Mutual influence of copper and paraquat on their adsorption in soil
Huanhua WANG,Zhiguo PEI,Guangcai CHEN and Baoshan XING
1National Engineering Laboratoryfor Lake Pollution Control and Ecological Restoration,Chinese Research Academyof Environmental Sciences,Beijing 100012(China)
2State Environmental Protection KeyLaboratoryfor Lake Pollution Control,Chinese Research Academyof Environmental Sciences,Beijing 100012(China)
3State KeyLaboratoryof Environmental Chemistryand Ecotoxicology,Research Center for Eco-Environmental Sciences,Chinese Academyof Sciences,P.O.Box 2871,Beijing 100085(China)
4Research Institute of Subtropical Forestry,Chinese Academyof Forestry,Hangzhou 311400(China)
5Stockbridge School of Agriculture,Universityof Massachusetts,Amherst MA 01003(USA)
ABSTRACT Paraquat and copper(Cu)are commonly used and detected in soil.Therefore,it is important to understand their mobility in the environment.In this study,the competitive effects of paraquat and Cu on their adsorption in five representative Chinese soils were investigated using batch adsorption equilibrium experiments and spectroscopic analysis.The results showed that the adsorption of paraquat in soil varied with soil type and was positively correlated with both soil cation exchange capacity and organic matter content.Paraquat exerted a more remarkable suppression effect on the adsorption of Cu than Cu on the adsorption of paraquat.In the presence of 0.12 and 0.19 mmol L-1 paraquat,Cu adsorption decreased by 16%and 22%in Heilongjiang soil and by 24%and 37%in Jiangxi soil,respectively.In the presence of 0.1 and 0.2 mmol L-1 Cu,paraquat adsorption decreased by 4%and 8%in Heilongjiang soil and by 15%and 19%in Jiangxi soil,respectively.Exchange selectivity involving symmetric cation(paraquat2+ and Cu2+)exchange is the probable basis for the suppression effect.The ultraviolet-visible absorption experiments suggested that the formation of Cu-paraquat complexes was unlikely to happen in a solution or at the soil surface.Copper K-edge X-ray absorption spectroscopy indicated that Cu in soil may have some water as hydration layers as the nearest neighbors,and each Cu atom was coordinated with five oxygen atoms.These findings greatly improve our knowledge of the molecular-scale adsorption mechanisms of paraquat and Cu in soil and can be used to predict the behavior,transport,and fate of paraquat and Cu in agricultural soils.
KeyWords: cation exchange capacity,competitive adsorption,heavy metal,herbicide,organic contaminant,soil organic matter,ultraviolet-visible absorption spectrophotometry,X-ray absorption spectroscopy
INTRODUCTION
Paraquat dichloride (1,1′-dimethyl-4,4′-bipyridinium dichloride) is one of the most widely used nonselective cationic herbicides because of its rapid,contact-dependent killing of weeds and plants and its inactivation upon reaching the soil.Adsorption has a critical effect on the transport,reactivity,and bioavailability of paraquat in soil.Adsorption of bipyridinium herbicides onto minerals and soil has been studied since the 1960s (Weber and Scott, 1966; Pateiro-Moureet al.,2007).Cation-exchange interaction is one of the main mechanisms of paraquat adsorption onto clays and organoclays(Seki and Yurdakoç,2005).The ionic bonding associated with the positively delocalized charge of paraquat was suggested to be the most favorable mechanism of bonding to the soil components(Spark and Swift,2002).Other processes such as van der Waals forces,formation of charge transfer complexes,and hydrogen bonding have also been reported to enhance the adsorption beyond the simple cationexchange interaction (Robertset al., 2002).For example,Weber and Scott(1966)observed that paraquat intercalated to an interlayer of montmorilloniteviaCoulombic and van der Waals forces,and also to kaoliniteviaCoulombic forces alone.
The presence of coexisting contaminants,such as heavy metals,may affect the individual adsorption and ultimate fate of the contaminants in the soil environment.However,multisolute systems have historically been ignored in laboratory adsorption studies.Glyphosate diminished the adsorption of cadmium(Cd)onto soil,while Cd increased the adsorption of glyphosate(Zhouet al.,2004).Copper adsorption was higher in two out of three soils in the presence of glyphosate,but was higher in the third soil in the absence of glyphosate(Morilloet al., 2002).In addition, a cationic pesticide chlordimeform decreased the adsorption of Cu,and Cu also suppressed the sorption of chlordimeform on two acid soils(Undabeytiaet al.,2002).Copper diminished the sorption of cationic difenzoquat to peat and soil (Peiet al., 2008).Cation exchange was proposed to explain the competitive adsorption between paraquat and benzalkonium chloride onto montmorillonite(Ilariet al.,2021).To date,there is no clear consensus on the interactions between heavy metals and cationic organic contaminants in soil.
Copper is an essential trace element for both plants and animals at low concentrations,but it can be toxic at high levels brought about by the application of fertilizers,fungicides,sewage sludge,or wastewater irrigation.Thus,paraquat and Cu coexist in soil,rendering studies on the interaction between Cu and paraquat in soil of an increasingly significant environmental concern.Pateiro-Moureet al.(2007) suggested that Cu(0—3.15 mmol L-1)had little effect on the adsorption of paraquat(0.056—0.389 mmol L-1)and diquat(0.056—0.389 mmol L-1)in two acid vineyard soils,while Cu adsorption was reduced by the presence of paraquat.Heavy metals can form complexes with soil minerals or organic matter(OM)and thus change soil chemistry(e.g.,soil surface area,porosity size,and surface charge density),influencing the adsorption of herbicides.However, to the best of our knowledge,there is no information on whether Cu and paraquat form complexes in soil solution and the mechanisms of the adsorption of Cu and paraquat in soil.Moreover, little information is available on the processes taking place when heavy metals and paraquat are present together, such as competition for the sorption sites or the formation of complexes,and the relevant mechanisms are speculative without direct spectroscopic evidence.
Therefore,the aims of this study were to i)investigate the mutual effects of Cu and paraquat on their adsorption in soil and ii)provide insight into the mechanisms using an ultraviolet-visible(UV)absorption spectrophotometry and synchrotron X-ray absorption spectroscopy(XAS).
MATERIALS AND METHODS
Materials
Paraquat was purchased from Sigma Chemical Co.(USA) with a purity of 99%, water solubility of 626g L-1(20◦C),and octanol-water partition coefficient of-4.5(log-transferred)at 20◦C.Cu(NO3)2,NaNO3,NaOH,and HNO3were of reagent grade.
Five surface soils(0—20 cm)were collected from Heilongjiang Province(northeastern China),Jiangxi Province(southern China),Anhui Province (southeastern China),Shanxi Province(northern China),and Xinjiang Province(northwestern China).They represent typical Chinese soils with various physiochemical characteristics(Table I).Soil samples were air-dried and ground to pass through a 2-mm mesh sieve.Soil pH was measured at a soil to water ratio of 1:2.5(weight:volume).Soil specific surface area(SSA)and pore characteristics were estimated by the Brunauere-Emmett-Teller method.Cation exchange capacity(CEC)ofthe soils with pH<7.0 was determined by the BaCl2method,while the CEC of soils with pH>7.0 was determined by the CH3COONa method.Soil organic matter was determined by the method described by Nelson and Sommers(1996).Dissolved organic carbon(DOC)was measured with a fully automated DOC analyzer(Phoenix 8000,Tekmar-Dohrmann Company,USA).Amorphous iron(Fe)oxide(AmorFe)was extracted by shaking 1 g soil in 30 mL acidified ammonium oxalate buffer(0.175 mol L-1(NH4)2C2H4+0.1 mol L-1H2C2O4, pH 3.0) for 2 h (Loeppert and Inskeep, 1996).Crystalline Fe(CryFe)and manganese(CryMn)oxides were determined by the oxalate-ascorbic acid extraction method(Shuman,1982).

TABLE I Selected propertiesa) of the soils collected from Heilongjiang Province(northeastern China),Jiangxi Province(southern China),Anhui Province(southeastern China), Shanxi Province (northern China), and Xinjiang Province(northwestern China)
Adsorption experiments
All equilibrium experiments were conducted in 40-mL glass centrifuge tubes sealed with aluminum foil-lined Teflon screw caps.Preliminary experiments showed that loss of paraquat by adsorption to glass centrifuge tube walls and 0.45-µm membrane filter was<1%and that apparent equilibrium was reached for the adsorption of paraquat in soil within the first 30 min.
To determine the equilibration time of paraquat adsorption,a batch adsorption equilibration method was performed by mixing 20 mL of 0.12 mmol L-1paraquat solution with 30 mg Heilongjiang or Jiangxi soil.The pH of the soil suspension was maintained at 5.0±0.1 to maintain paraquat in a cationic form.The suspension was then rotated continuously for 24 h at 20◦C, centrifuged at 3 000×gfor 15 min,and then filtered through a 0.45-µm membrane filter.The kinetics of paraquat adsorption has been reported to be biphasic,with an initially rapid absorption phase followed by a slower adsorption phase,to slow the diffusion to less readily accessible adsorption sites(Robertset al.,2002).The 24-h equilibration period was implemented as described by Constenlaet al.(1990)and Pateiro-Moureet al.(2007).
A portion of the supernatants was analyzed to determine the equilibrium concentration of paraquat using an UV spectrophotometer(U-3010,Hitachi Ltd.,Japan)at wavelength(λ) = 257 nm.The amount of paraquat adsorbed in soil at equilibrium was determined by the difference between the initial and equilibrium concentrations of paraquat.All adsorption experiments were performed in triplicate.
The effect of ionic strength on the adsorption of paraquat was investigated by mixing Heilongjiang and Jiangxi soils(30 mg each) separately with 0.12 mmol L-1paraquat in 0.001, 0.05, and 0.5 mol L-1NaNO3solution containing 0.2 g L-1NaN3as biocide.The concentrations of paraquat and Cu in the supernatant were determined by an UV spectrophotometer atλ=257 nm and inductively coupled plasma-atomic emission spectroscopy (Optima 2000 DV,Perkin-Elmer Co.,USA),respectively.Copper standard solution(100µg mL-1,GBW080122)purchased from National Institute of Metrology,China was used for calibration.
Heilongjiang and Jiangxi soils(30 mg each)were equilibrated with 20 mL of 0,0.02,0.06,0.12,0.19,0.31,and 0.39 mmol L-1paraquat in 0.01 mol L-1NaNO3in the absence or presence of 0.1, 0.2, and 0.5 mmol L-1Cu in 40-mL glass centrifuge tubes.Paraquat adsorption in the other three soils of Anhui,Shanxi,and Xinjiang was investigated only in the presence or absence of 0.5 mmol L-1Cu.The addition of 0.5 mmol L-1Cu2+represents a concentration near the third grade of Environmental Quality Standard for Soils(500 mg kg-1)in China assuming all of the Cu2+in solution is adsorbed by soil(Peiet al.,2008).The pH of the adsorbent suspension was maintained at 5.0±0.1.The suspension was rotated continuously for 24 h at 20◦C,centrifuged at 3 000×gfor 15 min,and then filtered through a 0.45-µm membrane filter.The concentrations of paraquat and Cu in the supernatant were determined.All adsorption experiments were performed in triplicate.
Data analysis
The logarithmic form of Freundlich equation was used to fit the sorption data as follows:
whereQ(mmol g-1)is the amount of paraquat adsorbed by soil,Ce (mmol L-1) is the equilibrium concentration in solution,Kfis the intercept of the Freundlich isotherm,indicating binding affinity,andN(dimensionless)is the slope of the Freundlich isotherm, describing the heterogeneity.The correlation between paraquat adsorption and various soil properties was analyzed using SPSS 14.0 for windows software package(SPSS Inc.,USA).
Zeta potential measurement
Zeta potential of the five soils at different pH values was measured with a ZetaSizer Nano Series instrument(Malvern Instruments Ltd.,UK).NaNO3solution(0.01 mol L-1)was used as background electrolyte,and 0.01 mol L-1HCl was used to adjust pH from 2.5 to 7.5.
Ultraviolet-visible spectrophotometric measurement
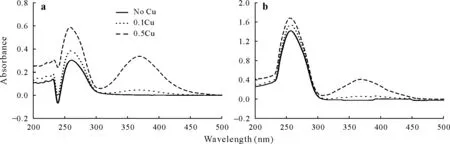
To clarify whether Cu and paraquat form Cu-paraquat complexes in soil suspension and whether the complex formation is responsible for the observed apparent suppression effect of Cu, the possible formation of Cu-paraquat complexes in the equilibrium solution was studied using the method developed by Oesset al.(1999).Specifically,30 mg of Heilongjiang or Jiangxi soil was mixed with 0.12 mmol L-1paraquat, 0.12 mmol L-1paraquat+0.1 mmol L-1Cu,or 0.12 mmol L-1paraquat+0.5 mmol L-1Cu,and then rotated continuously for 24 h at 20◦C and centrifuged at 3 000×gfor 15 min.The supernatants for each treatment were sampled for UV-visible spectrophotometric analysis.
Synchrotron XAS data collection and processing
Synchrotron XAS is an element-specific and bulksensitive method used to identify the formation of surface complexes and to obtain the coordination environment of Cu adsorbed in soil.The sample preparation conditions used for the XAS measurement were the same as described in the adsorption experiments.The initial solution concentration of Cu was 0.5 mmol L-1.The Cu K-edge XAS experiment was performed at the 1W1B beamline of Beijing Synchrotron Radiation Facility(China).The 7-period wiggler beamline provides monochromatic X-ray beams from 4 to 24 keV using a double Si(111)crystal monochromator,a collimating mirror,and a focusing mirror.During the experiment,the energy of storage ring was 2.2 GeV,and the beam current was approximately 80 mA.The high-order harmonic component was rejected by slightly detuning the double Si crystals.Energy calibration was made using a Cu foil.All reference samples(Cu foil,Cu(OH)2,Cu(NO3)2(aqueous),and Cu(CH3COO)2)were measured in transmission mode where two ionization chambers were employed.Soil samples were made into pellets,and a Cu(NO3)2(aqueous)sample was sealed in a cell with a proper thickness.Because of the low concentration,Cu adsorbed in soil was measured in fluorescence mode using a Lytle-type fluorescence detector(EXAFS Company,USA)with a Ni filter(EXAFS Materials,USA).
X-ray absorption fine structure (XAFS) analysis was performed using WinXAS 2.3 code(Ressler,1997).For each spectrum, the pre- and post-edge absorption background was fitted and subtracted using the polynomial function with a degree of 1 and 3,respectively.The extended XAFS(EXAFS)functions were normalized using the absorption edge jump and transferred tok-space.The functions of the Fourier transform toR-space withk2-weighting ink-space over the range of 20—115 nm-1(20—110 nm-1for Jiangxi soil)were then calculated.The fitting was performed in theR-space for the first Cu—O shell, where the coordination number(n),interatomic distance,energy offset(∆E0),and Debye-Waller factor were allowed to float freely.Phaseshift and backscattering amplitudes of the first Cu—O shell were extracted based on the theoretical crystallographic data of Cu(OH)2using FEFF6.0 code(de Leonet al.,1991).
RESULTS AND DISCUSSION
Effect of ionic strength on paraquat adsorption
Paraquat can be adsorbedviacation exchange, which can be verified by the decreased adsorption of paraquat with increasing ionic strength(Fig.1).Adsorption of paraquat was decreased by 10%—13%and 48%—52%in Heilongjiang soil and by 9%—14%and 41%—42%in Jiangxi soil when ionic strength increased from 0.001 to 0.05 mol L-1and to 0.5 mol L-1NaNO3,respectively.

Fig.1 Effect of ionic strength on kinetics of paraquat adsorption onto the soils collected from Heilongjiang Province (northeastern China) (a)and Jiangxi Province(southern China)(b).Error bars indicate the standard deviations of the mean(n=3).
Mutual effects of Cu and paraquat on their adsorption in soil
Paraquat adsorption isotherms in the presence and absence of Cu in Heilongjiang and Jiangxi soils were evaluated(Table II;Fig.2a,b).Paraquat adsorption in both soils was nonlinear.TheNvalues were far lower than 1(Table II),indicating that partition is not a mechanism responsible for paraquat adsorption in soil(Robertset al.,2002).Interestingly, Pateiro-Moureet al.(2007) reported the linear adsorption isotherm of paraquat for one soil type and two consecutive linear isotherms for another type of soil,indicating different adsorption behaviors of paraquat among various soils.With the addition of 0.5 mmol L-1Cu,the Freundlich isotherm fittedKfvalue for each soil decreased compared to that with no addition of Cu.Moreover,Heilongjiang soil had the highestKfvalue,while Xinjiang soil had the lowestKfvalue(Table II).Copper diminished the adsorption of paraquat in both soils,and the suppression effect intensified as the initial Cu equilibrium concentration increased.In the presence of 0.1 and 0.2 mmol L-1Cu,paraquat adsorption decreased by 4%and 8%in Heilongjiang soil,respectively,and decreased by 15%and 19%in Jiangxi soil,respectively.Similarly, adsorption of paraquat in Anhui, Shanxi, and Xinjiang soils also decreased in the presence of 0.5 mmol L-1Cu(Fig.3).
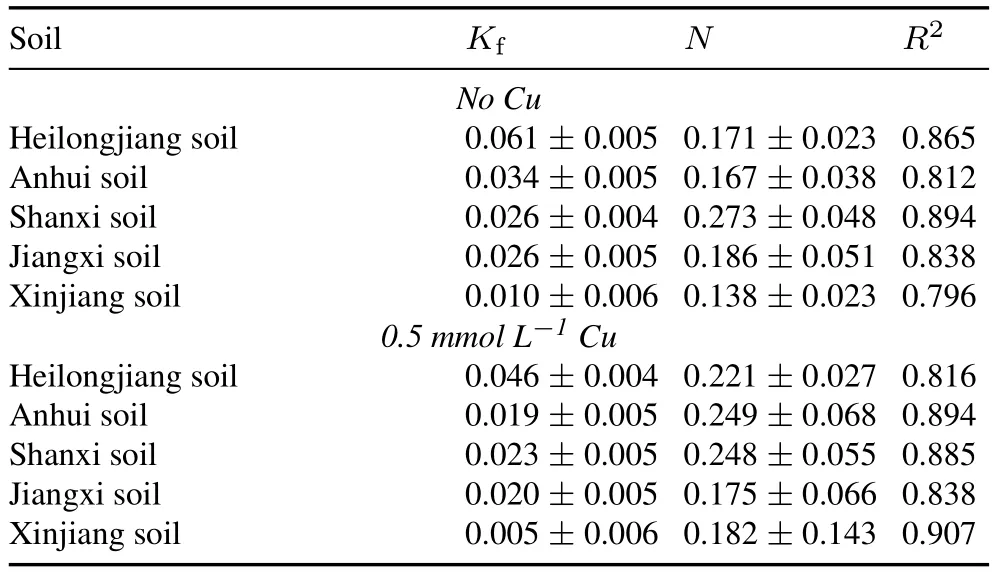
TABLE II Freundlich isotherm parametersa) of paraquat adsorption onto the soils collected from Heilongjiang Province(northeastern China),Jiangxi Province(southern China),Anhui Province(southeastern China),Shanxi Province(northern China), and Xinjiang Province (northwestern China) in the presence and absence of 0.5 mmol L-1 Cu

Fig.2 Mutual effects of Cu and paraquat added on their adsorption onto the soils collected from Heilongjiang Province(northeastern China)(a and c)and Jiangxi Province(southern China)(b and d).Error bars indicate standard deviations of the mean(n=3).0.1Cu,0.2Cu,and 0.5Cu=0.1,0.2,and 0.5 mmol L-1 Cu,respectively.

Fig.3 Mutual effects of Cu and paraquat added on their adsorption onto the soils collected from Anhui Province(southeastern China),Shanxi Province(northern China),and Xinjiang Province(northwestern China).Error bars indicate standard deviations of the mean(n=3).0.5Cu=0.5 mmol L-1 Cu.
Paraquat also suppressed the adsorption of Cu in Heilongjiang and Jiangxi soils(Fig.2c,d).In the presence of 0.12 and 0.19 mmol L-1paraquat,Cu adsorption decreased by 16%and 22%in Heilongjiang soil and by 24%and 37%in Jiangxi soil,respectively.In the presence of 0.39 mmol L-1paraquat,Cu adsorption decreased by 42%,37%,48%,50%,and 61%in Heilongjiang,Anhui,Shanxi,Jiangxi,and Xinjiang soils,respectively(Figs.2c,d and 3).According to Pateiro-Moureet al.(2007), although the concentration of paraquat(0.389 mmol L-1)was lower than that of copper(0.8.1.6,or 3.2 mmol L-1),copper adsorption was reduced in the presence of paraquat.In the present study,0.1 mmol L-1Cu and 0.12 mmol L-1paraquat have anca.1:1 stoichiometric ratio.In the presence of 0.1 mmol L-1Cu,paraquat adsorption decreased by 4%and 15%in Heilongjiang and Jiangxi soils, respectively, while in the presence of 0.12 mmol L-1paraquat, Cu adsorption decreased by 16%and 24%in Heilongjiang and Jiangxi soils,respectively.These results indicated that the degree of Cu release was not stoichiometrically related to the increase in herbicide retention(Pateiro-Moureet al.,2007).
The zeta potentials of the five soils decreased gradually with increasing solution pH (Fig.4).Over the pH range from 2.5 to 7.5,the zeta potentials were all negative except for Jiangxi soil, which was positive at pH 2.5.When pH was 5.5, the zeta potentials for Xinjiang, Shanxi, Jiangxi,Heilongjiang,and Anhui soils were-10.4,-15.6,-18.6,-23.3,and-24.6mV,respectively.The zeta potentials of the five soils increased in the order of Anhui Fig.4 Zeta potential as a function of pH in the soils collected from Heilongjiang Province(northeastern China),Jiangxi Province(southern China),Anhui Province(southeastern China),Shanxi Province(northern China),and Xinjiang Province(northwestern China). The primary rapid adsorption of paraquat was attributed to cation exchange,with the positively charged paraquat molecules being attracted to the negatively charged minerals and organic matter in soil.Adsorption of paraquat was positively correlated not only with CEC,but also with OM content,and the Pearson correlation coefficients were 0.96 and 0.85,respectively(P<0.05).Therefore,it should be noted that the adsorption of paraquat onto soil was solely determined by neither the clay content nor the OM content.Moreover,some of the OM associated with solid-state humic particles or clay aggregates may be inaccessible to paraquat,thus reducing the effective level of active OM(Barriuso and Koskinen,1996). Ultraviolet-visible spectra of the supernatant of 0.12 mmol L-1paraquat adsorbed in Heilongjiang and Jiangxi soils in the absence or presence of Cu are shown in Fig.5.The absorbance peaks of aqueous 0.1 and 0.5 mmol L-1Cu2+in Heilongjiang and Jiangxi soils were at 368 nm(data not shown).In the absence of Cu, the absorbance peaks of paraquat in Heilongjiang and Jiangxi soils occurred at 261 and 257 nm, respectively.In the presence of 0.1 and 0.5 mmol L-1Cu, the absorbance peaks of paraquat were at 260 and 257 nm in Heilongjiang and Jiangxi soils,respectively.In the presence of 0.12 mmol L-1paraquat,the absorbance peaks of 0.1 and 0.5 mmol L-1aqueous Cu2+in Heilongjiang and Jiangxi soils remained at 368 nm.These results suggested that Cu-paraquat complexation in the soil solution was unlikely to happen,which differed from the widely reported formation of Cu-glyphosate complexes.Sojo(1992)found no evidence for complexes between Cu2+and paraquat2+in solutions containing humic substances. Normalized Cu K-edge (8 979 eV) X-ray absorption near edge structure(XANES)spectra of the Heilongjiang and Jiangxi soils (Fig.6a) and the reference samples of aqueous Cu(NO3)2solution,Cu(OH)2,and Cu(CH3COO)2(Fig.6b) are presented.A small feature “A” on the rising edge,a main peak“B”,and a shoulder peak“C”occurred in the Heilongjiang and Jiangxi soils (Fig.6a), while the shoulder peak“C”was invisible in the aqueous Cu(NO3)2.The intensity of the shoulder “C” and the main peak “B”are related to the local symmetry and bond strength of Cu,which arise from the orbital splitting of 4p bonds for K-edge(Dupontet al.,2002).The presence of this shoulder peak suggests that the Cu—O coordination in the soil samples is different from that in the aqueous Cu(NO3)2,and the oxygen atoms are not centrosymmetrically configured around Cu. Fig.5 Ultraviolet-visible spectra of the supernatant of 0.12 mmol L-1 paraquat adsorbed onto the soils collected from Heilongjiang Province(northeastern China,a)and Jiangxi Province(southern China,b)in the absence or presence of Cu.0.1Cu and 0.5Cu=0.1 and 0.5 mmol L-1 Cu,respectively. Fig.6 Copper K-edge X-ray absorption near edge structure(XANES)spectra of the soils collected from Heilongjiang Province(northeastern China)and Jiangxi Province(southern China)(a)and reference samples of aqueous Cu(NO3)2,Cu(CH3COO)2,and Cu(OH)2 (b),normalized k2-weighted extended X-ray absorption fine structure(EXAFS)functions(k2χ(k))in the k-space of the soils and Cu(OH)2 (c),Fourier transform(FT)to R-space of the EXAFS function(d),and fitted curves in the R-space for the first Cu—O shell of the EXAFS data for the soil samples and Cu(OH)2 (e).A small feature“A”on the rising edge,a main peak“B”,and a shoulder peak“C”are labeled in a and b.A.U.=absorption unit;a.q.=aqueous. The normalizedk2-weighted EXAFS function,Fourier transform,and the fitted curve in theR-space are presented in Fig.6c—e.The normalizedk2-weighted EXAFS function of Jiangxi soil showed much noise above 0.7 nm(Fig.6c)due to the lower Cu content.The Fourier transforms reflect the relative radial distribution function of Cu with neighboring atoms located in the local coordination shells.The dominant peaks at 0.15 nm correspond to the first Cu—O shell.In Cu(CH3COO)2, a second peak at 0.24 nm was visible corresponding to the second Cu—C shell,where C is from the COO-ligand;however,this peak was not observed in the Heilongjiang and Jiangxi soils(Fig.6d).Additionally,in contrast to Cu(OH)2,the second Cu—Cu(O)peak atca.0.29 nm was not observed in the soil samples (Fig.6d).Compared with aqueous Cu(NO3)2,Cu in the soil samples may have some water as hydration layers as the nearest neighbors,but it may not be the same as Cu(NO3)2because of the different XANES features;some organic ligands in the soil samples may also be involved.The absence of high order coordination indicates that Cu in soil is highly dispersive.The fitting results of the EXAFS data using one shell model are presented in Table III.The first Cu—O shell data in the soil samples indicate that Cu was coordinated with 5.3 and 4.6 oxygen atoms at a bond length of 0.196and 0.194 nm,respectively.However,the aqueous Cu(NO3)2was coordinated with 6.3 oxygen atoms at a bond length of 0.193 nm.The five oxygen coordination is common in Cu compounds,such as Cu(OH)2and Cu(CH3COO)2.The neighboring atoms may contain some water molecules and these oxygen atoms are most likely not centrosymmetrically configured around Cu.The Cu coordination environment may also account for the mutual suppression effects of paraquat and Cu on their adsorption in soil. In a previous report (Wanget al., 2009), we have investigated the adsorption mechanisms of Cu2+and Pb2+in humic acids and wheat-burning ash using XAFS.The results suggested that Cu2+and Pb2+form complexes withcarboxylic,hydroxylic,and phenolic groups of humic acids and ash.This is consistent with Strawn and Baker(2008),who reported that Cu2+is mainly retained in OMviaformation of bidentate inner-sphere complexes with carboxyl ligands in a contaminated agricultural soil.The structure of Cu complexes formed on clay interlayer sites has also been investigated,and the results indicate that Cu adsorbed onto the interlayer as an outer-sphere complex(Brown and Kevan,1988;Hyunet al.,2000)and as an inner-sphere monomer or multinuclear complex on the edge of montmorillonite(Hyunet al.,2000).Furthermore,it was suggested that Cu formed outer-sphere associations in the interlayer at low pH,innersphere surface complexes on montmorillonite edge sites at intermediate pH,and dimers at high pH(Hyunet al.,2000).Cheahet al.(1998)observed that Cu2+forms dominantly mononuclear inner-sphere complexes with monodentate or bidentate coordination on amorphous silica,γ-Al2O3,and anatase at low Cu2+concentrations.Ternary complexes involving Cu(II)are likely to occur and to influence the binding and dissolution of Cu in clay-organic systems(Alcacioet al.,2001). TABLE III Fitting resultsa)of the first Cu—O shell to the extended X-ray absorption fine structure(EXAFS)data of the soils collected from Heilongjiang Province(northeastern China)and Jiangxi Province(southern China)and reference samples of aqueous Cu(NO3)2 and Cu(OH)2 CONCLUSIONS This study deals with an environmental problem posed by growing land application of paraquat and Cu-enriching materials.Two main findings were obtained from this study:i)paraquat had a stronger affinity for soil adsorption sites than Cu and ii)competitive adsorption of paraquat and Cu was responsible for the observed mutual suppression effects on their adsorption onto soil.The results showed that more Cu or paraquat tended to be released when they coexisted in soil than when they existed alone.Copper had higher mobility than paraquat due to the higher suppression effect of paraquat on Cu adsorption.Therefore, the resulting environmental risks may increase. ACKNOWLEDGEMENTS Beamline support at Beijing Synchrotron Radiation Facility is highly appreciated.The authors also thank Dr.Kristen Hychka for improving the English writing of the manuscript.
Possible formation of Cu-paraquat complexes
Coordination environment of Cu bysynchrotron X-rayabsorption spectroscopy


- Pedosphere的其它文章
- Drying-rewetting cycles reduce bacterial diversity and carbon loss in soil on the Loess Plateau of China
- Pedotransfer functions for predicting bulk density of coastal soils in East China
- Low soil C:N ratio results in accumulation and leaching of nitrite and nitrate in agricultural soils under heavy rainfall
- Free-living nematode community structure and distribution within vineyard soil aggregates under conventional and organic management practices
- Effects of rhamnolipids on bacterial communities in a dioxin-contaminated soil and the gut of earthworms added to the soil
- Biochar reduces uptake and accumulation of polycyclic aromatic hydrocarbons(PAHs)in winter wheat on a PAH-contaminated soil

