MoS2核壳球上氧掺杂的动力学调制及其电化学分解水效应
巩飞龙 刘静轩 刘梦梦 许三魁 李 峰*,,3
(1郑州轻工业大学河南省表界面科学重点实验室,郑州 450002)
(2河南工业大学材料学院,郑州 450002)
(3美洲先进纳米技术有限公司,休斯敦 77459)
Molybdenum disulfide(MoS2)is one of the promising substitutions to Pt catalysts for electrochemically splitting water in hydrogen evolution reaction (HER)[1].Because the HER activities of MoS2nanosheets mainly originate from sulfide edges[2-3], their practical applications therefore depend not only on activating their surfaces mainly exposed in inert (002) facets[4-7]but also on improving the cycle stability of 2D MoS2nanosheets of only one to several atomic layers in thickness[8].Toward this end, scientists have strived to tailor the microstructures and composition of 2D MoS2nanosheets to further boost their HER activities[9-15].It is demonstrated that O-doping can activate inert basal(002) facets of semiconductor MoS2nanosheets in the 2H phase[16].3D MoS2nanoboxes showed enhanced HER activities after being modified with MS (M: Ni and Co) shells onto their surfaces to form M-MoS3hybrids[17-18].The HER performance of the basal plane of MoS2can be highly improved by creating sulfur vacancies (MoS2-x)[19], which is attributed to the regulation of hydrogen adsorption - free energy (ΔGH) for strained surfaces[20].Pető′s group found that oxygen atoms spontaneously incorporate into the basal plane of MoS2single layers during ambient exposure, which results in substantially increasing the catalytic activity of the entire MoS2basal plane for HER[21].The HER performance from multilayered MoS2can be divided into two domains corresponding to “point defects” at low concentrations of surface sulfur vacancies and large regions under coordinated Mo atoms for high concentrations of surface sulfur vacancies[22].The extensive research activities, however, have mainly concentrated on tailoring the microstructures and composition of 2D MoS2nanosheets.To the best of our knowledge,there is no report in the literature on modulating the O-doping amount to activate 3D hierarchical MoS2materials.
1 Experimental
1.1 Materials
The main reagents included sodium molybdate dihydrate (Na2MO4·2H2O, 98.0%, AR, Aladdin), hexadecyl trimethyl ammonium bromide (CTAB, 98.0%,AR, Aladdin),n-butanol (C4H9OH, 98.0%, AR,Aladdin), ethylene glycol ((CH2OH)2, 98.0%, AR,Aladdin),hydrochloric acid(HCl,36%-38%,AR),thiourea (CH4N2S, 99.8%, AR, Aladdin).The deionized water (18.25 MΩ) was utilized for the entire experiment.All reagents were used without further purification.
1.2 Synthesis of MoS2 core - shell spheres with different O-doping amounts
Precursors (MoS2core-shell superspheres) composed of quasi-molecular superlattices were first synthesized by performing a one-pot approach developed in our lab[23-25].Typically, 300 mL aqueous solution of Na2MoO4(1 mmol, 0.242 0 g) was first added to 100 mLn-butanol solution of CTAB (15 mmol, 5.466 8 g)and stirred for 2 h.Ethylene glycol (50 mL) and concentrated hydrochloric acid (1.0 mL, 11.64 mol·L-1)were then introduced into the reactor in order and stirred for another 2 h.Thiourea (15 mmol, 1.141 8 g)was added into the solution and further stirred for 3 h.The milky white mixture was finally transferred into an autoclave with a polytetrafluoroethylene (PTFE) liner and kept at 220 ℃for 24 h.The black precipitates were separated by filtration, washed with distilled water and absolute ethanol three times each, and dried at 60 ℃for 24 h to obtain monodispersed MoS2coreshell superspheres (0.127 5 g;yield:85%).The precursor was subsequently annealed in Ar for 5 h by elevating the temperature to 900 ℃at a heating rate of 20,10,5,and 2 ℃·min-1to obtain MoS2core-shell spheres assigned as MoS2-O-20, MoS2-O-10, MoS2-O-5, and MoS2-O-2,respectively.
1.3 Characterization
The structures of the materials were characterized by using transmission electron microscopy (TEM,JEM-2100 operated at 200 kV, Japan).The field emission scanning electron microscopy (FESEM) was completed on a JSM-7001F operated at 15 kV.The thermogravimetric-differential thermal analysis (TG-DTA) of the materials was performed with a TG/DTA instrument(SDTQ600, Delaware) working at a constant heating rate of 2 ℃·min-1in Ar.To analyze the molecular structure and chemical composition of samples, FTIR spectra (Nicolet iS5, Thermo Fisher Scientific, USA)were collected in the range of 400-4 000 cm-1.The XPS spectra of the materials were collected on a Thermo Scientific Escalab (ESCALAB 250Xi, Massachusetts) with monochromatized AlKαX-ray as the excitation source.
1.4 Assemblies of electrodes and electrochemical tests
All electrochemical measurements were carried out at a CHI660E electrochemical workstation(Shanghai Chenhua Company) at room temperature.The precursor and as-prepared MoS2core-shell spheres were applied for assembling electrodes to investigate their HER performance in a standard three-electrode system with glassy carbon electrode coated with active materials as working electrode, Pt wire as counterelectrode, and Ag/AgCl electrode as reference electrode, respectively.The working electrodes were made by casting a colloid solution of active materials (1 mg·mL-1) to glassy carbon electrodes of 3 mm diameter,then evenly dispersing 10 µL Nafion-water solution(0.01%,w/w) and finally keeping at room temperature overnight to dry their surfaces.The loading amount of the materials was 0.32 mg·cm-2.All polarization curves were measured in 0.5 mol·L-1H2SO4(pH=0)solution at a scan rate of 5 mV·s-1from -0.3 to 0.1 V(vs RHE).The closed cyclic voltammetry (CV) curves of the materials with different scanning rates from 20 to 200 mV·s-1were performed to investigate their doublelayer capacitance in acid media (0.5 mol·L-1H2SO4).All electrochemical impedance spectroscopy (EIS)curves were measured in an acidic solution of 0.5 mol·L-1H2SO4with an initial potential of 0.1 V (vs RHE)and a frequency of 10-2-106Hz.The measured Tafel slope of 28 mV·dec-1for the Pt/C electrode agrees well with the values reported previously[26].
2 Results and discussion
Our group has developed a one-pot technique for producing functional materials with well - controlled size and shape, such as MoS2core-shell superspheres composed of quasi-molecular superlattices[23].The special molecular superlattices assembled with surfactant molecules (CTAB)2Sy(y=1 or 2) and MoS2atomic layers offer a unique platform for us to further tailor their microstructures and compositions.The MoS2core-shell superspheres were therefore synthesized with the onepot approach by simply enlarging the scale (Fig.S1,Supporting information) and applying it as precursors to produce MoS2core-shell spheres.While the precursor superspheres produced at an enlarged scale maintained a similar size and morphology (Fig.S2) in comparison with those reported previously, the oxygen doped into the precursors dramatically increased toca.23.1% based on XPS analyses (Fig.S3).The MoS2coreshell spheres with different O-doping amounts were produced by regulating the heating rates.The low- and high-magnification FESEM images (Fig.S4a-S4i)reveal that the as-prepared materials retained highly uniform spherical structures at the heating rate of 10, 5, and 2 ℃·min-1, in contrast to the collapsed spheres at the heating rate of 20 ℃·min-1(Fig.S4j-S4l).The materials were further analyzed with XPS (Fig.1a and Fig.S5-S7).Specifically, the Mo3dXPS spectra of MoS2-O-20,MoS2-O-10, MoS2-O-5, and MoS2-O-2 (Fig.1a) mainly consist of two strong peaks at 229.08 and 232.28 eV,corresponding to Mo4+3d5/2and Mo4+3d3/2of 2H-MoS2,respectively.A couple of weak peaks at 232.52 and 235.72 eV ascribe to Mo6+3d5/2and Mo6+3d3/2of MoO3,indicating that oxygen atoms have incorporated into the lattices of 2H-MoS2nanosheets.The dynamic evolution of the O-doping amount (Fig.1b) in MoS2core-shell spheres can be evaluated with the deconvolution of Mo element corresponding to MoO3and 2H-MoS2in Mo3dXPS spectra.It is interesting to find out that the O-doping amounts (Fig.S3) linearly declined from 23.1% of the precursors to 5.5% of MoS2-O-5, as regulating the heating rate from 20 to 5 ℃·min-1.The O-doping amount, however, approached a terrace at 5 ℃·min-1,and it maintained at a similar level of 6.2% further lowering the rate to 2 ℃·min-1.Compared to the precursors,the O-doping amount has been successfully modulated to a lowered level in all MoS2core-shell spheres after annealing at 900 ℃.It is convenient and effective to tune the O - doping amount in MoS2core - shell spheres by adjusting the heating rate.The lower rate results in a lower concentration of O doped in MoS2core-shell spheres.The inset in Fig.1b shows TEM images of precursors and as-prepared materials.All of the particles inherit the core-shell spherical structures of precursors(Fig.S8-S10),except MoS2-O-20(Fig.S11)collapsed at a too-high heating rate.The EDS spectra of the materials also confirm the tuned O - doping amount and sulfur amount (Fig.S8e, S9e, S10e, S11e,and S12-S14).The results agree well with those from XPS.It is noteworthy that while all of the materials were annealed in Ar,the O-doping amount can be modulated without additional sulfur resources introduced into the processes.The decrease of the O - doping amount can be only attributed to the anion exchange reactioninsitutaking place inside the precursors.Therefore, it is necessary to scrutinize the special microstructures of precursors to better understand the dynamic modulation of O-doping.
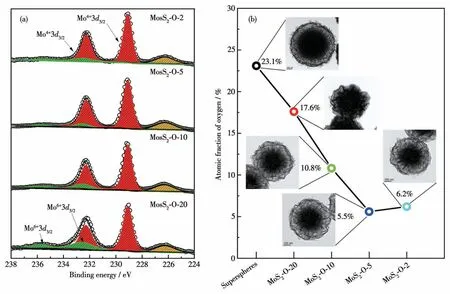
Fig.1 (a)High-resolution Mo3d XPS spectra of MoS2 core-shell spheres;(b)O-doping amounts in MoS2 core-shell spheres evaluated with deconvolution of the XPS spectra
The TG-DTA profile(Fig.2)of precursors revealed that they lost their weight byca.13.1% by elevating the temperature to 350 ℃, which can be attributed to the decomposition of organic surfactant molecules(CTAB)2Sy(y=1 or 2)incorporating between MoS2atomic layers to form quasi-molecular superlattices.In addition, the characteristic absorptions ascribed to surfactant molecules also disappeared in the FTIR spectrum(Fig.3)of MoS2-O-2.The results are consistent with our previous observation on the formation of quasi-molecular superlattices consisting of (CTAB)2Sy(y=1 or 2) and MoS2atomic layers.Therefore, we suggest that anion exchange reaction directly takes place in the heating process, because sulfur-related species could be released by the decomposition of (CTAB)2Sy(y=1 or 2)andin-situreact with O-dopants.The higher the heating rate, the faster the decomposition of (CTAB)2Sy,which results in a lower chance forin-situperforming anion exchange reaction.The O-doping amounts therefore linearly declined from 23.7% of precursor to 5.5% of MoS2-O-5 with the decreasing of heating rate.Both the surfactant molecules incorporated into MoS2atomic layers and the initial amount of O-doped onto precursors could dominate the final level of O-doping amounttogether in the annealing process.The O-doping amount finally approached a terrace to MoS2-O-5 and it cannot be further reduced, while the heating rate was lowered to 2 ℃·min-1.Based on thein-situanion exchange reaction, the amount of O has been successfully modulated by simply adjusting the heating rate.The finding could open a new pathway for tailoring the microstructures and composition of 3D hierarchical MoS2materials to further optimize their functionalities.
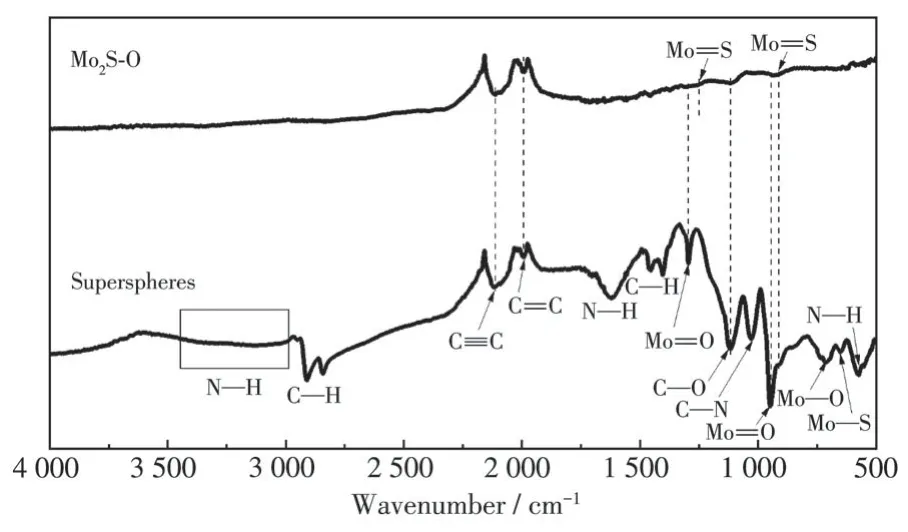
Fig.3 FTIR spectra of precursor super spheres and MoS2-O-2
The electrochemical performance of materials was evaluated in acid media to investigate the effect of the O-doping amount on their HER activities.Fig.4a shows the linear sweep voltammetry (LSV) curves of precursors,MoS2-O-2,MoS2-O-5,MoS2-O-10,and MoS2-O-20.Impressively, MoS2-O-2 and MoS2-O-5 electrodes had similar polarization profiles and both presented the highest current density in comparison with the precursors, MoS2-O-10, and MoS2-O-20 electrodes.The very close overpotentials of MoS2-O-2(246 mV)and MoS2-O-5(247 mV) at 10 mA·cm-2were the lowest, compared to MoS2-O-10 (259 mV), MoS2-O-20 (283 mV), and precursors (385 mV).Fig.4b shows the Tafel curves of the electrodes.Compared to precursor (184 mV·dec-1),MoS2-O-10 (102 mV·dec-1), and MoS2-O-20 (123 mV·dec-1),MoS2-O-2 and MoS2-O-5 also showed the lowest Tafel slopes (90 and 91 mV·dec-1, respectively).The TEM and HRTEM images (Fig.S15) showed the structural stability of MoS2-O-2 after the LSV tests.In addition,the MoS2-O-2 and MoS2-O-5 electrodes also exhibited superior catalysis activities in comparison with MoS2micro boxes consisting of single crystallineoriented MoS2nanosheets with overpotential of 300 mV at 10 mA·cm-2and Tafel slope of 134 mV·dec-1, as well as MoS2/C hollow spheres with overpotential of 479 mV at 10 mA·cm-2and Tafel slope of 274 mV·dec-1[27].The double-layer capacitance (Cdl) (Fig.4c)was also calculated from CV curves to estimate the electrochemically active surface area (ECSA) (Fig.S16)of the different electrodes.The MoS2-O-2 electrodes exhibited the largestCdl(5.7 mF·cm-2),and MoS2-O-20 delivered the lowestCdlvalue.The EIS spectra of the materials (Fig.4d) can help to further understand the electrode kinetics over a frequency range from 100 kHz to 0.1 Hz at an amplitude of 5 mV.The spectra all consist of a depressed semicircle of charge transfer resistance (Rct) in the high-frequency region and a slope of Warburg impedance (Zw) in the low-frequency region.The MoS2-O-2 electrode exhibited the lowestRct, compared to others with higher O-doping amounts,suggesting that a suitable O-doping amount can substantially boost the charge transfer from catalyst to electrode.The slope of the MoS2-O-2 electrode at the lowfrequency region was higher than 45°, indicating that MoS2-O-2 can store charges efficiently.The cycle stabilities of MoS2-O-2 electrodes were also evaluated by continuously conducting linear CV.The potential and current density of the electrodes all remained unchanged before and after 2 000 cycles at a scan rate of 20 mV·s-1(Fig.S17).The excellent long-term cycling stabilities of the electrodes can be attributed to the stable 3D hierarchical structures of the materials.The results indicate that the electrochemical performance of MoS2core-shell spheres is closely related to the O-doping amount,and it can be optimized and highly improved by tuning the O-doping amount and tailoring their 3D hierarchical structures.O-doping can affect the electrochemical performance of MoS2materials significantly.
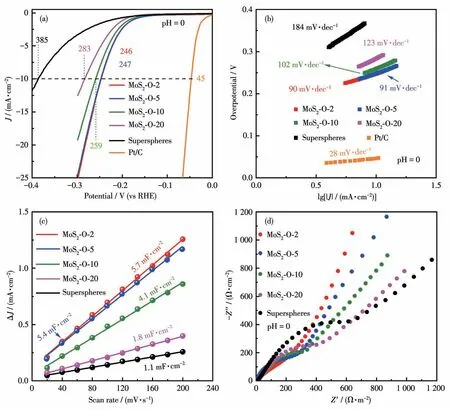
Fig.4 (a)LSV curves,(b)Tafel plots,(c)Cdl values,and(d)EIS spectra of materials
3 Conclusions
In summary, based on thein-situanion exchange reaction, the O - doping amount of MoS2core-shell spheres can be tuned dynamically by regulating the heating rate.The lower rates result in lower O-doping amounts.While it is still a challenge to thoroughly understand the mechanism of modulating the O-doping amount and further tune it to a lower level, this approach may open a novel pathway to modulate diverse dopants onto 3D hierarchical materials through creating molecular superlattices to performin-situreactions.
Supporting information is available at http://www.wjhxxb.cn

