C-reactive protein to albumin ratio predict responses to programmed cell death-1 inhibitors in hepatocellular carcinoma patients
Bai-Bei Li,Lei-Jie Chen,Shi-Liu Lu,Biao Lei,Gui-Lin Yu,Shui-Ping Yu
Abstract BACKGROUND Over the years,programmed cell death-1 (PD-1) inhibitors have been routinely used for hepatocellular carcinoma (HCC) treatment and yielded improved survival outcomes.Nonetheless,significant heterogeneity surrounds the outcomes of most studies.Therefore,it is critical to search for biomarkers that predict the efficacy of PD-1 inhibitors in patients with HCC.AIM To investigate the role of the C-reactive protein to albumin ratio (CAR) in evaluating the efficacy of PD-1 inhibitors for HCC.METHODS The clinical data of 160 patients with HCC treated with PD-1 inhibitors from January 2018 to November 2022 at the First Affiliated Hospital of Guangxi Medical University were retrospectively analyzed.RESULTS The optimal cut-off value for CAR based on progression-free survival (PFS) was determined to be 1.20 using x-tile software.Cox proportional risk model was used to determine the factors affecting prognosis.Eastern Cooperative Oncology Group performance status [hazard ratio (HR)=1.754,95% confidence interval (95%CI)=1.045-2.944,P=0.033],CAR (HR=2.118,95%CI=1.057-4.243,P=0.034) and tumor number (HR=2.932,95%CI=1.246-6.897,P=0.014) were independent prognostic factors for overall survival.CAR (HR=2.730,95%CI=1.502-4.961,P=0.001),tumor number (HR=1.584,95%CI=1.003-2.500,P=0.048) and neutrophil to lymphocyte ratio (HR=1.120,95%CI=1.022-1.228,P=0.015) were independent prognostic factors for PFS.Two nomograms were constructed based on independent prognostic factors.The C-index index and calibration plots confirmed that the nomogram is a reliable risk prediction tool.The ROC curve and decision curve analysis confirmed that the nomogram has a good predictive effect as well as a net clinical benefit.CONCLUSION Overall,we reveal that the CAR is a potential predictor of short-and long-term prognosis in patients with HCC treated with PD-1 inhibitors.If further verified,CAR-based nomogram may increase the number of markers that predict individualized prognosis.
Key Words: C-reactive protein to albumin ratio;Hepatocellular carcinoma;Programmed cell death-1 inhibitors;Prognosis;Nomogram
INTRODUCTION
Hepatocellular carcinoma (HCC) is one of the most common and difficult-to-treat cancers worldwide[1].According to the Global Cancer Statistics 2020,HCC ranks sixth in incidence and third in mortality among all cancers,with annually approximately 906000 newly diagnosed cases and 830000 deaths annually[2].Given the lack of specific symptoms and rapid progression,most patients with HCC are diagnosed at an advanced stage[3].Late diagnosis,limited therapeutic options and l high recurrence rate are the main reasons for the poor prognosis of HCC[4-6].
In recent years,immunotherapy for HCC at immune checkpoints has made remarkable progress,especially antibodies targeting programmed cell death-1 (PD-1) and programmed cell death ligand-1 (PD-L1) pathway have promoted the development of systemic comprehensive therapy for HCC[7-10].Immune checkpoint blockers targeting PD-1 have been approved for systemic integrative treatment of HCC with favorable clinical responses and survival benefits[11,12].However,because the efficacy of PD-1 inhibitors varies widely among individuals,only a small percentage of patients benefit from anti-PD-1 therapy[13].In clinical trials using Nivolumab and Pembrolizumab alone to treat patients with advanced HCC,the objective effective rate was only 17% to 20%[11,14].Moreover,Pembrolizumab's phase III trial failed to reach its main endpoint,suggesting the need to identify subgroups of patients most likely to benefit from PD-1 inhibitors[15].Immune checkpoint inhibitors are also extremely costly agents.Therefore,finding practical and robust prognostic predictors to identify HCC patients who may benefit from PD-1 therapy has recently become a research hotspot.
To date,the biomarkers that have been widely studied to predict the response of PD-1 inhibitors and improve the prognosis of tumor patients are tumor mutation load (TMB) and targeted PD-L1 expression[16-18].Many studies have demonstrated that TMB sequencing by a polygenic cancer panel or whole exome can predict the efficacy of PD-1 inhibitor therapy in patients with multiple types of cancer,but there is little data on TMB being meaningful in patients with HCC[19,20].The expression level of PD-L1 has been proved to be a stratification factor for random grouping of various cancer types or a selective marker for immuno-oncology subgroup analysis[21,22].The expression level of PD-L1 affects the function of T cells in tumor microenvironment,which is related to the prognosis of patients with HCC[14,23].However,because the detection of PD-L1 expression level is expensive,PD-L1 expression level as a marker has not been widely used in clinical practice.Recent studies have shown that the increase of inflammatory characteristics in HCC tumors is associated with improved response and overall survival (OS)[24].As peripheral blood T cells and tumor infiltrating lymphocytes have been found to be related to the efficacy of tumor patients treated with PD-1 inhibitors[25,26].More and more studies have begun to explore the predictive value of biomarkers derived from peripheral blood,because these biomarkers are universal,economical,fast,efficient and so on[27,28].
Many studies have found that systemic inflammatory responses affect the occurrence and development of malignant tumors by regulating the biological microenvironment[29-33].Based on this concept,several simple and stable inflammatory markers have been shown to be associated with prognosis in malignancy,including platelet-to-lymphocyte ratio (PLR),systemic immune-inflammation index,neutrophil-to-lymphocyte ratio (NLR),prognostic nutritional index and Glasgow Prognostic Score[34-38].Recently,a novel inflammatory marker,the C-reactive protein (CRP) to albumin ratio (CAR),has been reported as an independent prognostic factor in various malignancies.Renet al[39] reported that CAR predicted mortality and recurrence rates after resection of HCC.Huanget al[40] reported that the CAR could predict the outcomes of patients with nasopharyngeal carcinoma treated with chemotherapy.Based on the prognostic role of CAR in patients with other malignant tumors,we speculate that CAR may be related to the prognosis of HCC patients treated with PD-1 inhibitors.However,the role of the CAR in HCC patients treated with PD-1 inhibitors has not been evaluated.Therefore,we conducted this study.
The present study assessed the prognostic performance of the CAR in HCC patients treated with PD-1 inhibitors.We also constructed nomogram models based on the results of Cox multifactorial analysis for prognostic prediction of OS and progression-free survival (PFS) in HCC patients treated with PD-1 inhibitors.
MATERIALS AND METHODS
Patients
We conducted a retrospective analysis of patients with HCC who received PD-1 inhibitors in the first affiliated Hospital of Guangxi Medical University from January 2018 to November 2022.The inclusion criteria for this study were as follows: (1) Aged at least 18 at diagnosis;(2) clinical or pathological diagnosis of HCC;(3) treatment with PD-1 inhibitors for at least 3 cycles;(4) Eastern Cooperative Oncology Group performance status (ECOG PS) score of 0-1;and (5) child-Pugh Class A or B.We initially enrolled 213 patients in the study.The exclusion criteria were as follows: (1) Diagnosis of secondary liver malignancy or mixed liver malignancy;(2) treatment with PD-1 inhibitors <3 cycles;(3) child-Pugh Class C;(4) severe immune-related disease;(5) incomplete baseline data or follow-up information;and (6) suffer from inflammatory disease or blood system disease.Fifty-three patients were excluded,and 160 patients with HCC were finally included for analysis.
PD-1 blockers were administered intravenously at standard doses: Sintilimab 200mg per 2 wk;toripalimab at 3 mg/kg body weight per 2 wk;tislelizumab at 200 mg per 3 wk;and camrelizumab at 3 mg/kg body weight per 3 wk.Adverse events (AEs) were assessed according to the Common Terminology Criteria for Adverse Events v5.0.Patients were treated according to the treatment plan until disease progression (PD) or AEs occurred.
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Medical Ethics Committee of the First Affiliated Hospital of Guangxi Medical University (2023-E332-01).
Data collection
All data,including basic patient information,treatment strategy,laboratory test results and device results,were obtained from the electronic medical records and follow-up by phone calls.Serologic results measured within 5 d prior to the patient's first PD-1 inhibitor treatment were selected.Radiographic response was assessed by computed tomography or magnetic resonance imaging approximately every 6-12 wk after the start of treatment.Follow-up was performed by phone call every 2 mo after the start of treatment.The final data collected included age,gender,history of hepatitis virus,Child-Pugh,laboratory test results,ECOG PS,tumor size,tumor number,macrovascular invasion,extrahepatic metastases,prior medical history,history of smoking,history of alcohol consumption,Barcelona Clinic Liver Cancer (BCLC) stage,previous treatment,and survival data.The Child-Pugh classification was assessed according to ascites,hepatic encephalopathy,albumin,bilirubin and prothrombin time.The CAR was obtained by dividing the CRP (mg/L) by albumin (g/L).The remaining ratios were obtained as follows: PLR=platelets (109/L)/lymphocytes (109/L);lymphocyte to CRP ratio (LCR)=lymphocytes (109/L)/CRP(mg/L);NLR=neutrophils (109/L)/lymphocytes (109/L).PFS was defined as the time from initiation of PD-1 inhibitors to recurrence of HCC,PD,or death of the patient from HCC.OS was defined from initiation of PD-1 therapy until death.
Statistical analysis
R software (version 4.2.2) and SPSS (version 26) were used for statistical analyses.X-tile software was used to obtain the best cut-off values for continuous variables.Continuous variables were analyzed usingt-tests or non-parametric tests,and categorical variables were analyzed using Pearson's chi-square test or Fisher's exact test.Univariate and multivariate Cox regression analysis were used to determine the independent predictor variables for PFS and OS.The risk-stratified survival curves were expressed as Kaplan-Meier curves and analyzed using Log-rank tests.The nomogram and calibration plots were plotted using the "rms" package,the C-Index was calculated using the "survcomp" package,and the ROC curves were plotted using the "pROC" package.For all tests,a two-sidedP<0.05 was considered statistically significant.
RESULTS
Patient characteristics
The characteristics of HCC patients included in this study are shown in Table 1.A total of 160 patients were included in this study,including 134 males (83.8%) and 26 females (16.3%).Thirty-nine (24.4%) patients were older than 60 years;131 (81.9%) had previous hepatitis virus infection;85 (53.1%) had cirrhosis;121 (75.6%) had multiple tumors;71 (44.4%) had tumor invasion of the portal vein;and 83 (51.9%) had extrahepatic metastasis of the tumor.According to BCLC staging,most patients presented with BCLC C (n=120,75.0%),followed by BCLC B (n=30,18.8%) and BCLC A (n=10,6.3%).Using X-tile software,the best cut-off value of CAR was determined to be 1.20 according to PFS.Based on this cut-off value,patients were included in the low CAR group (n=94) and high CAR group (n=66).The correlation analysis of clinicopathological factors with CAR is shown in Table 1.CAR was associated with gender (P=0.045),macroscopic vascular invasion (P=0.013) and smoking history (P=0.013).There was no significant correlation between CAR and age,hepatitis infection,Child-Pugh grade,alpha-fetoprotein level,ECOG PS,tumor size,tumor number,extrahepatic metastasis,diabetes,hypertension,alcohol drinking,cirrhosis,previous ablation,previous transcatheter arterial chemoembolization (TACE),previous surgery and BCLC stage (P>0.05).

Table 1 The correlations between the C-reactive protein to albumin ratio and clinicopathological factors,n (%)
Comparison of low and high CAR survival curves
The median overall survival was 466 d and the median PFS was 168 d.A total of 88 patients (55%) died and 140 (87.5%) had confirmed programmed death at the last follow-up visit.The Kaplan-Meier method and log-rank test were used tocompare the survival curves between the low and high CAR groups (Figure 1).The results showed that OS was significantly shorter in patients with high CAR compared to those with low CAR (P<0.001).Similarly,PFS was significantly worse in patients with high CAR compared to patients with low CAR (P<0.001).

Figure 1 Kaplan-Meier survival curve of hepatocellular carcinoma in low C-reactive protein to albumin ratio group and high C-reactive protein to albumin group.A: Overall survival;B: Progression-free survival.CAR: C-reactive protein to albumin ratio.
Correlation of CAR with patient survival
Multiple clinicopathological factors were included in the Cox proportional hazards model for survival analysis.Univariate Cox regression analysis showed that ECOG PS (P=0.005),protein induced by vitamin K absence II (P=0.014),CAR (P=0.000),tumor size (P=0.002),tumor number (P=0.004),macrovascular invasion (P=0.042),smoke (P=0.024),previous operation (P=0.012),CRP (P=0.005),albumin (P=0.013),and aspartate aminotransferase (P=0.000) were significantly correlated with OS.However,during multivariate Cox regression analysis,only ECOG PS [hazard ratio (HR)=1.754,95% confidence interval (95%CI)=1.045-2.94,P=0.033],high CAR (HR=2.118,95%CI=1.057-4.243,P=0.034) and multiple tumor number (HR=2.932,95%CI=1.246-6.897,P=0.014) were the independent factors affecting the prognosis of OS (Table 2).Similarly,univariate Cox regression analysis showed that CAR (P=0.000),Tumor number (P=0.028),CRP (P=0.000),albumin (P=0.004),LCR (P=0.026),NLR (P=0.036) were significantly associated with PFS.Multivariate Cox regression analysis showed that high CAR (HR=2.730,95%CI=1.502-4.961,P=0.001),multiple tumor number (HR=1.584,95%CI=1.003-2.500,P=0.048) and NLR (HR=1.120,95%CI=1.022-1.228,P=0.015) were independent predictors of PFS (Table 3).

Table 2 Univariate and multivariate Cox regression analyses of risk factors for overall survival

Table 3 Univariate and multivariate Cox regression analyses of risk factors for progression free survival.
Establishment and validation of OS nomogram
Based on the results of multivariate Cox regression,a nomogram was established based on CAR,ECOG PS and tumor number to predict the OS of patients with HCC treated with PD-1 inhibitors.The nomogram was used to estimate the overall survival of patients with HCC at 12,18 and 24 mo by calculating the sum of the factor scores (Figure 2A).Then,the prediction ability of the nomogram was verified by the concordance index (C-index),receiver operating characteristic (ROC) curve,calibration plot and decision curve analysis (DCA).The C-index of OS nomogram was 0.721 (95%CI: 0.573-0.832).The nomogram predicted an area under the ROC curve (AUC) of 0.696 for 12-mo OS,0.765 for 18-mo OS,and 0.735 for 24-mo OS (Figure 3A).In addition,the calibration plots show the best agreement between the nomogram and the actual observations (Figure 4A).DCA revealed that the nomogram model provided a high clinical net benefit for predicting OS at 360,540 and 720 d (Figure 5A).

Figure 3 The area under the receiver operating characteristic curve was utilized to weigh up the performance of overall survival and progression-free survival nomogram models. A: Receiver operating characteristic (ROC) curves for 12-mo,18-mo,and 24-mo overall survival;B: ROC curves for 9-mo,12-mo,and 15-mo progression-free survival.AUC: Area under the receiver operating characteristic curve.

Figure 4 Calibration curves for nomogram models related to overall survival and progression-free survival. A: Calibration curves of overall survival at 12 mo,18 mo and 24 mo;B: Calibration curves of progression-free survival at 9 mo,12 mo and 15 mo.OS: Overall survival;PFS: Progression-free survival.

Figure 5 Decision curve analysis of overall survival and progression-free survival nomograms. A: Decision curve analysis (DCA) of overall survival at 12,18,and 24 mo;B: DCA of progression-free survival at 9,12,and 15 mo.
Establishment and validation of PFS nomogram
CAR,NLR and tumor number were identified as independent prognostic factors for PFS by multifactorial Cox regression analysis.Independent prognostic factors were incorporated to create a nomogram to predict PFS in patients with HCC (Figure 2B).The C-index of the PFS nomogram was 0.665 (95%CI: 0.552-0.762).The AUC was used to measure the performance of the PFS nomogram model.The AUC was 0.741,0.787 and 0.804 for predicting the PFS at 9,12 and 15 mo(Figure 3B).The calibration plot of PFS shows a good consistency between the predicted values and the actual values (Figure 4B).The DCA curve shows a huge net benefit at different time points,indicating that nomogram has a good potential clinical validity for predicting PFS (Figure 5B).
Establishment of risk classification system
Based on the nomogram,each patient received a personalized risk score.We use the X-tile software to determine the cutoff value of the risk score (Figure 6).According to OS nomogram and cutoff value,all patients were divided into low risk (score: 0-116.32),middle risk (score: 155.96-160.36) and high risk groups (score: 216.32-216.32) according to the OS nomogram.Similarly,all patients were classified into low risk (score: 3.65-54.01),middle risk (score: 54.26-91.33) and high risk groups (score: 91.40-142.67) according to the PFS nomogram and cut-off value.The Kaplan-Meier curves showed that this risk classification system had good stratification and differentiation ability (Figure 7).

Figure 6 The X-tile software is used to determine the cut-off value of the risk score. A: The cut-off value of the overall survival nomogram risk score;B: The cut-off value of the progression-free survival nomogram risk score.
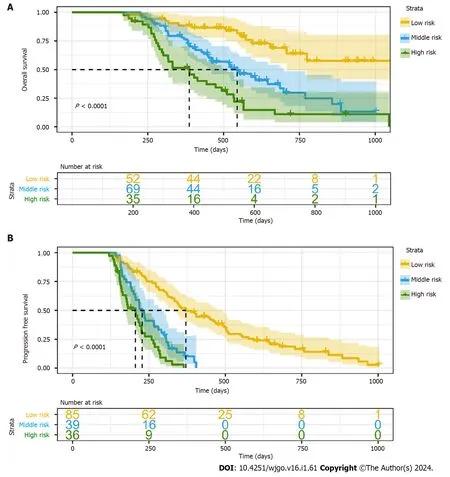
Figure 7 Kaplan-Meier curves for risk classification system based on nomogram. A: Risk classification system of overall survival nomogram;B: Risk classification system of progression-free survival nomogram.
DISCUSSION
This study investigated the relationship between clinicopathological factors and prognosis in patients with HCC treated with PD-1 inhibitors.Multivariate COX regression analysis showed that CAR,tumor number and ECOG PS were independent prognostic factors for OS.CAR,tumor number and NLR were independent prognostic factors for PFS.In Kaplan-Meier survival curve analysis,OS and PFS were significantly better in the low-CAR group than in the high-CARgroup,which also indicated that CAR could predict patient prognosis.Previous studies have shown that CAR can be used as a prognostic factor to predict the prognosis of patients with pancreatic cancer,gallbladder cancer,non-small cell lung cancer,neck squamous cell carcinoma,and cholangiocarcinoma[41-43].At present,the mechanism of the effect of CAR on the prognosis of HCC patients is not completely clear,but there are some possible explanations.CAR is a complex biomarker composed of CRP and albumin,which is affected by both CRP and albumin.
CRP is an acute phase protein produced after inflammation,tissue injury,malignant tumor and other changes[44].It has been established that the CRP recognizes exogenous substances or necrotic cells,activates the complement system,and enhances phagocytosis[45].CRPs are regulated by cytokines such as IL-1,IL-6 and tumor necrosis factor,which are co-secreted by malignant tumor cells and host immune cells and are associated with tumor growth,invasion,metastasis and chemotherapy resistance[46].Therefore,the increase of CRP may mean a poor prognosis of malignant tumors.
Albumin is synthesized by the liver,accounting for about 50% of total plasma protein,maintaining body nutrition and osmotic pressure,and is one of the main markers reflecting the nutritional status of the body[47,48].Trauma,malignant tumors and systemic inflammatory reactions can cause a decrease in albumin synthesis by the liver,promote albumin catabolism,aggravate capillary infiltration and cause hypoproteinemia[49].Hypoproteinemia causes decreased collagen synthesis,increased granuloma formation and hypercellularity,which suppresses the body's immune response and triggers tumor invasion and drug resistance[50-53].Previous studies have also demonstrated that albumin is closely related to the prognosis of patients with HCC[54,55].Compared with other tests,we have made some progress in this test.Compared with the expression of TMB and PD-L1,serum CAR not only responds to the inflammatory and immune status of the body,but also to the nutritional status of the body,which can predict the prognosis of patients with malignant tumors.Not only that,CAR can be obtained from routine blood tests with no additional medical costs.Therefore,CAR is affordable,convenient and effective for both clinicians and patients.
We also found that tumor number,ECOG PS and NLR are associated with the prognosis of patients with HCC,which is consistent with previous studies.Katayamaet al[56] confirmed that tumor number can predict the efficacy and prognosis of patients with HCC after TACE.In a multicenter retrospective study,ECOG PS was confirmed to be a prognostic factor in patients with unresectable HCC who received PD-1 inhibitors combined with anti-angiogenic therapy[57].A meta-analysis showed that NLR is a predictor of outcome after treatment for HCC[58].
We constructed two prognostic nomograms to predict clinical outcomes based on the independent prognostic factors determined by multivariate Cox regression analysis.The C-index index and calibration plots confirmed that our nomograms were reliable prediction tools.ROC curve confirms that OS nomogram can well predict patients' OS at 12,18,and 24 mo,and also confirms that PFS nomogram has an advantage in predicting PFS at 9,12,and 15 mo.In addition,we have established a new risk classification system based on nomogram to help assess the risk level of patients with HCC treated with PD-1 inhibitors,resulting in individualized treatment and accurate prognosis.Moreover,it is gratifying to note that ECOG PS is obtained by observing patients,NLR is obtained by routine blood tests,and tumor number is obtained by routine imaging tests.These markers are simple,easily accessible,non-invasive,repeatable and cost-effective,and are beneficial to the individual patients as well as the health care system.
It is worth noting that there are some limitations to our research.First of all,this is a retrospective study,and selection errors are inevitable.Secondly,only 160 cases were included in this study,and multicenter,larger sample studies are needed in the future to verify our conclusions.
CONCLUSION
In this study,the CAR was found to be a potential predictor of short-and long-term prognosis in patients with HCC treated with PD-1 inhibitors.The nomogram based on CAR achieved individualized prediction for patients.
ARTICLE HIGHLIGHTS
Research background
Over the years,programmed cell death-1 (PD-1) inhibitors have been routinely used for hepatocellular carcinoma (HCC) treatment and yielded improved survival outcomes.Nonetheless,significant heterogeneity surrounds the outcomes of most studies.Therefore,it is critical to find biomarkers that predict the efficacy of PD-1 inhibitors in patients with HCC.This may also help in meaningful and cost-effective use of this very costly therapy.
Research motivation
The role of the C-reactive protein to albumin ratio (CAR),whose prognostic value was suggested for various other malignancies had not yet been in HCC patients treated with PD-1 inhibitors has not been evaluated.
Research objectives
This study aimed to investigate the performance of the CAR in assessing the efficacy of patients receiving PD-1 inhibitors for HCC.
Research methods
The clinical data of 160 patients with HCC treated with PD-1 inhibitors from January 2018 to November 2022 at the First Affiliated Hospital of Guangxi Medical University were retrospectively analyzed.
Research results
Our study confirmed that Eastern Cooperative Oncology Group performance status,CAR and tumor number were independent prognostic factors for overall survival,while CAR,tumor number and neutrophil-to-lymphocyte ratio were independent prognostic factors for progression-free survival.Nomogram based on CAR can well evaluate the risk level of patients with liver cancer treated with PD-1 inhibitors.
Research conclusions
In this study,the CAR was found to be a potential predictor of short-and long-term prognosis in patients with HCC treated with PD-1 inhibitors.The CAR-based construct of nomogram achieved individualized prediction for patients.
Research perspectives
Further multi-center,large-sample clinical and randomized controlled studies are still needed to help identify additional risk factors for HCC in patients treated with PD-1 inhibitors.
FOOTNOTES
Co-first authors:Bai-Bei Li and Lei-Jie Chen.
Author contributions:Li BB,Chen LJ and Yu SP designed the study;Lu SL,Lei B and Yu GL collected the data;Li BB,Lu SL and Yu SP assembled the data;Li BB,Chen LJ and Yu SP analysed,interpreted the data and wrote the article;all authors read and approved the final manuscript.Bai-Bei Li and Lei-Jie Chen contributed efforts of equal substance throughout the research process.The choice of these researchers as co-first authors acknowledges and respects this equal contribution,while recognizing the spirit of teamwork and collaboration of this study.
Supported bythe Key Laboratory of Early Prevention and Treatment for Regional High Frequency Tumor (Guangxi Medical University),Ministry of Education,No.GKE-ZZ202117 and No.GKE-ZZ202334.
Institutional review board statement:This study was approved by the Ethics Committee of the First Affiliated Hospital of Guangxi Medical University (Approval No.2023-E332-01).
Informed consent statement:All participants provided informed written consent prior to study enrollment.
Conflict-of-interest statement:The authors declare that they have no competing interests.
Data sharing statement:The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is non-commercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Bai-Bei Li 0000-0002-6133-9361;Lei-Jie Chen 0000-0001-5275-4638;Shi-Liu Lu 0000-0002-8020-0488;Biao Lei 0000-0002-3010-0972;Gui-Lin Yu 0009-0007-9768-3555;Shui-Ping Yu 0000-0002-1581-7342.
S-Editor:Lin C
L-Editor:A
P-Editor:Yu HG
 World Journal of Gastrointestinal Oncology2024年1期
World Journal of Gastrointestinal Oncology2024年1期
- World Journal of Gastrointestinal Oncology的其它文章
- Comprehensive analysis of the role of ubiquitin-specific peptidases in colorectal cancer: A systematic review
- Colonoscopy plays an important role in detecting colorectal neoplasms in patients with gastric neoplasms
- Analysis of the potential biological value of pyruvate dehydrogenase E1 subunit β in human cancer
- Colorectal cancer’s burden attributable to a diet high in processed meat in the Belt and Road Initiative countries
- Emerging role of liquid biopsy in rat sarcoma virus mutated metastatic colorectal cancer: A case report
- Comprehensive evaluation of rare case: From diagnosis to treatment of a sigmoid Schwannoma: A case report
