin,zonula occludens-1 (ZO-1),and ZO-2,which increased the tight junction between BMECs and alleviated the increase in BMEC injury induced by high glucose.These effects may be mediated by the inhibition of the RhoA/ROCK2 signaling pathway,which prevented the downregulation of junctional proteins and inhibited endothelin-1 and inflammatory cytokine secretion,ultimately alleviating the increase in BBB permeability induced by LPS[102,103].These results suggest that catalpol may have a protective effect on the BBB and the NVU,potentially contributing to its therapeutic potential for cerebral vascular diseases.
In summary,catalpol has demonstrated a strong protective effect against cerebral vascular diseases.This effect is mainly achieved by promoting angiogenesis and maturation,preventing vascular edema,and protecting BMECs and the BBB.These effects require the activation of several signaling pathways,including JAK2/STAT3,HIF-1α/VEGF,Notch,PI3K/AKT,and MEK1/2-ERK1/2,as well as inhibition of the RhoA/ROCK2 signaling pathway.
4.3.Myocardial ischemia and myocardial infarction
Myocardial ischemia is a condition caused by the obstruction of coronary arteries,which results in inadequate oxygen supply to cardiomyocytes.Prolonged ischemia can further progress to myocardial infarction[104].Catalpol,known for its protective effect against ischemic injury and ischemia-reperfusion injury in various noncardiac tissues [105-110],has also shown a similar protective effect in myocardial tissue (Table 2) [61-71].


Catalpol has been shown to have a preventative effect on myocardial ischemia-reperfusion injury.Song et al.[61]found that catalpol could effectively prevent acute myocardial ischemiainduced myocardial injury in a dose-dependent manner.The researchers further confirmed that catalpol inhibits cardiomyocyte apoptosis by enhancing the expression of Bcl-2 while downregulating the expression of Bax and Caspase-3[61].Another study demonstrated that pretreatment with catalpol in adult male Wistar rats prior to the induction of myocardial infarction significantly reduced the percentage of apoptotic cells[62].Additionally,catalpol pretreatment was reported to significantly improve cardiac function and reduce myocardial infarction,apoptosis,and necrosis of cardiomyocytes in male rats subjected to 30 min of myocardial ischemia and 3 h of reperfusion [63].The study further revealed that the mechanism of catalpol in the treatment of cardiac ischemia-reperfusion injury might be related to attenuated oxidative/nitrative stress by blocking peroxynitrite (ONOO-) formation.A recent study found that catalpol significantly inhibited myocardial ischemia-reperfusion injury and protected oxygen-glucose deprivation/reoxygenation-treated cardiomyocytes by suppressing various inflammatory markers and oxidative stress.In terms of the mechanism,catalpol inhibited the damage caused by inflammation and oxidative stress in myocardial ischemia-reperfusion injury by activating the Nrf2/HO-1 signaling pathway[64].
Catalpol has also been shown to have beneficial effects on myocardial infarction.Bi et al.[65,66] found that catalpol pretreatment could prevent isoproterenol-induced myocardial injury in rats,which might be achieved by maintaining endogenous anti-oxidant enzyme activities and alleviating the inflammatory response.In addition,they also demonstrated that catalpol reduced cardiomyocyte apoptosis by regulating the apelin/APJ signaling pathway and reducing caspase-9 and caspase-3 activities,altering the expression of Bax and Bcl-2 in the hearts of rats with isoproterenol-induced myocardial infarction [62].A study by Zeng et al.[67] also showed that the protective effect of catalpol on the myocardium in rats with isoprenaline-induced myocardial infarcts might involve angiogenesis through mobilization of endothelial progenitor cells and activation of the Notch1/Jagged1 pathway.Bone mesenchymal stem cell (BMSC) transplantation is considered as an effective treatment for myocardial infarction.Catalpol pretreatment has been shown to enhance the survival and VEGF secretion of BMSCs and to improve their therapeutic effect on myocardial infarction [111].
The protective effect of catalpol on cardiomyocytes has also been demonstrated in some in vitro studies.Lin et al.[112]found that catalpol protected glucose-deprived H9c2 cells by reducing apoptosis and oxidative damage.In addition,the expression of autophagic lysosomes,autophagic flux,autophagic vesicles,and mitochondria-related proteins was significantly increased in catalpol-treated cells.Administration of 3-methyladenine (an autophagy inhibitor) and tamoxifen (an ER blocker) completely blocked the effects of catalpol [112].These data indicated that catalpol inhibited apoptosis and oxidative stress in glucosedeprived H9c2 cells by promoting cell mitophagy and regulating the ER,thus supporting that catalpol could be a novel medicine candidate against myocardial ischemia for the treatment of cardiovascular diseases.Catalpol could also reduce the release of lactate dehydrogenase and malondialdehyde (MDA) in endothelial cells and cardiomyocytes induced by H2O2,increase the concentration of superoxide dismutase,and increase the cell clearance of ROS [113].These effects were partially antagonized by the PI3K-specific inhibitors wortmannin and LY294002,which indicated that catalpol could inhibit H2O2-induced cardiomyocyte injury by activating the PI3K/Akt-Bad pathway.Recently,in an AC16 cell injury model induced by hypoxia/reoxygenation,catalpol was found to promote the viability of AC16 cells,reduce cell apoptosis,and inhibit the release of inflammatory cytokines and the leakage of myocardial injury markers,and further demonstrated that catalpol alleviated hypoxia/reoxygenation-induced AC16 cell injury by regulating the miR-22-3p/DPP4 axis [114].
In conclusion,catalpol plays a protective role in myocardial ischemia and ischemia-reperfusion injury through its anti-oxidant,anti-inflammatory,anti-apoptotic,and proangiogenic effects.In this process,catalpol inhibited oxidative stress,inflammation,and apoptosis in cardiomyocytes by upregulating the Apelin/APJ,PI3K/Akt,and Nrf2/HO-1 signaling pathways,activating autophagy and regulating the miR-22-3p/DPP4 axis,while the activation of the Notch/Jagged signaling pathway induced by catalpol was involved in stimulating angiogenesis after myocardial infarction.
4.4.Cardiac hypertrophy and heart failure
Heart failure is a complex clinical syndrome characterized by abnormal changes in cardiac structure or function,leading to pulmonary congestion,systemic circulation congestion,and organ hypoperfusion.Currently,several studies have revealed the ability of catalpol to prevent heart failure (Table 2) [62,68].In 2011,Chen et al.[115] successfully applied for a patent (Application No.: CN201010529674.2;Publication No.: CN102008497A)in China,which pointed out that catalpol had new pharmacodynamic activity against heart failure,and made catalpol into a traditional Chinese medicine injection for the treatment of heart failure.Bi et al.[62] found that pretreatment with catalpol significantly enhanced myocardial contractility and improved cardiac function in rats with isoproterenol-induced myocardial infarction.Specifically,catalpol significantly inhibited isoproterenol-induced alterations in hemodynamic parameters and prevented a decline in systolic,diastolic,and mean blood pressure.Moreover,catalpol significantly prevented left ventricular contractile dysfunction,as demonstrated by improvements in left ventricular end-systolic pressure (LVESP) and left ventricular maximum rate of positive or negative pressure development (±LVdp/dtmax).Cui et al.[68] investigated whether catalpol could inhibit isoproterenol-induced cardiac hypertrophy in mice.Catalpol significantly reduced the heart weight index and left ventricular weight index and decreased the contents of MDA,cyclic adenosine monophosphate (cAMP),and Ang II in the myocardium [68].These results suggested that catalpol might interfere with cardiac hypertrophy by inhibiting oversecreted neuroendocrine factors and scavenging free radicals,which had important effects on the occurrence and development of cardiac hypertrophy.However,the mechanism by which catalpol protects against cardiac hypertrophy has not been fully elucidated.Together,these studies suggested that catalpol could inhibit heart hypertrophy and improve heart function,making it a potential new drug for the treatment of heart failure.
4.5.Others
Moreover,in addition to its protective effects against myocardial ischemia and ischemia-reperfusion injury,catalpol has demonstrated potential in mitigating diabetic cardiovascular complications,thrombosis,and other cardiovascular events [51,69].Further information on these findings can be found in the Sections 4-6 in the Supplementary data[65,82,84,85,94-99,108-110,116-135].
5.Future directions and recommendations for catalpol in CVD research and treatment
To further explore the potential of catalpol in treating CVDs,the following recommendations and future research directions should be considered.
5.1.In-depth mechanistic studies
Although various studies have demonstrated the protective effects of catalpol on the cardio-cerebrovascular system,its precise molecular mechanisms still need further elucidation.Conducting in-depth mechanistic studies will help understand the precise role of catalpol in CVD treatment and provide more comprehensive evidence for its clinical application.CVDs often involve damage to multiple systems.Identifying a shared therapeutic pathway for preventing and treating these conditions is essential.For example,the activation of the PI3K/Akt signaling pathway may be a vital mechanism in catalpol's anti-CVD effects,as it is involved in various roles,including against vascular endothelial cell apoptosis,enhancing cerebrovascular formation,and protecting myocardial cells [56,85,113].Consequently,more in-depth molecular research targeting the PI3K/Akt pathway may uncover additional cardiocerebrovascular protective effects and the “commonality mechanism” of catalpol.
5.2.Investigating synergistic effects
As an active compound derived from traditional herbal medicines,catalpol has been proven to have synergistic effects with other active compounds present in other Chinese herbal sources [136,137].Utilizing synergies between drugs can more significantly improve the therapeutic effect than a single drug.For example,the effects of the combination of catalpol and berberine were better than monotherapy in improving insulin resistance and reducing the deposition of lipid substances in cells [138].Liu et al.[139] confirmed that catalpol and puerarin freeze-dried powder can promote the proliferation of neural stem cells in rats after permanent focal cerebral ischemia,increase microvessel density on the ischemic side of the brain,and facilitate angiogenesis.Another study also showed that catalpol and puerarin freeze-dried powder at all tested doses(65.4,32.7,and 16.4 mg/kg) significantly reduced neurological deficiency,infarct volume,and apoptotic cells in ischemia/reperfusion rats [59].In vitro studies have found that the protective effect of catalpol and puerarin freeze-dried powder on primary brain vascular endothelial cells depends on ERK/HIF-1a and PI3K/AKT/mTOR/HIF-1α [60].It is suggested that catalpol and puerarin freeze-dried powder may be a new drug for the treatment of cerebral ischemia.Identifying and investigating these synergistic effects at the global and cellular levels could potentially enhance the therapeutic effects and improve the overall effectiveness of treatment strategies for CVDs.
5.3.Efficacy and toxicity studies
The efficacy of a drug is largely related to the dosage of the drug used and depends on the administration route.We summarized the dosage and effects of catalpol used in previous studies (Table 2)[46-71].The dosage ranges of catalpol for intragastric,intraperitoneal,and intravenous administration were 2.5-200,1-10,and 2.5-10 mg/kg,respectively.Interestingly,we found that a dose of 5 mg/kg catalpol was widely used and has been shown to produce significant anti-CVD effects,whether administered intragastrically or intraperitoneally/intravenously [47,49,50,52-58,61-66,70,71].In addition,a small number of studies have also reported that a dose of 2.5 mg/kg catalpol had therapeutic effects[54,56,65,66,70,71].For intraperitoneal injection,a dose of 1 mg/kg catalpol also had therapeutic effects [58,61].
To date,Catalpol has been proven to be relatively safe for rodents,with no apparent side effects.In an animal toxicity study[140],Kunming mice and Wistar rats were utilized to assess the safety of catalpol.The acute toxicity test of catalpol in Kunming mice showed that the median lethal dose (LD50) and 95% confidence limit of intraperitoneally administered catalpol were 206.5 and 190.7-223.6 mg/kg,respectively.After a long-term intravenous injection of catalpol (10,20,and 40 mg/kg/day;90 days),Wistar rats exhibited no changes in appearance,hematology,blood biochemistry,or toxicity of major organs,indicating that the longterm application of catalpol had no apparent toxic or side effects.Dong et al.[141] studied the acute toxicity of catalpol and found that it had no significant acute toxicity in mice.Specifically,Institute of Cancer Research(ICR)mice received a gavage of catalpol at the maximum dose of 1000 mg/kg within one day,and the mice did not display noticeable poisoning symptoms or death.After two weeks of continuous observation,none of the tested mice died,and their eating and activities appeared normal.The body weight did not significantly differ from that of the control group within 10 days.Additionally,the safety pharmacological study of catalpol and puerarin freeze-dried powder demonstrated that the tail vein injection of catalpol and puerarin freeze-dried powder in Kunming mice at low (52.3 mg/kg),medium (104.6 mg/kg),and high(209.2 mg/kg) doses did not impact their general behavior,autonomic activity,coordination function,or the rate of sleep induced by a subthreshold hypnotic dose of pentobarbital.After injecting Sprague-Dawley (SD) rats with low (32.7 mg/kg),medium(65.4 mg/kg),and high(130.8 mg/kg)doses of catalpol and puerarin freeze-dried powder,indicators such as respiratory rate,respiratory amplitude,electrocardiogram,heart rate,systolic blood pressure,and diastolic blood pressure did not change significantly [142].These results indicate that catalpol and puerarin freeze-dried powder has no considerable effect on the central nervous system,respiratory system,or cardiovascular system within the therapeutic dose range.Recently,a pilot clinical trial assessed the safety of catalpol in treating colon cancer patients[121].The study involved 345 patients undergoing surgical resection of locally advanced colon cancer who were treated with an intraperitoneal injection of 10 mg/kg catalpol twice daily for 12 weeks.At the end of the 48-month follow-up period,only mild nonfatal treatment-related adverse events were observed in patients receiving catalpol treatment,supporting its safe use in human subjects.In conclusion,catalpol has been shown to be well tolerated and nontoxic.
There is a lack of clinical trials investigating the cardiocerebrovascular protective effects of catalpol.To bridge the gap between preclinical and clinical research,well-designed clinical trials should be conducted to evaluate the efficacy,safety,and optimal dosing of catalpol in human subjects.Additionally,further toxicity studies should be performed to establish the safety profile of catalpol in the long term.
5.4.Development of novel catalpol derivatives and formulations
Research and development of novel catalpol derivatives,as well as new pharmaceutical formulations,could improve the bioavailability,efficacy,and safety of catalpol.By modifying the chemical structure or developing novel delivery systems,the therapeutic potential of catalpol in treating CVDs could be further enhanced[143].
Despite having the same catalpol core structure,derivatives with different substituents at various positions can exhibit significant differences in efficacy.For instance,6-O-substituted catalpol derivatives from Scrophularia dentata demonstrated superior inhibition of TNF-α,interleukin (IL)-1β,and IL32 expression levels through the NF-κB pathway compared to catalpol itself [14,144].Furthermore,compounds with low-polarity substituents at the 6-Oposition of catalpol showed higher NF-κB inhibitory potency[145].The 6-O-acyl catalpol not only displayed more extensive bioactivity than catalpol but also exhibited better pharmacological activity in some cases [14].Appropriate preparation technologies may also enhance the utilization efficiency of catalpol.Tang et al.[146]found that inclusion complex could remarkably increase the oral absorption of catalpol compared with nanocrystal,and the brain targeting index of inclusion complexes and self-microemulsions were both higher than that of nanocrystals,suggesting that inclusion complexes could significantly promote the absorption of catalpol into blood and penetrate the BBB to enhance the efficacy of catalpol.
Modifying the chemical structure of catalpol to create new derivatives or new preparation technologies could potentially expand its range of effects and may lead to a more significant impact on cardiovascular protection in the future.
5.5.Personalized medicine approach
Considering the individual differences in the response to treatment,a personalized medicine approach could be employed to optimize the use of catalpol in CVD management.By taking into account the genetic background,lifestyle factors,and disease progression of individual patients,tailored treatment strategies can be developed to maximize the therapeutic benefits of catalpol [147].Advancements in systems biology technologies,including genomics,proteomics,and metabolomics,have greatly contributed to the personalization of treatment in Chinese medicine.These technologies enable the effective identification of biomarkers for specific diseases,laying the foundation for the development of innovative targeted therapeutics [148].A targeted drug derived from catalpol may offer diverse cardiovascular and cerebrovascular protective effects tailored to the unique needs of different patient groups.
By addressing these recommendations and future research directions,a more comprehensive understanding of catalpol's role in CVD treatment can be achieved,potentially leading to the development of novel therapeutic options for patients with CVDs.
6.Conclusions
In conclusion,multiple domestic and foreign studies have provided scientific evidence for catalpol's protective effect on the cardio-cerebrovascular system.As an active compound obtained from traditional herbal medicines,catalpol exerts anti-CVD effects mainly through its anti-oxidant,anti-inflammatory,and anti-apoptotic activities in a coordinated “multitarget and multipathway” manner.For example,the PGC-1α/TERT,PI3K/Akt,AMPK,Nrf2/HO-1,ER,Nox4/NF-κB and GRP78/PERK signaling pathways were related to the vascular protection of catalpol;the apelin/APJ,PI3K/Akt,Nrf2/HO-1,miR-22-3p/DPP4,ROS-NF-κBNeat1,and Neat1/miR-140-5p/HDAC4 signaling pathways were involved in the myocardial protection of catalpol.Although there is no clinical study on the cardio-cerebrovascular protective effects of catalpol,it has been currently used clinically to treat tumors.Safety studies showed that catalpol was a well-tolerated compound with no toxicity.Thus,catalpol may provide a new medicine treatment option for patients with CVDs.Moreover,indepth research on catalpol may open a new door to enable the use of various natural catalpol derivatives and other iridoids for the treatment of CVDs.
CRediT author statement
Zixi Zhang:Conceptualization,Methodology,Writing-Original draft preparation,Software;Yongguo Dai:Software,Visualization,Writing -Reviewing and Editing;Yichao Xiao:Visualization,Supervision,Writing -Reviewing and Editing;Qiming Liu: Supervision,Project administration,Funding acquisition,Writing -Reviewing and Editing.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
Graphic Abstract was created using the image bank of Servier Medical Art (http://smart.servier.com/),licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/).This work was supported by the National Natural Science Foundation of China (Grant Nos:.82070356 and 81770337),the Key Project of Hunan Provincial Science and Technology Innovation,China (Grant No.:2020SK1013),and the Hunan Provincial Natural Science Foundation of China (Grant No.:2021JJ30033).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2023.06.010.
 Journal of Pharmaceutical Analysis2023年10期
Journal of Pharmaceutical Analysis2023年10期
- Journal of Pharmaceutical Analysis的其它文章
- Recent advancements in single-cell metabolic analysis for pharmacological research
- SPME as a green sample-preparation technique for the monitoring of phytocannabinoids and endocannabinoids in complex matrices
- Monoclonal antibody targeting mu-opioid receptor attenuates morphine tolerance via enhancing morphine-induced receptor endocytosis
- Protective effects of dioscin against Parkinson's disease via regulating bile acid metabolism through remodeling gut microbiome/GLP-1 signaling
- Histone deacetylase inhibitor pracinostat suppresses colorectal cancer by inducing CDK5-Drp1 signaling-mediated peripheral mitofission
- Dihydroartemisinin ameliorates innate inflammatory response induced by Streptococcus suis-derived muramidase-released protein via inactivation of TLR4-dependent NF-κB signaling
