Performance Improvement and Antibacterial Mechanism of BiOI/ZnO Nanocomposites as Antibacterial Agent under Visible Light
Jing Kong ,Jingui Zhang ,Sufen Zhang ,Juqun Xi ,Ming Shen ,3,*
1 Medical College,Yangzhou University,Yangzhou 225009,Jiangsu Province,China.
2 College of Chemistry and Chemical Engineering,Yangzhou University,Yangzhou 225002,Jiangsu Province,China.
3 Jiangsu Huicheng Medical Technology Co.,Ltd.,Yangzhou 225108,Jiangsu Province,China.
Abstract: Bacterial infections cause various serious diseases including tuberculosis,meningitis,and cellulitis.Moreover,there is an increase in the number of drug-resistant bacterial strains,which has caused a global health issue.Thus,it is highly essential to develop more effective antibacterial agents.Currently,zinc oxide (ZnO) is commonly used as an inorganic antibacterial agent,but with a notable limit in efficiency.In this work,to improve ZnO antibacterial activity under visible light,bismuth oxyiodide (BiOI) with a narrow bandgap of 1.8 eV was used as a suitable refinement to ZnO.Four different BiOI/ZnO nanocomposites were designed and synthesized via a simple mechanical stirring method in an atmospheric environment;these were denoted as BiOI/ZnO-2.5%,BiOI/ZnO-5%,BiOI/ZnO-10%,and BiOI/ZnO-20%.The successful synthesis of the BiOI/ZnO nanocomposites was verified through X-ray powder diffraction,energy-dispersive X-ray analysis,scanning electron microscopy (SEM),transmission electron microscopy (TEM),and X-ray photoelectron spectroscopy (XPS).A unique BiOI/ZnO heterojunction was also observed for the nanocomposites through high-resolution TEM,XPS,and selected area electron diffraction.Ultraviolet-visible diffuse reflectance spectroscopy revealed that all four BiOI/ZnO nanocomposites exhibited improved visible light absorption and possessed narrower bandgaps than the ZnO nanoparticles(nano-ZnO).Furthermore,the antibacterial activities of all BiOI/ZnO nanocomposites were investigated under visible light against both gram-positive and gram-negative bacteria strains.The results indicated a significant improvement in the antibacterial activities of BiOI/ZnO-10% and BiOI/ZnO-20% against both Staphylococcus aureus (S.aureus) and Escherichia coli (E.coli).Strong light exposure was found to be attributable to an increase in the antibacterial activity against S.aureus.In addition,the antibacterial mechanistic investigation was conducted upon visible light activation.The SEM images showed completely broken bacterial cell walls for both bacteria strains after treatment with the BiOI/ZnO nanocomposites.Hydroxyl radicals (•OH),which are strong reactive oxygen species,generated by the BiOI/ZnO nanocomposites under visible light,were also trapped by 5,5-dimethyl-1-pyrroline-N-oxide.Furthermore,zeta potential analysis revealed the presence of more positively charged BiOI/ZnO nanocomposite surfaces than the surfaces of nano-ZnO.The metal ions released from the BiOI/ZnO nanocomposites under visible light were also studied through inductively coupled plasma mass spectrometry.Based on the above results,BiOI/ZnO nanocomposites were found to exhibit antibacterial mechanism similar to that of nano-ZnO.In the dark,E.coli growth was only inhibited by Zn2+ released from both BiOI/ZnO nanocomposites and pure nano-ZnO.After visible light activation,•OH generated from the BiOI/ZnO nanocomposites mainly contributed to the bacterial cell death of both E.coli and S.aureus.This study proposes an effective strategy to enhance the antibacterial activity of nano-ZnO under visible light upon the formation of nanocomposites with BiOI.Besides,this study indicates that the ZnO-based nanocomposites can be used as a more effective antibacterial agent in clinical applications.
Key Words: BiOI/ZnO nanocomposites; Photocatalysis; Antibacterial activity; Visible light activation;Reactive oxygen species
1 Introduction
Microbial contaminations and infections threaten global health and environmental safety.These problems have become a worldwide challenge for all humans1–3.Antibiotics are active antimicrobial substances that can inhibit bacterial growth or kill bacteria.In general,there are two major types: organic antibiotics and inorganic antibiotics4.Organic antibiotics are more widely used for treating microbial infections in clinics and other areas5.The rising of organic-antibiotic-resistant bacterial strains reduces the accessible choices for the treatment of the corresponding infections6,7.After decades of application,it is able to detect organic antibiotics in the water supply.And the extremely low concentration of organic antibiotics in regular tap water may induce bacterial resistance after enough time of contact8,9.There is an urgent requirement for efficient and green antimicrobial agents and materials,especially antimicrobial materials which are capable of reducing the burden of harmful infections and pollution.
In recent years,photocatalytic nanoparticles attract lots of attention as promising inorganic antibacterial agents for their advantages including the wide antibacterial spectrum,no secondary pollution,and good stability10–13.Most of these photocatalytic nanoparticles can also work as good semiconductors14,15.Under ultraviolet (UV) light or visible light exposure,semiconductors can generate electrons and holes in the conduction band and the valence band,respectively.That is because the energy provided by the light is larger than the corresponding bandgap14.Once in contact with water or air,these electrons and holes can produce reactive oxygen species(ROS) including hydrogen peroxide (H2O2),hydroxyl ions(OH-),hydroxyl radicals (•OH),and superoxide anions (•O-2).ROS can break the microbial cell wall and cell membrane,and further cause cell content leakage and cell death16.Once these ROS pass through the cell membrane and cell wall,they also can inhibit cell growth and metabolism.As good oxidation reagents,ROS species can further oxidize and break down bacterial metabolites and intracellular substances.This process can also kill bacteria17.In general,ROS production is a key antibacterial mechanism of photocatalytic nanoparticles.Zinc oxide (ZnO) is a promising semiconductor that shows a wide range of applications: photocatalysts,sensors,UV lasers,and creams in the pharmaceutical and cosmetic industries.These various applications are related to its chemical stability,light stability,and being environmentally friendly18.The ZnO nanoparticles also show mild antibacterial activity against a wide range of bacteria strains after being activated by UV light19–21.The wide bandgap of the ZnO determines that to activate the antibacterial activity of the ZnO nanoparticles,more energy is required.Visible light cannot provide enough energy22.But UV light activation is not convenient for daily applications in clinics.A new type of visible light activatable ZnO-based nanoparticles with improved antibacterial activity against both Gram-negative and Gram-positive bacteria strains would provide more possible clinical applications.Doping or forming heterojunctions with other metals,metal oxides,and non-metallic materials can lead to bandgap narrowing or expansion23–25.Doping metals and non-metallic materials with low bandgaps can introduce impurity levels which can be a trap position for charge carriers and lead to narrowing the bandgap26.Forming composites with metal oxides (BiOI27,28and Cu2O23) can also lower the bandgap energy and increase the corresponding absorbance under visible light29,30.
For a long time,bismuth-based organic drugs (bismuth subsalicylate,colloidal bismuth subcitrate,and colloidal bismuth tartrate) have been used in clinics for the treatment ofHelicobacterpylori(H.pylori) infections.At the same time,the broad antibacterial activity of bismuth is also reported31.It is believed that once enough bismuth enters the bacterial cell,the bismuth would inactivate proteins that are essential for pathogenic virulence32.Most of the time,the applications of these bismuth organic drugs are limited.Meanwhile,bismuthbased inorganic semiconductors attract lots of attention for their special electron structure,unique photocatalytic activity,and wide applications in other areas33–35.Dibismuth trioxide(Bi2O3),bismuth vanadate (BiVO4),bismuth titanate(Bi4Ti3O12),and bismuth oxyhalides (BiOX,X = Cl,Br,I) are bismuth-containing semiconductors with wide Bi-6s hybridized orbital.These materials are well-studied for their applications in the photocatalytic area33,35,36.Within these materials,bismuth oxyhalide (BiOX) is a type of chemical with a tetragonal matlockite layered crystal structure formed by four oxygen atoms and four halogen atoms surrounding one central bismuth ion33.Based on published articles,the bandgap of BiOX material decreases as the ionic radius of the halogen increases from chlorine to iodine37.Bismuth oxyiodide (BiOI) has the narrowest bandgap of around 1.8 eV and displays the strongest absorbance under visible light compared to all other BiOX35.Although BiOI shows the best visible light photocatalytic activity than other bismuth oxyhalides,the narrow bandgap limits its actual applications.Besides the narrow bandgap,the applications of BiOI are also affected by the high photo-induced electron-hole recombination rate and low electron transfer ability38.These shortages make the BiOI a suitable material for the composition formation with zinc oxide,an n-type semiconductor with a bandgap of around 3.5 eV39.Besides the physical and chemical properties,the antibacterial activities of BiOI-based materials also attract our attention during the design of ZnO-based visible light activable materials.Jamiletal.reported that nano-sized BiOI material can killEscherichiacoli(E.coli) after visible light activation40.The resultant composite would have a chance to show physical properties from both materials and a high chance to exhibit a synergistic impact on Gram-positive and Gram-negative bacteria under visible light27,41,42.
Based on the compatible bandgap values between ZnO and BiOI,a new set of photocatalytic antibacterialp-ntype BiOI/ZnO nanocomposites was designed and synthesizedviaa mechanical stirring method.The obtained BiOI/ZnO-2.5%,BiOI/ZnO-5%,BiOI/ZnO-10%,and BiOI/ZnO-20% nanocomposites were first characterized with X-ray powder diffraction (XRD),energy dispersive X-ray (EDX) analysis,scanning electron microscopy (SEM),transmission electron microscopy (TEM),X-ray photoelectron spectroscopy (XPS),and high-resolution transmission electron microscopy(HRTEM).The optical properties of the BiOI/ZnO nanocomposites were also investigated.To estimate the antibacterial activity of BiOI/ZnO nanocomposites,Staphylococcusaureus(S.aureus) andE.coliwere selected as examples of the Gram-positive bacteria and Gram-negative bacteria,respectively.The corresponding evaluations were performed under simulated visible light.The antibacterial activity of the ZnO and the BiOI nanoparticles were also investigated to compare the activity improvement.Antibacterial activities of the BiOI/ZnO-10% nanocomposite under different strengths of simulated visible light were explored.The antibacterial mechanisms of the BiOI/ZnO nanocomposites were evaluated through morphology change,Zeta potential analysis,photocatalytic reactive oxygen species generation,and metal ion releasement analysis under simulated visible light.
2 Experimental
2.1 Regents and chemicals
Zinc oxide nanoparticles (nano-ZnO) were purchased from Paukert and used indirectly in all assays (Nanjing,China).Bismuth(III) nitrate pentahydrate (Bi(NO)3∙5H2O),potassium iodide (KI),sodium hydroxide (NaOH),and ethanol (EtOH)were purchased from Sinopharm Chemical Reagent Co.,Ltd.5,5-Dimethyl-1-pyrroline-N-oxide was purchased from Aladdin® (Shanghai) Co.,Ltd.Tryptone and yeast were used as the analytic grade.The bacteria strains covered in this paper were purchased from the microbiology department of Yangzhou University.All water used in all analyses was deionized water.
2.2 Preparation of the BiOI/ZnO nanocomposites
0.074 g of Bi(NO)3∙5H2O was dissolved in 50.0 mL of water.After stirring and fully dissolving,another 0.038 g of KI was added to the same beaker.After 30 min of stirring,the viscous and clear gel of BiOI was formed.Then,0.50 g of ZnO nanoparticles was added to 25.0 mL of water to form a ZnO nanoparticles suspension.The ZnO nanoparticle suspension was slowly added to the BiOI gel.After 1 h of stirring,the resultant solution was vortexed to obtain the pellet.The pellet was washed with water and ethanol six times.The BiOI/ZnO nanocomposites were obtained after drying out the pellet in the oven for 12 h at 60 °C.BiOI/ZnO-2.5%,5%,10%,and 20% samples were prepared according to this protocol with 2.5%,5%,10%,and 20% (molar percentage) of BiOI compared to the amount of ZnO nanoparticles used in this method.And the general process was also illustrated in Fig.1.
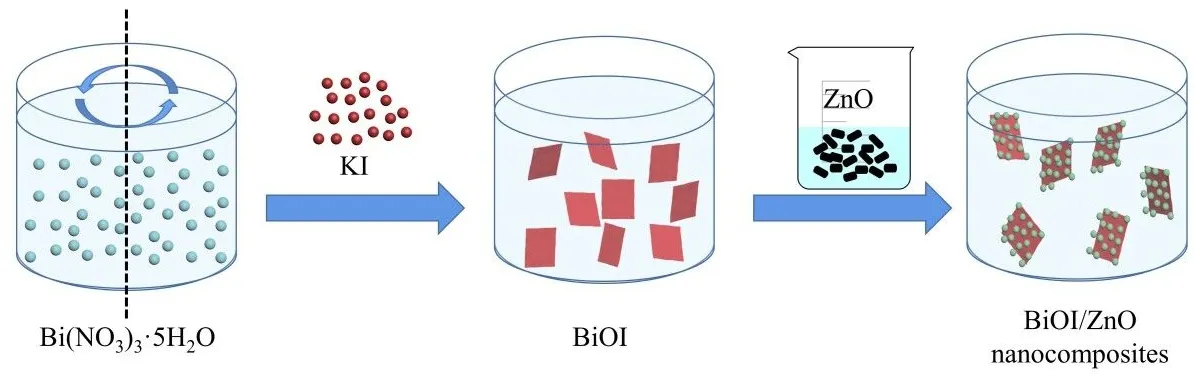
Fig.1 The general preparation scheme of BiOI/ZnO nanocomposites.
2.3 Characterization of BiOI/ZnO nanocomposites
The crystal phases of ZnO,BiOI,and BiOI/ZnO nanocomposite materials were recorded through the X-ray powder diffractometer (XRD,D8 Advance) within a 2θrange of 10°–80° at a wavelength (λ) of 0.15406 nm.Ultraviolet-visible diffuse reflectance spectroscopy (UV-Vis DRS,Cary 5000) was used to obtain the UV-Vis DRS spectrum utilizing BaSO4as a baseline.The measurement was done at a scanning range of 200–800 nm.Scanning electron microscopy (SEM,SUPRA 55),transmission electron microscopy (TEM,JEM-2100),and highresolution TEM (HRTEM,Tecnai G2 F30 S-TWIN) were utilized to depict the structure and morphology of BiOI/ZnO nanocomposite.The SEM (Gemini SEM 300,Carl Zeiss) was also applied to observe the morphology change of the bacteria.X-ray photoelectron spectroscopy (XPS,ESCALAB 250Xi,and Thermo Scientific,USA) was used to analyze the sample.The obtained binding energy was compared to the C 1sbinding energy at 284.8 eV.The electrochemical analyzer (CHI-760E,CH Instruments) was applied to perform the photocurrent analysis.The 250 W Xenon lamp was used as the light source with the 0.2 mol∙L-1Na2SO4solutions as the electrolyte solution.Electric spin resonance (ESR,Bruker A300-10/12) was utilized to capture the free radical signals.A Zeta potentiometer (Nano Series,Nano-ZS90) was used to measure the surface charge of the sample.The ion release and the doping amount of ions released from ZnO nanoparticles and BiOI/ZnO nanocomposites were measured by inductively coupled plasma mass spectrometer (ICP-MS) (ICP,Thermo,iCAPTM QC).
2.4 Preparation of bacterial stock solution
The bacterial stock solution was prepared and used fresh as follows:Staphylococcusaureus(S.aureus,29213) andEscherichiacoli(E.coli,44102) were first cultured on two different Luria-Bertani (LB) agar plates.After 14 h of culture,a single colony from each plate was transferred to 5.0 mL of Lysogeny broth (LB) medium for incubation under 37 °C shaking at 150 r∙min-1.After 16 to 18 h,100 μL of the bacteriacontaining LB medium was transferred to 900 μL of fresh LB medium.The resulting broth was cultured for another 2 to 3 h to obtain a final optical density (O.D.) value of around 0.7 at 600 nm.
2.5 Antibacterial evaluations of BiOI/ZnO nanocomposites
The antibacterial abilities of different BiOI/ZnO nanocomposites againstS.aureusandE.coliwere determinedviathe number of colony-forming units (CFUs) by the plate counting method.Briefly,the strains were transferred to LB liquid medium and further incubated in the shaker overnight at 37 ± 1 °C.After which the bacteria (O.D.= 0.7–0.8) were diluted 10 times to keep the final concentration of bacteria in the range of 1 × 107CFU∙mL-1.Then take 100 μL culturedE.coliorS.aureusstock solution,as well as 500.0 μg∙mL-1of BiOI/ZnO nanocomposites,were dispersed into 0.1 mol∙L-1autoclaved sodium acetate solution (pH = 4.55) and incubated for 30 min at room temperature.The Xenon lamp (CEL-HXF300) was used as the visible light source for testing groups.The control group was tested without any nanocomposites.After incubation,100 μL of the resulting suspension was spread evenly on a solid agar plate.After 15 h of incubation,the number of bacterial colonies on each plate was determined as the colony-forming units (CFUs).
To investigate how light strength affects the antibacterial activity of the nanocomposites,two different Xenon lamps (140 and 210 W) were used as the light source.Both two Xenon lamps were worked under the same 14 V working voltage.For the 140 W Xenon lamp,the 10 A electric current was applied.While the 15 A electric current was applied to the 210 W Xenon lamp.And the bacterial culture was performed as described before onS.aureus.The control group was tested without any treatment.
2.6 Bacteria morphology change investigation
The morphology change of treated bacteria was observed by scanning electron microscope.The control group was tested without adding any nanocomposites.First,the bacterial suspensions were treated with BiOI/ZnO nanocomposite for 30 minutes and fixed with 2.5% glutaraldehyde overnight.The bacterial cells were further dehydrated with the sequential treatment of 30%,50%,70%,80%,90%,and 100% ethanol for 15 min then dried by critical point drying.Finally,the dried bacteria were sputter-coated with gold for imaging using scanning electron microscopy.
2.7 Zeta potential analysis
The Zeta potentiometer (Nano Series,ZEN 3690,Malvern Instruments Ltd.,UK) was used to measure the surface charge of the ZnO nanoparticles and BiOI/ZnO nanocomposites.A 5.0 mg sample was dissolved in 5.0 mL ultra-pure water to obtain the 1.0 mg∙mL-1solution.From which 1.0 mL was transferred into the potential sample pool,and the Zeta potential was measured with a Zeta potentiometer.
2.8 Photocatalytic reactive oxygen species (ROS)generation analysis
The CHI-760E electrochemical analyzer was applied to measure the photoelectrochemical ability of the BiOI/ZnO nanocomposites and ZnO nanoparticles.A saturated calomel electrode was used as the counter electrode,the platinum plate was used as the reference electrode.0.20 mol∙L-1Na2SO4aqueous solution was used as the electrolyte.
All the ESR analyses were performed at room temperature according to a general procedure as follows: glass capillary tubes containing BiOI/ZnO nanocomposites or ZnO nanoparticles(1.0 × 10-2mol∙L-1,50.0 μL) were inserted into the electron spin resonance (ESR) cavity to record different signals under visible light exposure or at dark.The 5,5-dimethyl-1-pyrroline-N-oxide(DMPO) served as the spin trap for ROS species.
2.9 Metal ion releasement analysis
The inductively coupled plasma mass spectrometry (ICP-MS)was utilized to test the contents of Zn2+and Bi3+released from ZnO nanoparticles and BiOI/ZnO nanocomposites.The 0.020 g sample was dispensed in 4.0 mL NaAc (pH = 4.55) buffer solution and placed in a 37.0 °C shaker.Then,a 0.020 g sample was also placed in neutralized water for shaking at 37.0 °C.After shaking at 150 r∙min-1for 30 min,both mixtures were centrifuged.The resulting two supernatants were transferred to measure the ion release.
3 Results and discussion
3.1 SEM and TEM analysis
The morphology of the BiOI,the ZnO,and the synthesized BiOI/ZnO-10% nanocomposites was observedviaSEM,TEM,and HRTEM.Fig.2a shows the SEM micrograph of the purchased ZnO nanoparticles.From this picture,irregularshaped ZnO nanoparticles are observed with an average 30 nm diameter.A typical flake morphology of the synthesized BiOI flakes is observed in Fig.2b.From the SEM micrograph (Fig.2c),the BiOI/ZnO-10% nanocomposites exhibit a complicated morphology that tiny ZnO nanoparticles are scattered on the BiOI flakes.To further explore the detailed morphology of the BiOI/ZnO-10% nanocomposites,TEM and HRTEM is also utilized.The HRTEM micrograph of the BiOI/ZnO-10%nanocomposite sample is shown in Fig.2d.It is very obvious that the BiOI flakes and the ZnO nanoparticles are binding closely to each other.And this result is also proved by the SEM result (Fig.2e).Both HRTEM and SEM micrographs can verify the successful formation of the nanocomposite structure.In Fig.2e,the two lattice structures of the ZnO and the BiOI are also observed.The 0.26 nm lattice fringe spacing is corresponding to the (002) facet of the ZnO.And the 0.28 nm lattice fringe spacing is corresponding to the (110) facet of the BiOI.At the same time,a distinct interface is clearly observed which indicates the tightened combination between the ZnO nanoparticle and the BiOI flake.The selected area electron diffraction (SAED)micrograph of the BiOI/ZnO-10% sample at the specific area is shown in Fig.2f.The uniform and evenly spread SAED pattern indicates that the ZnO nanoparticles and the BiOI flakes are tightly attached.The typical bright ring SAED pattern is observed.And the d-spacing values match with thed-spacing values of the (100),(002),(101),(102),(110),and (103) crystal planes of the ZnO43.The evenly spread spotty pattern also matches with the d-spacing values of the (110) and (200) crystal planes of the BiOI44.Overall,this new type of BiOI/ZnO nanocomposites shows good crystallinity.

Fig.2 SEM micrographs of different samples.
Noticeably,the unevenly sized ZnO nanoparticles were observed in Fig.2a.Since the ZnO nanoparticles were utilized directly after purchase,the obtained BiOI/ZnO composites kept the uneven ZnO particles.As the size of the ZnO nanoparticles decreases,the corresponding antibacterial activity actually increases20.Eventually,the uneven ZnO particles may affect the general antibacterial activity of BiOI/ZnO composites against both Gram-negative and Gram-positive bacteria strains.
3.2 EDS and XRD analysis
Fig.3a shows the EDS images of the BiOI/ZnO-10%nanocomposites.From the image,the Bi,I,O,and Zn elements are evenly spread within the BiOI/ZnO-10% sample.This result indicates that the surface chemical elements of the synthesized sample are the same as the designed BiOI/ZnO nanocomposites.The XRD analysis is also performed to further verify the obtained BiOI/ZnO samples.And the result of XRD patterns of the ZnO nanoparticles,BiOI flakes,BiOI/ZnO-2.5%,BiOI/ZnO-5%,BiOI/ZnO-10%,and BiOI/ZnO-20% samples are shown in Fig.3b.With only 2.5% of BiOI in the BiOI/ZnO-2.5% nanocomposites,visible BiOI diffraction peaks are detected.In the BiOI/ZnO-2.5% XRD patterns,the diffraction peaks at 2θ= 31.76°,34.42°,36.25°,47.53°,56.59°,62.85°,66.37°,67.94°,and 69.08° are observed.These diffraction peaks are ascribed to the (100),(002),(101),(102),(110),(103),(200),(112),and (201) crystal planes of hexagonal single-phase wurtzite structure of ZnO,respectively (JCPDS No.99-0111).On the other side,the rest diffraction peaks at 2θ= 29.65°,31.66°,37.06°,39.37°,45.38°,51.34°,and 55.15° are also observed.These diffraction peaks are ascribed to the (102),(110),(103),(004),(200),(114),and (212) crystal planes of the standard tetragonal BiOI (JCPDS No.10-0445).From the XRD patterns,impurity diffraction peaks belonging to Bi2O3and BiI3are not found.This result indicates that the synthetic method is highly selective which only leads to the formation of BiOI.Comparing the XRD patterns of all four BiOI/ZnO nanocomposite samples,as the amount of BiOI increases from 2.5% to 20%,the intensity of diffraction peaks ascribed to the BiOI also increases.At the same time,the intensity of ZnOrelated diffraction peaks decreases.For the BiOI/ZnO-20%sample,there is only very weak ZnO-related diffraction peaks are observed.This kind of diffraction peak intensity change may be due to the physical nature of the BiOI and ZnO particles.The BiOI particles usually have good crystallinity and strong diffraction peaks with relatively large particle sizes.While the ZnO particles have a relatively smaller particle size,wider full width at half maxima (FWHM),and relatively weaker diffraction peak intensity.With the EDS and XRD results,the existence of BiOI and ZnO in the BiOI/ZnO nanocomposites is verified.

Fig.3 EDS and XRD results of the following samples.
3.3 XPS analysis
To further prove that the synthesized sample is obtained as designed,the XPS analysis was performed to check the surface element on the synthesized BiOI/ZnO nanocomposite sample.The full XPS survey spectra of the BiOI/ZnO-10% sample reveals the existence of Zn,O,Bi,and I element.In the Bi 4fhigh-resolution spectra (Fig.4b),the two strong peaks at 159.20 and 164.48 eV are assigned to Bi 4f7/2and Bi 4f5/2of Bi3+45.While the Zn 2phigh-resolution spectra (Fig.4c) show two peaks at 1021.5 and 1044.6 eV which are assigned to Zn 2p3/2and Zn 2p1/2.As shown in Fig.4d,the two peaks at 618.78 and 630.28 eV are attributed to the I 3d5/2and I 3d3/2of the I46.In the high-resolution XPS spectra of O 1s,the stronger peak at 530.6 eV is assigned to the O which coordinates with Zn in the ZnO.While the weaker peak at 532.5 eV is due to the ZnO’s absorption of oxygen in the hypoxic region47.Another weak peak at 529.78 eV is assigned to the lattice oxygen in the BiOI48.Based on the XRD and the XPS results,the obtained nanocomposites are a combination of the ZnO nanoparticles and the BiOI flakes.
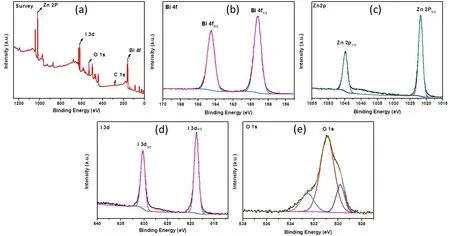
Fig.4 XPS spectra of the BiOI/ZnO-10% sample.
3.4 Optical property evaluation
The UV-Vis DRS analysis was applied to evaluate the optical properties of ZnO,BiOI/ZnO-2.5%,BiOI/ZnO-5%,BiOI/ZnO-10%,BiOI/ZnO-20%,and the BiOI flakes.All four BiOI/ZnO nanocomposites show better absorption under UV light and visible light than the ZnO nanoparticles (Fig.5a).Nanocomposites with more BiOI show better absorption under visible light compared to the absorption under UV light.This result indicates that the as-synthesized BiOI/ZnO nanocomposites show better visible light absorption.Interestingly,the BiOI sample shows an absorbance between the BiOI/ZnO-5% and BiOI/ZnO-10% nanocomposite sample under visible light.This result indicates that the BiOI/ZnO nanocomposites have a combined visible light absorption ability(BiOI) and UV light absorption ability (ZnO).At the same time,the color of the obtained nanocomposites gets darker as the amount of BiOI increases during the preparation.This color change also gives a hint that adding more BiOI into the nanocomposites can increase visible light absorption.

Fig.5 Optical property evaluation results.
UV-Vis DRS analysis was also performed to calculate the bandgap of the ZnO,BiOI/ZnO-2.5%,BiOI/ZnO-5%,BiOI/ZnO-10%,BiOI/ZnO-20%,and the BiOI samples.Bandgap energy gives information about how much energy needs to activate electrons of the materials from the valence band to the conduction band.The result of the UV-Vis DRS spectroscopy is shown in Fig.5b.And the corresponding bandgap energy of each sample was calculated according to Tauc’s equation49:αhν=A(hν-Eg)n/2. From which,theα,h,ν,A,andEgare the absorption coefficient,the Planck constant,light frequency,a constant,and bandgap energy,respectively.The exponent ‘n’ is determined by the type of transitions and usually has a certain value either equal to 1 (direct semiconductor) or 4 (indirect semiconductor).ZnO and BiOI are both indirect semiconductors,then= 4 is used directly in equation50.The calculated bandgap of the ZnO sample,the BiOI sample,BiOI/ZnO-2.5%,BiOI/ZnO-5%,BiOI/ZnO-10%,and BiOI/ZnO-20% nanocomposites are 3.14,1.84,3.06,3.01,2.97,and 2.94 eV,respectively.The BiOI/ZnO nanocomposites exhibit bandgap energy between the ZnO and the BiOI particles.The lower bandgap energy is observed for nanocomposites with a higher amount of BiOI.BiOI/ZnO-20% nanocomposites have a bandgap energy of around 2.94 eV and show the largest absorbance under visible light.From these results,the bandgap energy gets lower for samples with more amount of BiOI.Lower bandgap energy indicates the specific material requires lower energy to be activated.This is corresponding to the nanocomposites’ absorption increasing trend under visible light.
3.5 Antibacterial activity evaluation against S.aureus
To evaluate the antibacterial activity of the obtained series of BiOI/ZnO nanocomposites,the colony-forming units per milliliter (CFU∙mL-1) were used as the standard to compare the specific antibacterial activity.The different antibacterial activities of the synthesized BiOI/ZnO nanocomposites,ZnO nanoparticles,and BiOI flakes were evaluated either with simulated visible light (Xenon lamp) or without any light againstS.aureus.The results were shown in Fig.6.As observed in Fig.6b,there were quite a lot of colonies on each petri dish after being treated with different samples.The CFU∙mL-1was converted to the logarithm of CFU∙mL-1(lg(CFU∙mL-1)) for easier comparison.Without light activation,the treated groups(Fig.6a),including ZnO,BiOI,and the BiOI/ZnO nanocomposites,show no antibacterial activity compared to the none treated control group.The number of lg(CFU∙mL-1) of the ZnO and BiOI particles shows no change before and after the visible light exposure.This result indicates that visible light exposure cannot activate both ZnO and BiOI particles.The antibacterial activity of the obtained BiOI/ZnO nanocomposites shows obvious improvement after visible light activation.The BiOI/ZnO-20% treatment group shows a 3.5 lg(CFU∙mL-1)reduction from 6.4 to 2.9 lg(CFU∙mL-1) after visible light exposure.This is the highest lg(CFU∙mL-1) reduction within four BiOI/ZnO nanocomposites.A 2.1 lg(CFU∙mL-1) reduction is observed for the BiOI/ZnO-2.5% treatment group.Comparing all four nanocomposites,the antibacterial activity increases as the amount of BiOI increases during the nanocomposite preparation after visible light activation.Meanwhile,these four BiOI/ZnO nanocomposites show better antibacterial activity than ZnO and BiOI particles under visible light.This result reveals that the heterojunction forming between BiOI and ZnO can improve antibacterial activity.And this improvement can be activated by visible light.
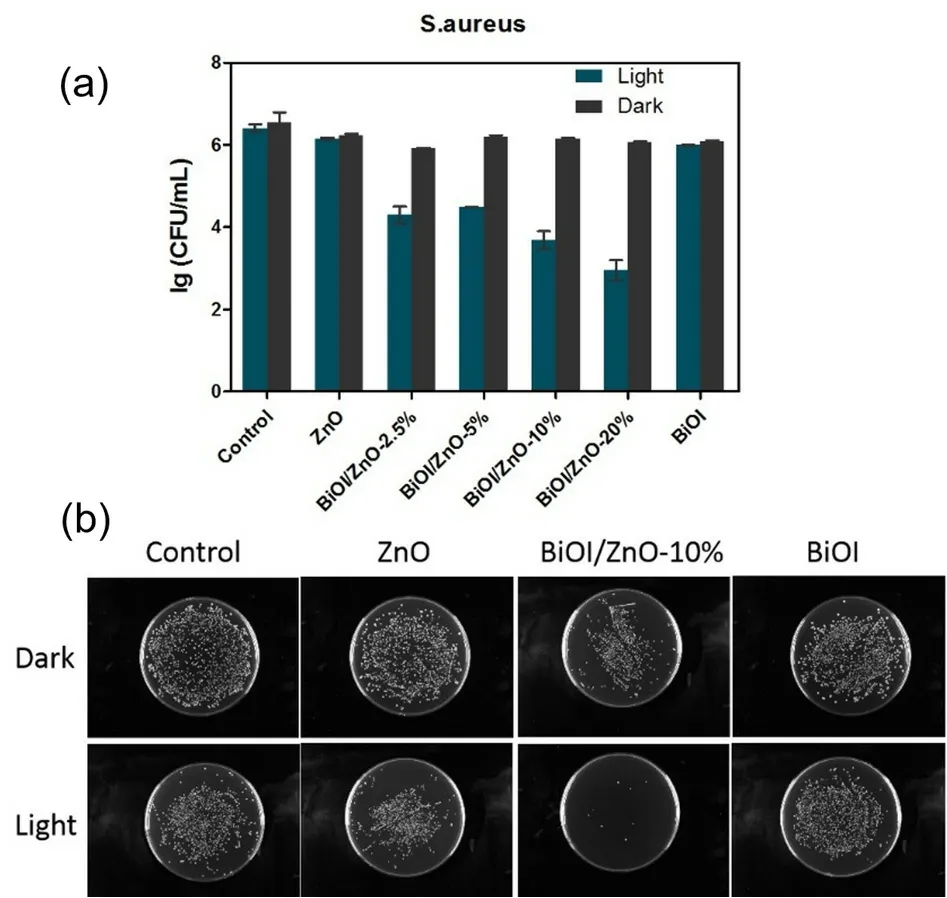
Fig.6 The antibacterial activity of the following samples against S.aureus.
3.6 Antibacterial activity evaluation against E.coli
E.coliwas selected as the representative Gram-negative bacterial strain for the evaluation of the antibacterial activity of the synthesized nanocomposites.And the results were shown in Fig.7.Without any light activation,the ZnO and different BiOI/ZnO nanocomposites show antibacterial activity againstE.colias compared to the control group.In contrast,the BiOI particles did not show antibacterial activity in the dark.While after simulated visible light activation,improved antibacterial activities are observed for the ZnO nanoparticles and different BiOI/ZnO nanocomposites.A similar situation is also observed for the BiOI flakes.The BiOI/ZnO-10% and BiOI/ZnO-20%nanocomposites show similar antibacterial activity to the ZnO nanoparticles which are around 2.0 lg(CFU∙mL-1) reduction before and after the visible light activation.Around 1.3 lg(CFU∙mL-1) reduction is observed for the BiOI particles.Overall,the BiOI/ZnO-20% composites show better antibacterial activity than the BiOI flakes and a slightly improved antibacterial activity as compared to the ZnO nanoparticles againstE.coli.A decreasing antibacterial activity trend is observed as the percentage of BiOI in the BiOI/ZnO composites also decreases.This result is due to the low antibacterial activity of the BiOI component40.At the same time,the antibacterial activity of the BiOI/ZnO composites is also influenced by visible light activation.The presence of 20% of BiOI in BiOI/ZnO composites is sufficient to supplement the antibacterial effect of reduced ZnO compared with the ZnO nanoparticles.
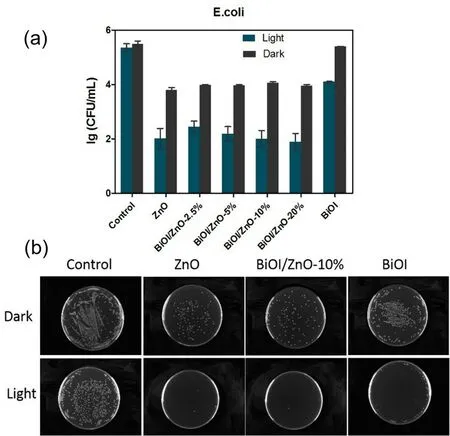
Fig.7 The antibacterial activity of the following samples against E.coli.
As reported the antibacterial mechanism of ZnO nanoparticles involves: (1) direct contact between the ZnO nanoparticles and the bacterial cell wall,(2) the cell integrity change after membrane damage,(3) the releasement of the Zn2+ions,(4) the ROS generation51.ForS.aureus,the ZnO nanoparticles do not show any antibacterial activity without visible light.While a different result is observed on theE.colitest,the ZnO nanoparticles show antibacterial activity againstE.coli.Jianget al.reported that the membrane damage caused by metal ions plays an important role in killingE.coli52.The Zn2+ion released from the ZnO nanoparticles can influence bacterial active transportation,amino acid metabolism,and enzyme system disruptions51.And theE.colibacterial cell wall is more negatively charged than theS.aureusbacteria53.The more negatively chargedE.colicell wall may have a stronger electrostatic interaction effect on the Zn2+ions which would induce theE.colicell wall damage.In this assay,the ZnO nanoparticles and the BiOI/ZnO nanocomposites show similar antibacterial activity againstE.coliin the dark.That is because the release of the zinc ions from the BiOI/ZnO nanocomposites may not be affected by the formation of the heterojunction.A similar amount of Zn2+would have a high chance to produce a similar antibacterial activity againstE.coli.While the cell wall ofS.aureusis less negatively charged and has less strong electrostatic interaction with the Zn2+ions.This may be the reason that both ZnO nanoparticles and BiOI/ZnO nanocomposites show no antibacterial activity againstS.aureuswithout visible light.
3.7 Effect of light strength on the antibacterial activity
From theS.aureusantibacterial evaluation results,the BiOI/ZnO nanocomposites can be activated by the simulated visible light.To further investigate whether the light strength affects the antibacterial activity of the BiOI/ZnO nanocomposites,the antibacterial test onS.aureuswas performed with two different Xenon lamp light sources with 30 min of light exposure.The BiOI/ZnO-10% nanocomposites were selected for this analysis for their good antibacterial activity similar to the BiOI/ZnO-20% nanocomposites.For economic consideration,the BiOI/ZnO-10% nanocomposites would be more suitable for further evaluation.
The results were shown in Fig.8.From the none treatment control group,the light intensity increase can increase the antibacterial activity a little bit.This result may be due to the increased bacterial ROS production in the cell after stronger light exposure16.The BiOI/ZnO-10% nanocomposites decrease theS.aureusgrowth from 5.25 lg(CFU∙mL-1) to 3.63 lg(CFU∙mL-1)under a 140 W Xenon lamp at 10 A working current.A larger reduction,around 2.11 lg(CFU∙mL-1),is observed whenS.aureusis treated with the same BiOI/ZnO-10% nanocomposites under a 210 W Xenon lamp.Based on this result,the light intensity increase can slightly affect the antibacterial activity of the BiOI/ZnO nanocomposites.One reason for this result is the formation of the electron-hole pair.The stronger light means a stronger energy which can prompt the generation of electronhole pair.Electron-hole pair generation can also induce ROS production,which is a key bacterial killing mechanism27.This result also indicates that photocatalytic ROS production may be an important antibacterial mechanism of the BiOI/ZnO nanocomposites.
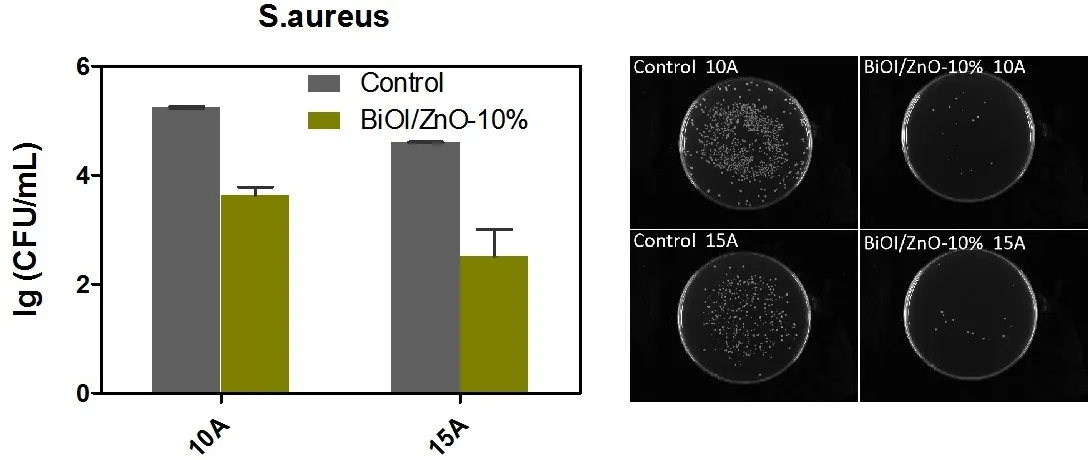
Fig.8 The antibacterial results of BiOI/ZnO-10% nanocomposites against S.aureus under two different Xenon lamps at two different working currents: 10 A (140 W) and 15 A (210 W).
3.8 Antibacterial mechanistic exploration
As mentioned before,many different mechanisms of action are related to the ZnO nanoparticles.Photocatalytic ROS generation,ion releasement,nanoparticles’ indirect contact with the bacterial cells,and cell wall damage are four important mechanisms that affect the antibacterial activity of the ZnO nanoparticles51,54.It is necessary to check which of these mechanisms are also involved in the BiOI/ZnO nanocomposites antibacterial process.
3.8.1 Bacterial morphology change
Since cell wall damage is an important antibacterial mechanism of the ZnO nanoparticles,it is worth checking whether the BiOI/ZnO nanocomposites have the same cell wall damage ability as the ZnO nanoparticles.TheS.aureusandE.coliwere first treated with the BiOI/ZnO-10% nanocomposites.After 30 min of visible light activation,the morphology changes ofS.aureusandE.coliwere monitored.The untreated cell morphology was also monitored and shown in Fig.9a and 9b.It is very clear that without any treatment,theS.aureuscells show a round shape and theE.colicell shows a typical rod shape.After being treated for 30 min under visible light,theS.aureuscell wall loses its original shape.Rough,wrinkled,dented,and/or damagedS.aureuscell walls are observed (Fig.9b).Similar situation is also observed in theE.colitreatment group.In Fig.9d,theE.colicell walls become distorted,reformatted,and seriously ruptured after treatment.Interestingly,the nanocomposites are found attached to both cell walls in Fig.9b and Fig.9d.This can prove that the contact between the cell wall and the nanocomposites may play an important role in killing both bacteria.

Fig.9 The SEM pictures of the following samples.
3.8.2 Zeta potential analysis
Since the nanocomposites can attach to bothS.aureusandE.colicell walls,it is necessary to investigate whether the contact between the nanocomposites and the bacterial cell walls is caused by the surface charges of the nanocomposites.The Zeta potential analyzer was applied for the measurement.As shown in Fig.S1 (Supporting Information),the surface charge of the ZnO nanoparticles is positively charged and the value is around+13.3 mV.The BiOI/ZnO nanocomposites have a more positively charged surface with a value of around +18.9 mV(Fig.S1 in Supporting Information).While the surface charge of the BiOI particles is around -6.99 mV which is a negative charge.The bacterial cell wall is usually negatively charged55.The positively charged nanoparticles can attach to the cell wallviaelectrostatic interaction.The more positively charged BiOI/ZnO nanocomposites can attract more bacterial cells and lead to greater antibacterial activity.Based on this result,the electrostatic deposition of the BiOI/ZnO nanocomposites on the surface of the bacteria is another important factor leading to the enhancement of antibacterial activity.
3.8.3 Photocatalytic reactive oxygen species generation analysis
The photocatalytic reactive oxygen species generation activity is an important mechanism of action of nanoparticles that affect the antibacterial activity.At the same time,the photocatalytic activity of nanoparticles is a key physical property that determines the degradation ability of organic pollutants56.Based on the UV-Vis DRS spectra,the BiOI/ZnO nanocomposites can absorb both UV light and visible light.From the light strength evaluation analysis,the results indicate that the antibacterial activity of the BiOI/ZnO nanocomposites improved after visible light activation.Through the quantitative analysis of the photocatalytic degradation activity on rhodamine B (RhB),the BiOI/ZnO nanocomposites exhibit improved photocatalytic activity under simulated visible light than the ZnO nanoparticles(Fig.S2b in supplementary information).Around 98% of the RhB was degraded by the BiOI/ZnO sample after 90 min of simulated visible light exposure (Fig.S2c in Supporting Information).The detailed analysis protocol and results of the rhodamine B degradation assay were shown in the supplementary information.
As a good photocatalytic catalyst,the BiOI/ZnO nanocomposites can store energyviathe production of photogenerated charge carriers and electron-hole pairs once activated by visible light.Then,the electron-hole pairs generated by photoactivation interact with the water (H2O) or free oxygen(O2) on the surface of the nanocomposites.Through a series of redox reactions,different reactive oxygen species (ROS)including hydroxyl radicals (•OH),hydroxyl peroxide (H2O2),and superoxide (•O-2) are generated57.The photocatalytic ROS generation ability of the BiOI/ZnO nanocomposites was investigated in two parts: the generation of the photogenerated charge carriers and the capture of the ROS species.The production of photocurrents was investigated as a way to evaluate the separation efficiency of photogenerated charge carriers.As shown in Fig.10a,the transient photoelectric response curve of the ZnO,BiOI,and different BiOI/ZnO nanocomposite samples are present.From the curves,all samples have a relatively stable current density which indicates all these samples have a stable photoelectric ability.For different BiOI/ZnO nanocomposites,the photocurrent intensity of the nanocomposites gets stronger as the amount of BiOI in the BiOI/ZnO nanocomposites increases.The curves also indicate the increased light absorption ability as the nanocomposites’BiOI-containing amount increases.The BiOI and the ZnO form ap-ntype heterojunction which can prompt the separation of the photogenerated charge carriers.Normally,the more BiOI,the more heterojunction formation which leads to more photogenerated charge carriers.And the results in Fig.10a also agree with it.The only exception is the BiOI/ZnO-20% (mol percentage) sample which shows a lower current density than the BiOI/ZnO-10% sample.It may be due to the excessive BiOI on the ZnO surface hindering the transfer of photoelectrons between ZnO and BiOI.Another possible reason for this phenomenon is that BiOI may reduce the oxidation reaction site on the ZnO surface58.All BiOI/ZnO nanocomposites have higher current density than the ZnO nanoparticles which is corresponding to the increased antibacterial activity.This shows that the photocatalytic activity increase may be a factor that affects the antibacterial activity against both Gram-negative and Gram-positive bacteria.

Fig.10 Photocatalytic activity of the BiOI/ZnO nanocomposites and the control samples.
To investigate the obtained BiOI/ZnO nanocomposites generate which of the ROS species,electron spin resonance(ESR) spectra were performed to capture the free radicals with the simulated visible light.As shown in Fig.10b,no signals are captured for the BiOI/ZnO nanocomposites and the ZnO nanoparticles in the dark.With the visible light activation,only the DMPO-•OH signals are captured for ZnO and BiOI/ZnO nanocomposites (Fig.10b).No other ROS species signals are captured.The BiOI/ZnO nanocomposites show stronger intensity peaks than the ZnO nanoparticles.And the peak intensity actually increases as more BiOI is added to the nanocomposite.The samples’ peak intensities are also corresponding to the photocurrent analysis results.Through this analysis,the BiOI/ZnO nanocomposites can generate hydroxyl radicals.ZnO nanoparticles only generate a minimum amount of hydroxyl radicals which correlates to the less active degradation of the RhB.While the BiOI/ZnO-10% and the BiOI/ZnO-20%nanocomposites can produce much more hydroxyl radicals.And this phenomenon matches up with the 99% degradation of the RhB.This result indicates that the hydroxyl radicals may contribute to the photocatalytic degradation of RhB.
ROS species are known to affect bacterial growth by attacking the bacterial cell wall.And this process can further lead to bacterial cell death54.Here,the hydroxyl radicals produced by the BiOI/ZnO nanocomposites can also degrade the bacterial cell wall through a series of chain redox reactions.The intensity of the DMPO-•OH signals of different nanocomposites correlates to the general antibacterial activity against bothS.aureusandE.coli.This result indicates that photocatalytic ROS production is another important mechanism of action of the BiOI/ZnO nanocomposites.
3.8.4 Metal ion releasement analysis
The metal ion released from the nanoparticles is another important antibacterial mechanism.Metal ions like Zn2+and Bi3+can actually increase the growth of microbes at low concentrations.The reduction of microbial growth could only be observed under a high concentration of metal ions including Zn2+and Bi3+51.The inductively coupled plasma mass spectrometry(ICP-MS) was applied to measure the Zn2+and Bi3+released from ZnO nanoparticles and BiOI/ZnO nanocomposites at both neutralized solution and acidic solution.In the neutralized ultrapure water,no metal ion releasement was detected after long-time exposure.While the detailed results of the acidic condition are shown in Table S1 (included in Supporting Information).The highest Zn2+concentration is measured from the BiOI/ZnO-2.5% sample.The BiOI/ZnO-10% sample shows a similar Zn2+releasement as the ZnO nanoparticles.And the BiOI/ZnO-10% sample can release the largest concentration of Bi3+among all test samples.The BiOI/ZnO-20% sample shows the best antibacterial activity against bothS.aureusandE.coliunder visible light.But,the metal ion release ability of the BiOI/ZnO-20% sample is not the best.This phenomenon may be affected by the fully formed heterojunctions within the BiOI/ZnO-20% sample.As mentioned before,the Zn2+concentration affects the antibacterial activity of ZnO-based nanoparticles without visible light.Here the Zn2+released from the ZnO and different nanocomposites are within a similar level.This result is corresponding to the similar antibacterial activity of the ZnO and different nanocomposites under dark conditions.On the other hand,the Gram-negative bacterial growth is mainly affected by the Zn2+ion releasement.Generally,Zn2+ion releasement may not be the key factor affecting the corresponding antibacterial activity under visible light.
Overall,with the above antibacterial mechanistic explorations,the BiOI/ZnO nanocomposites exhibit similar antibacterial mechanisms as the ZnO nanoparticles except for the ROS production ability after visible light activation.The SEM photographs give details of the ruptured bacterial cell walls ofS.aureusandE.coliafter treatment under visible light.The more positively charged nanocomposite surface may contribute to the attachment between the nanocomposites and the bacterial cell wall.Once the BiOI/ZnO nanocomposites interact with the bacterial cell wallviathe electrostatic interaction,it can cause cell wall damageviathe photocatalytic generation of hydroxyl radicals (Fig.11).The metal ions (Zn2+,Bi3+) released from the BiOI/ZnO nanocomposites only affect the survival ofE.colicells rather than theS.aureuscells in the dark situation.

Fig.11 The proposed general mechanisms of action of the BiOI/ZnO nanocomposites under visible light.
4 Conclusions
Inorganic antibacterial agents are important for the rising of infectious diseases.BiOI/ZnO nanocomposites were designed and synthesized through a simple mechanical stirring method to obtain a type of ZnO-based antibacterial agent with improved antibacterial activity under visible light.After being characterized with XRD,EDX,SEM,TEM,and XPS,the obtained nanocomposites were proven to be pure without any impurities.With the UV-Vis DRS results,the decreasing bandgaps of the ZnO,BiOI/ZnO-2.5%,BiOI/ZnO-5%,BiOI/ZnO-10%,and BiOI/ZnO-20% were observed from 3.14,3.06,3.01,2.97 to 2.94 eV,respectively.Compared with the ZnO nanoparticles,the BiOI/ZnO nanocomposites exhibited enhanced visible light absorption as indicated by the UV-Vis DRS result.The increased antibacterial activity of the BiOI/ZnO nanocomposites was demonstrated by treatingS.aureusandE.coliwith visible light activation,while the pure ZnO nanoparticles and the pure BiOI flakes showed no or mild antibacterial activity against both bacteria strains.Contracted and ruptured bacterial cells were observed in both treated bacteria.Through different antibacterial mechanistic evaluations,the enhanced visible light activatable photocatalytic ROS production led to bacterial cell damage after the BiOI/ZnO nanocomposites contacted the bacterial cell walls.While the Zn2+and Bi3+released from the BiOI/ZnO nanocomposites could only play a role in killing both Gram-positive and Gramnegative bacterial strains without any light.In general,the design strategy of incorporating BiOI into the ZnO-based nanocomposites brings out an effective successful improvement of the antibacterial activity and the visible light absorption ability of the ZnO nanoparticles.At the same time,the obtained BiOI/ZnO nanocomposites have a high potential to be promising clinical antibacterial agents.Moreover,the simple synthetic method makes this nanocomposite convenient for industrial manufacturing.
Supporting Information: available free of chargeviathe internet at http://www.whxb.pku.edu.cn.
- 物理化学学报的其它文章
- Recent Advances in Self-Supported Transition-Metal-Based Electrocatalysts for Seawater Oxidation
- 用于高灵敏快速核酸检测的荧光碳点
- Introducing Novel,Multiple Cd Coordination Modes into Gold Nanoclusters by Combined Doping for Enhancing Electrocatalytic Performance
- 电催化二氧化碳还原催化剂、电解液、反应器和隔膜研究进展
- 利用多氟丙烯酸酯添加剂提升准二维钙钛矿发光二极管性能
- 电子自旋效应在电催化剂中的作用