Tissue distribution of cadmium and its effect on reproduction in Spodoptera exigua
Honghua Su ,Menglu Wu ,Yong Yang ,Yan Deng ,Yizhong Yang# ,Qingming Sun
1 College of Plant Protection,Yangzhou University,Yangzhou 225009,China
2 Institute of Fruit Tree Research,Guangdong Academy of Agricultural Sciences/Key Laboratory of South Subtropical Fruit Biology and Genetic Resource Utilization,Ministry of Agriculture and Rural Affairs/Guangdong Provincial Key Laboratory of Tropical and Subtropical Fruit Tree Research,Guangzhou 510640,China
Abstract Vegetable fields are often contaminated by heavy metals,and Spodoptera exigua is a major vegetable pest which is stressed by heavy metals mainly by feeding.In this study,cadmium accumulation in the tissues of S.exigua exposed to cadmium and its effects on the growth and development of the parents and the offspring were investigated.Under the stress of different concentrations of cadmium (0.2,3.2,and 51.2 mg kg-1),the cadmium content in each tissue of S.exigua increased in a dose-dependent manner.At the larval stage,the highest cadmium accumulation was found in midgut in all three cadmium treatments,but at the adult stage,the highest cadmium content was found in fat body.In addition,the cadmium content in ovaries was much higher than in testes.When F1 S.exigua was stressed by cadmium and the F2 generation was not fed a cadmium-containing diet,the larval survival,pupation rate,emergence rate and fecundity of the F2 generation were significantly reduced in the 51.2 mg kg-1 treatment compared to the corresponding F1 generation.Even in the F2 generation of the 3.2 mg kg-1 treatment,the fecundity was significantly lower than in the parental generation.The fecundity of the only-female stressed treatment was significantly lower than that of the only-male stressed treatment at the 3.2 and 51.2 mg kg-1 cadmium exposure levels.When only mothers were stressed at the larval stage,the fecundity of the F2 generation was significantly lower than that of the F1 generation in the 51.2 mg kg-1 treatment,and it was also significantly lower than in the 3.2 and 0.2 mg kg-1 treatments.The results of our study can provide useful information for forecasting the population increase trends under different heavy metal stress conditions and for the reliable environmental risk assessment of heavy metal pollution.
Keywords: heavy metal pollution,cadmium,Spodoptera exigua,tissue distribution,reproduction
1.Introduction
In recent years,the rapid development of industrialization and agricultural modernization has caused serious heavy metal pollution in the soil,water bodies and atmosphere,and these trends are getting worse year by year.Occupational poisoning and environmental pollution events caused by cadmium have occurred frequently in China,and the exceedance rate of cadmium pollution sites for soil nationwide is 7% (MEP 2014).The cadmium concentrations in soil reported in the literature have all exceeded the standard for non-contaminated agricultural lands,contaminated agricultural lands,industrial lands,aquatic sediments,and urban lands (Ashrafetal.2021).Cadmium exists in the soil in water-soluble,exchangeable and organically bound states,and plants can take it up in the water-soluble and exchangeable states.Some studies have shown that the enrichment of cadmium in plants mainly occurs in the roots,with small amounts transferred to other parts;for example,Nishizonoetal.(1987) found that 70-90% of the cadmium in plant roots was enriched in the root tip cell wall.
The cadmium absorption and enrichment capacities of vegetables vary greatly depending on the species.The enrichment and absorption capacities of fruit and root vegetables are generally weaker than those of leafy vegetables (Smigieletal.1994).In animals,cadmium mainly comes from food and affects their growth and development.Cadmium enrichment in the kidneys of animals affects the reproductive system and fecundity(Monsefietal.2010),while cadmium stress also increases the risk of embryonic malformation and can even cause death (Thompson and Bannigan 2008).
Herbivorous insects play vital roles in the food chains and food webs in agroecosystems,which are inevitably exposed to heavy metals (Galletal.2015;Lietal.2018).Herbivorous insects usually absorb cadmium from the environment through feeding and respiration,and the accumulation is continuous.Recent studies have shown that the heavy metal contents in herbivorous insects vary significantly among different tissues,ages,sexes and generations of insects,thereby affecting their vital parameters such as fecundity and oviposition (Plachetka-Bozeketal.2018b;Luoetal.2019;Meseetal.2022).For example,the Ni2+contents in different tissues of 6th instar larvae ofSpodopteralituraafter feeding on Nicontaining artificial diets were in the order of midgut>fat body>epidermis,and the accumulation increased with time and the Ni2+dosage (Sunetal.2008).The same significant dose-response relationship was found inS.exiguastressed by Pb2+,where the concentration of Pb2+in each insect state differed significantly between treatments in the same generation,and the concentration of Pb2+basically increased with an increase in the stressed generations.The Pb2+concentrations in each developmental stage were in the order of 5th instar larvae>adults>pupae (Huetal.2014).The survival rates of larvae,pupae and adults decreased significantly with an increase in the Pb2+concentration when it exceeded 100 mg kg-1(Zhouetal.2012a).
Cadmium accumulation in the herbivorous insect body affects growth,development and reproduction,such as changes in the larval,pupal or adult survival,shortening/extending the duration,stimulation/inhibition of fecundity,and an increase/decrease in hatching rate (Plachetka-Bozeketal.2018b).Exposure to high concentrations of cadmium (100 and 250 mg kg-1) has been shown to prolong the developmental duration and inhibit the growth rate of the fourth instar larvae ofLymantriadispar(Mikkola and Rantala 2010).Cerveraetal.(2004) found that after cadmium stress,the life cycle ofOncopeltusfasciatuslarvae changed,the larval duration became longer,the weight of newly emerged adults was reduced,and the fecundity of females was reduced.Cadmium also affected the fertility of the next generation.In addition,herbivorous insects have evolved ecological adaptation strategies to cope with adversity in the face of long-term heavy metal stress (Kafeletal.2012a,b,2014;Augustyniaketal.2017;Plachetka-Bozeketal.2018a).Once they have developed adaptations to heavy metals,their tolerances for other adversities will also improve (Zhouetal.2012a,b;Augustyniaketal.2016,2017;Plachetka-Bozeketal.2018b),which in turn leads to outbreaks of pest populations,and hence threatens food security and ecological safety.
In this study,we investigated the distribution of cadmium in larvae and adults ofS.exiguawhich were stressed for two consecutive generations,and determined the effects on development and reproduction.Our results may provide a theoretical basis for exploring the effects of heavy metals on herbivorous insects in the field and a useful reference for the environmental risk assessment of heavy metal pollution.
2.Materials and methods
2.1.Insects
Spodopteraexiguawere provided by the Institute of Plant Protection,Chinese Academy of Agricultural Sciences.They were reared on an artificial diet in the laboratory at (27±1)°C and (70±7)% RH,with a 14 h L:10 h D photoperiod.
2.2.Artificial diet preparation
The artificial diet was prepared according to Suetal.(2021).CdCl2was added to 1 kg of liquid artificial diet and thoroughly mixed to obtain the Cd-containing artificial diets (at 0.2,3.2,and 51.2 mg kg-1).The same volume of double distilled water (ddH2O) was added to the control diets (CK is used to represent the control group that has not been treated with heavy metals below).
2.3.Determination of cadmium in various tissues of S.exigua
Larvae were fed with cadmium-containing diet (0.2,3.2 and 51.2 mg kg-1) until pupation.The 5th instar larvae and freshly emerged adults were selected,and washed with ddH2O.The epidermis,midgut,hemolymph and fat body of the 5th instar larvae and the head,thorax,abdomen (midgut,hemolymph,fat body,ovaries or testis),legs and wings of the freshly emerged adults were collected and washed again with ddH2O.Artificial diet with no cadmium was used as the control.The concentrations of cadmium in different tissues were determined by atomic fluorescence spectrophotometry.Samples were stored in 30 mm×50 mm weighing bottles for later use.The different tissue samples were dried in an oven at 120°C for 4 h and then cooled in the dryer immediately.The 0.1 g samples of dried tissue were weighed,digested in an HNO3+HClO4system,transferred to a colorimetric tube,and then the cadmium content in each sample was measured by the flame absorption method using an AFS-2202E atomic fluorescence photometer (Pangetal.2011)with three replicates per treatment.
2.4.Survival,pupation,and emergence rates
Newly hatched larvae were reared on diets containing different concentrations of cadmium (0,0.2,3.2,and 51.2 mg kg-1) in disposable cartons and transferred to 12-well plates with one larva per well at the end of 2nd instar.Fresh artificial diet was provided daily.Within 24 h after pupation,pupae were placed individually in plastic cups.The time of emergence and number of adults were recorded.The following formulas were used to calculate the rates:
Survival rate (%)=Number of 5th instar larvae/Number of tested neonate larvae×100
Pupation rate (%)=Number of pupae/Number of 5th instar larvae×100
Emergence rate (%)=Number of adults/Total number of pupae×100
7. That which thou hast promised must thou perform: After changes made by the Grimms, the primary moral of the story is to keep a promise once it is made. The king, being a good father, emphasizes this lesson to his royal daughter, insisting she keep her promise to the frog.Return to place in story.
2.5.Number of eggs in the paired treatments
After emergence,one female and one maleS.exiguawere paired and placed in a disposable plastic box(30 cm×30 cm×30 cm).Ten pairs were used in each treatment,and the paired combinations are shown in Table 1.Egg-laying paper and skimmed cotton ballssaturated with 5-10% honey water were placed in each plastic box which was sealed with a gauze net.The egglaying paper was replaced daily,and the egg masses were kept in transparent sealed bags.The number of eggs laid each day was counted under a dissecting microscope.

Table 1 Females paired with males exposed to different cadmium concentrations (mg kg-1)1)
2.6.Effect of cadmium-treated F1 females on the reproduction of the F2 generation
Larvae of the first generation were reared on diets containing different concentrations of cadmium (0,0.2,3.2 and 51.2 mg kg-1).Then the collected eggs were used as the insect source of the next generation.All larvae of the second generation in each treatment were fed with diet containing no cadmium.The rearing protocol was the same as that of the first generation.
2.7.Data analysis
The cadmium accumulation data for different tissues were subjected to one-way ANOVA,and then the Tukey method was used to analyze the significance of differences.The data for growth and development were statistically analyzed using ANOVA with the DPS15.10 statistical software.The LSD test was used to analyze the significance of differences between treatments.
3.Results
3.1.Cadmium contents in larval tissues
The highest cadmium concentrations in the three cadmium treatments were found in the midgut,followed by the fat body and the epidermis.Hemolymph accumulated the lowest concentrations.Significant differences in the cadmium contents were found between the midgut and fat body,and highly significant differences were found between the epidermis and hemolymph (Table 2).
3.2.Cadmium contents in adult tissues
Table 3 shows a similar pattern for males and females at the different concentrations,with the accumulated concentrations in the abdomen being significantly higher than in the head,thorax,legs and wings at all concentrations.At the same cadmium exposure,the cadmium contents in head,thorax and wings were significantly higher in females than in the corresponding tissues of males,while there were no significant differences in the cadmium contents in abdomen and legs between males and females (Table 3).
3.3.Cadmium contents in the various abdominal tissues of adults
The cadmium content in the fat body of males was significantly higher than those in the midgut,hemolymph and testes for all cadmium treatments.The cumulative concentrations in fat body and ovaries in the abdomens of females were significantly higher than those in the midgut and hemolymph at all concentrations.The concentrations of cadmium in the ovaries were significantly higher than those in the testes at a given concentration,while there were no significant differences in the fat body,midgut and hemolymph between females and males (Table 4).
3.4.Effects on the F1 and F2 generations when only F1 S.exigua was exposed to cadmium stress
The survival rates of the F1and F2generations (68-75%)at three concentrations (0,0.2 and 3.2 mg kg-1) were not significantly different,and the three survival rates were in the sequence of 0.2>0>3.2 mg kg-1.The survival rates at 51.2 mg kg-1(51-57%) were significantly lower than those at the other concentrations,and the survival of the F2generation was significantly lower than that of the F1generation (Fig.1).

Fig.1 Survival rates of the F1 and F2 generations of Spodoptera exigua under different cadmium stresses.Data are mean±SE(n=10).Different lowercase letters in the figure indicate significant differences between different concentrations(P<0.05).* and ** indicate P<0.05 and P<0.01,respectively.

Fig.2 Pupation rates of the F1 and F2 generations of Spodoptera exigua under different cadmium stresses.Data are mean±SE (n=10).Different lowercase letters in the figure indicate significant differences between different concentrations(P<0.05).* and ** indicate P<0.05 and P<0.01,respectively.
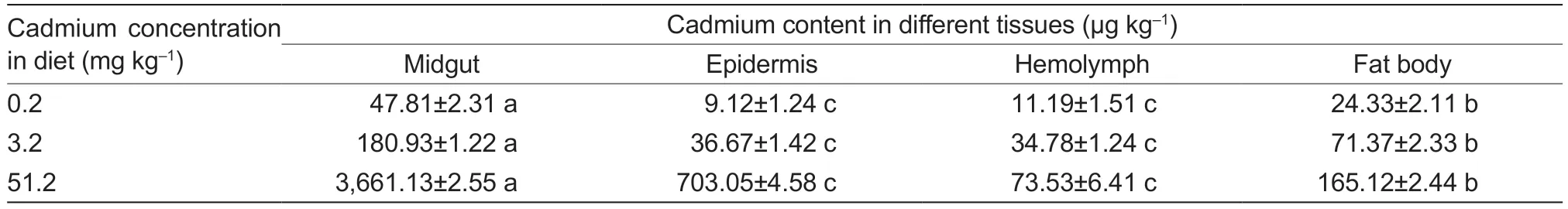
Table 2 Cadmium contents in larval tissues of Spodoptera exigua exposed to different cadmium concentrations
The emergence rates of the F2generations (86 and 87%) whose parents were exposed to 0 and 0.2 mg kg-1,respectively,were slightly higher than those of the F1generations (84 and 86%);and the emergence rates of the F2generations (67 and 33%) whose parents were exposed to 3.2 and 51.2 mg kg-1,respectively,were lower than those of the F1generations (71 and 65%).The emergence rates in the 0,0.2 and 3.2 mg kg-1cadmium treatments were not significantly different from those of the F2generations,but the emergence rates of the F1generations at 51.2 mg kg-1were significantly different from those of the F2generations.The emergence rates of the F1generations at 51.2 mg kg-1were extremely significantly higher than those of the F2generations (Fig.3).

Fig.3 Emergence rates of the F1 and F2 generations of Spodoptera exigua under different cadmium stresses.Data are mean±SE (n=10).Different lowercase letters in the figure indicate significant differences between different concentrations(P<0.05).* and ** indicate P<0.05 and P<0.01,respectively.
The number of eggs in the F1and F2generations showed decreasing trends with increases in the cadmium concentration.The number of eggs in the F1generations(348 and 312) were slightly higher than those in the F2generations (346 and 309) when they were exposed to 0 and 0.2 mg kg-1cadmium,respectively;while the number of eggs in the F2generations (171 and 143)whose parents were exposed to 3.2 and 51.2 mg kg-1,respectively,were extremely significantly lower than in the corresponding F1generations (231 and 189) (Fig.4).

Fig.4 Number of eggs laid by the F1 and F2 generations of Spodoptera exigua under different cadmium stresses.Data are mean±SE (n=10).Different lowercase letters in the figure indicate significant differences between different concentrations(P<0.05).* and ** indicate P<0.05 and P<0.01,respectively.
3.5.The number of eggs laid by S.exigua from different pairing combinations
Among the three pairings,the following trends were found:(1) The number of eggs laid byS.exiguastressed by higher concentrations of cadmium were significantly lower than those by lower concentrations.(2) Regarding the pairing of males without cadmium exposure and females treated with other concentrations,the number of eggs laid by females at 0.2 mg kg-1was significantly higher than those of the other four concentrations,while the number of eggs laid by females exposed to 51.2 mg kg-1was significantly lower than those of the other four concentrations,which decreased with an increase in the cadmium concentration.(3) Regarding the pairing of females without cadmium exposure and males stressed by the three cadmium concentrations,the number of eggs laid by females paired with males at 0.2 and 3.2 mg kg-1were significantly higher than the other two treatments.In addition,when the males and females were both exposed to 0.2 mg kg-1cadmium,the number of eggs was significantly higher than those of only males or females under stress.In the 3.2 mg kg-1treatment,the number of eggs laid by the parents,both of which were stressed,was less than that when only males were stressed,which was less than that when only females were stressed.There were significant differences among the above combinations.In the 51.2 mg kg-1treatment,the sequence for the number of eggs was only female stressed>both male and female stressed>only male stressed (Fig.5).

Fig.5 Effect of cadmium on the number of eggs laid by Spodoptera exigua after pairings with different concentrations.Data are mean±SE (n=10).Different lowercase letters in the figure indicate significant differences between different concentrations (P<0.05).* and ** indicate P<0.05 and P<0.01,respectively.
3.6.Effects on the reproduction of the F2 generation when only females were exposed to cadmium
As shown in Fig.6,the survival rate,pupation rate,emergence rate and egg production of the F2generation showed decreasing trends as the cadmium concentration increased,and all of these parameters were lower in the three cadmium treatments than in the control,except at the 0.2 mg kg-1concentration (Fig.6).

Fig.6 Indicators of the F2 generation of females under continuous stress.Data are mean±SE (n=10).Different lowercase letters in the figure indicate significant differences between different concentrations (P<0.05).* and ** indicate P<0.05 and P<0.01,respectively.
4.Discussion
Heavy metal ions enter herbivorous insects mainly through epidermal contact,respiration and feeding.The survival,body weight,developmental duration and fecundity of insects are affected by heavy metal exposure(Kramsetal.2015).The enrichment of heavy metals in herbivorous insects is a continuous dynamic process which is mainly influenced by stress time,the amount of feeding,the content of heavy metals in the food and their own metabolic capacity.When the uptake rate of heavy metals by insects exceeds their rate of metabolism,the heavy metals are enriched in herbivorous insects and transmitted throughout the food chain (Gintenreiteretal.1993).Our results showed that the content of cadmium inS.exiguawas positively correlated with the cadmium content in the artificial diet,which may indicate that the accumulation of heavy metals in insects is related to the concentration of heavy metals in food.Wuetal.(2010)showed that the cadmium accumulation inBoettcherisca peregrinalarvae varied with the concentration of cadmium in the diet.Crawfordetal.(1996) fedLocustamigratoriawith cadmium-contaminated wheat seedlings,and found that the cadmium content in tissues increased significantly with an increase in the cadmium content in wheat seedlings,with significant differences among the different treatments.Similar results were found inS.lituraFabricius after feeding on Ni2+containing food (Sunetal.2008).
In this study,compared with the tissues of larvae exposed to 0.2 mg kg-1cadmium,the cadmium contents in the corresponding tissues ofS.exigualarvae exposed to 3.2 mg kg-1cadmium increased by about two-fold;in the 51.2 mg kg-1cadmium treatment it increased in the midgut by 75.58-fold,in the epidermis by 76.09-fold,and in the hemolymph and fat body by 5.57-and 5.79-fold,respectively.These results indicate that the midgut has an outstanding capacity for cadmium accumulation.Among the tissues of the adultS.exigua,the abdomen had the highest cadmium accumulation;the cadmium content of the fat body was about 3 times higher than that of the midgut,and the cadmium content in the 3.2 mg kg-1cadmium treatment was about 3 times higher than that in the 0.2 mg kg-1cadmium treatment.In the 51.2 mg kg-1cadmium treatment,the cadmium contents of the fat body,midgut and hemolymph were 85.45,71.20 and 76.45 times higher,respectively,than in the 0.2 mg kg-1treatment.The reason for the differences in cadmium contents between larvae and adults may be the complete metamorphosis of these insects,in which most tissues and organs of the larvae are disintegrated and the adult organs are reconstructed during the pupal stage (Suzukietal.1984).In this period,heavy metal ions in the body were also allocated accordingly.Spodopteraexigualarvae require a large amount of food,and the midgut of herbivorous insects is the main site for the digestion and absorption of nutrients,thus we can speculate that the cadmium content in the midgut would be significantly higher than in other body parts,which is consistent with the results of our experiment.The midgut plays an important role in ion regulation and mineral absorption because the metal particles in the columnar cells and the lysosomes in the midgut cells can deposit large amounts of metal ions (Ballan-Dufrancais 2002).Excessive heavy metal ions can pass through the basement membrane of midgut epithelial cells and are transportedviathe hemolymph to the fat body and epidermis,so cadmium could be detected in those tissues,the contents of which increased with increases in the cadmium concentration in the diet.However,the forms of cadmium deposited in the midgut cells and differential distribution patterns in different tissues need further investigation.
Numerous studies have shown that heavy metals promote the growth and development of herbivorous insects at low concentrations and inhibit them at high concentrations (Jiangetal.2020).For example,the developmental duration ofNilaparvatalugensnymphs feeding on rice contaminated with low concentrations of cadmium was significantly shorter compared to the control,but significantly longer whenN.lugensnymphs were exposed to high concentrations of cadmium (Yuetal.2008).Our results showed that excess cadmium in food can accumulate in various tissues ofS.exigua,such as the midgut,and the development and reproduction of the mothers and offspring were affected accordingly.In the 51.2 mg kg-1treatment,the survival,pupation and emergence rates were significantly reduced,and the egg number was also significantly lower after exposure to a high (51.2 mg kg-1) cadmium concentration.
Significant differences were found in the testes and ovaries of adultS.exigua.In the three treatments,the cadmium content in the ovaries was about 5 times higher than in the testes.The enrichment capacities of females were also significantly higher than that of males in the heads,thoraxes and wings.Devkota and Schmidt(2000) found that female locusts also had a significantly higher enrichment capacity for cadmium than males,but much higher cadmium levels were detected in males ofChorthippusbrunneusthan in females (Hunteretal.1987).The same was true for nickel inGilpiniasociaKlug andNeodiprionsertiferGeoffroy,and Wilczeketal.(2004) also found that males had a stronger accumulation capacity.These studies suggest that there may be some differences in the uptake and metabolic capacities of different heavy metals between males and females.The results of the experiment in this study showed that although the larvae of the F2generation were not exposed to cadmium,the survival rate,pupation rate,and number of eggs laid by the F2generation were significantly lower than those of the F1generation in the 51.2 mg kg-1treatment,indicating that high cadmium concentrations inhibit the population increase in the long run.Heavy metals have also been shown to stimulate the fecundity of female insects,and through the egg-laying process the heavy metals in mothers could be transferred to the eggs(Kubotaetal.2002).Therefore,more in-depth studies are needed to reveal the mechanisms of differences in the abilities of male and female insects to enrich heavy metals and the transfer process during egg laying.
The survival rate,pupation rate,emergence rate and the number of eggs decreased with an increase in the cadmium concentration.In each of the three treatments(0,0.2 and 3.2 mg kg-1),there were no significant differences in the above parameters between the F1and F2generations,but in the 51.2 mg kg-1treatment,the above parameters of F1were significantly different from those of F2.The toxic effects of cadmium on insects differ at different concentrations.Low cadmium concentrations(<1.0 mmol L-1) had limited effects on the growth and development of the houseflyMuscadomestica,but the emergence rate,survival rate,pupation rate,and average weight were negatively affected when the cadmium concentration was more than 1.0 mmol L-1(Niuetal.2000).We can conclude that different species have different tolerances to the same heavy metal stress.In this study,high cadmium stress (51.2 mg kg-1) led to severe harm not only to F1,but also to F2which was not exposed to any cadmium.This meant that the high cadmium exposure of F1impacted the embryonic development of F2.
Cadmium contamination can lead to adverse effects in herbivorous insects,such as reduced fecundity.Vitellogenin is synthesized by the fat body and secreted into the hemolymph,and selectively taken up by developing oocytes to become yolk protein (Engelmann 1979).Vitellogenin (Vg) synthesis is the basis of yolk,which is critical for egg maturation and embryonic development,and yolk is central to the regulation of reproduction in herbivorous insects (Bebasetal.2008).The synthesis of vitellogenin is impaired in femaleOncopeltusfasciatuswhen exposed to cadmium (Cerveraetal.2005).This impairment may be due to changes in the compositions and functions of the fat body and hemolymph when subjected to heavy metal stress,which weaken the reproductive behavior ofS.exigua,or it may be due to the oxidative stress of heavy metals in the insects.The latter would disrupt the nervous system and lead to disruption of the endocrine system,so a series of physiological responses would be affected,such as reduced secretion of reproduction-related hormones and increased DNA repair.Ultimately,this would lead to a reduction in Vg synthesis and vitellin accumulation related to reproduction.The external manifestations of this would be reduced egg production and reduced egg quality.
5.Conclusion
WhenS.exiguawas exposed to different concentrations of cadmium (0.2,3.2,and 51.2 mg kg-1),the cadmium content in each tissue ofS.exiguaincreased in a dosedependent manner.The highest cadmium accumulation was found in midgut at the larval stage and in fat body at the adult stage in all the three cadmium treatments.Cadmium affected the fecundity of both F1and F2generations.A high cadmium concentration (51.2 mg kg-1) resulted in significantly lower fecundity of the F2generation than the F1generation,even though F2was not fed with cadmium-containing diet.
Acknowledgements
We thank Ms.Connie Alison (Geneva,New York,USA)very much for her careful proofreading of this manuscript.This study was partially supported by the Open Project Program from the Key Laboratory of South Subtropical Fruit Biology and Genetic Resource Utilization (Ministry of Agriculture and Rural Affairs),China (212103).
Declaration of competing interests
The authors declare that they have no conflict of interest.
 Journal of Integrative Agriculture2024年1期
Journal of Integrative Agriculture2024年1期
- Journal of Integrative Agriculture的其它文章
- Advances in DNA methylation and its role in cytoplasmic male sterility in higher plants
- Assessment of molecular markers and marker-assisted selection for drought tolerance in barley (Hordeum vulgare L.)
- 3D genome organization and its study in livestock breeding
- SUPER WOMAN 2 (SPW2) maintains organ identity in spikelets by inhibiting the expression of floral homeotic genes OsMADS3,OsMADS58,OsMADS13,and DROOPING LEAF
- Genetic dissection and validation of a major QTL for grain weight on chromosome 3B in bread wheat (Triticum aestivum L.)
- Coupling of reduced inorganic fertilizer with plant-based organic fertilizer as a promising fertilizer management strategy for colored rice in tropical regions
