Synergistic coupling of 0D–2D heterostructure from ZnO and Ti3C2Tx MXene-derived TiO2 for boosted NO2 detection at room temperature
Hong-Peng Li,Jie Wen,Shu-Mei Ding,Ji-Bo Ding,Zi-Ho Song,Cho Zhng,**,Zhen Ge,Xue Liu,Rui-Zheng Zho,Feng-Cho Li
a College of Mechanical Engineering,Yangzhou University,Yangzhou,225127,China
b Yangtze Delta Region Institute (Huzhou),University of Electronic Science and Technology of China,Huzhou,313001,China
c School of Materials Science and Engineering,National Institute for Advanced Materials,Nankai University,Tianjin,300350,China
d Laboratory of Advanced Materials,Shanghai Key Laboratory of Molecular Catalysis and Innovative Materials,and School of Chemistry and Materials,Fudan University,Shanghai,200433,China
e Key Laboratory of Spin Electron and Nanomaterials of Anhui Higher Education Institutes,School of Mechanical and Electronic Engineering,Suzhou University,Suzhou,234000,China
Keywords: MXene derivative Heterostructure Gas sensors TiO2 ZnO
ABSTRACT 2D MXenes are highly attractive for fabricating high-precision gas sensors operated at room temperature(RT)due to their high surface-to-volume ratio.However,the limited selectivity and low sensitivity are still long-standing challenges for their further applications.Herein,the self-assembly of 0D–2D heterostructure for highly sensitive NO2 detection was achieved by integrating ZnO nanoparticles on Ti3C2Tx MXene-derived TiO2 nanosheets(designated as ZnO@M-TiO2).ZnO nanoparticles can not only act as spacers to prevent the restacking of M-TiO2 nanosheets and ensure effective transfer for gas molecules,but also enhance the sensitivity of the sensor the through trapping effect on electrons.Meanwhile,M-TiO2 nanosheets facilitate gas diffusion for rapid sensor response.Benefiting from the synergistic effect of individual components,the ZnO@M-TiO2 0D–2D heterostructure-based sensors revealed remarkable sensitivity and excellent selectivity to low concentration NO2 at RT.This work may facilitate the sensing application of MXene derivative and provide a new avenue for the development of high-performance gas sensors in safety assurance and environmental monitoring.
1.Introduction
The rapid development of global industrialization has led to significant environmental challenges [1].Nitrogen dioxide (NO2),a common toxic air pollutant,which can combine with other chemical compounds in the atmosphere to produce acid rain,adversely affecting the ecosystem[2–6].Consequently,designing reliable and high-precision sensors to monitor,detect,and provide timely feedback on the hazardous levels of NO2gas is of great significance to the ecological environment and human health [7,8].As one of the most investigated gas sensors,the electrically-transduced gas sensor is simple and easy to fabricate using cost-effective techniques [9].The sensing mechanism of the chemiresistive gas sensors is based on conversion of the response of sensitive materials to gas into signals of resistance to change upon adsorption and reaction with the target gas molecules [10].As the core of a chemiresistive gas sensor,the sensitive material should meet all the following criteria [11–13]: (1) large exposed surface available for the material-analyte interaction;(2) abundant active sites for effective and selective binding of molecular analytes;and(3)low power consumption.Therefore,rational design and innovative synthesis of sensitive materials is crucial to improve the performance of gas sensors.
Two-dimensional (2D) materials with nanoscale thickness and extremely high surface-to-volume ratio have facilitated the development of highly sensitive gas sensors in the past few years [14].Significantly,the large surface-to-volume ratio ensures the availability of a large number of active sites for stronger adsorption between sensitive material and target analyte,which increases the sensitivity even at extremely low analyte concentrations [15].Until now,graphene,phosphorene,hexagonal boron nitride,transition metal dichalcogenides,2D metal-organic frameworks,covalent organic frameworks,transition metal oxides,and transition-metal carbides/nitrides(MXenes)have been successfully utilized to fabricate high-performance gas sensors [9,14,16–20].Among them,2D MXenes have recently shown great potential in high-quality gas sensor technologies due to their intriguing surface chemistry and high electrical conductivity [20–22].For instance,Jung and coworkers fabricated a gas sensor based on 2D Ti3C2for acidic gases at room temperature (RT) with high limit of detection and excellent signal-to-noise ratio [23].Nevertheless,the pure Ti3C2TxMXene gas sensors also suffer from the shortcomings of very low response values and long-term service stability.At the same time,the aggregation and self-stacking of 2D nanomaterials may lead to a substantial loss of accessible surface area and the number of activation sites,resulting in decreased intensity of gas recognition[24,25].Consequently,the design and development of MXene-based NO2sensors with excellent selectivity and high sensitivity used at RT is still a challenge.
One effective strategy for further improving the sensitivity is to construct hetero-structures using guest functional components [26–29].On the one hand,the hetero-interfacial effects between host and guest sensitive materials enhances the interfacial charge transfer and synergistic effect[30].On the other hand,MXenes have also been considered as promising two-in-one starting materials for the synthesis of transition metal oxides (TiO2) with fast electronic transmission,high capacity for oxygen adsorption,and sufficient atomic utilization [31].Therefore,incorporating MXene-derived 2D transition metal oxide with guest functional materials to construct heterostructure is an effective strategy to further improve the MXene-based NO2sensing performance at RT.
In this work,we report a highly active nanocomposite comprising Ti3C2TxMXene-derived TiO2nanosheets and ZnO nanoparticles (herein referred to as ZnO@M-TiO2) for NO2detection with high sensitivity.The increased surface-to-volume ratio of leads to larger interface areas with the target gas molecule and shorter diffusion path of gas within the nanostructured channels.The ZnO nanoparticles not only serve as spacers to effectively support adjacent MXene-derived TiO2(M-TiO2)nanosheets,providing more diffusion paths and accessible surface for the target gas molecules,but also jointly improve gas sensing performance a functional component.Furthermore,the numerous heterojunction interfaces between ZnO nanoparticles and M-TiO2nanosheets contribute to enhanced detection sensitivity.The resulting ZnO@M-TiO2based gas sensor exhibits superb sensitivity,ultra-low limit of detection,and high selectivity towards NO2.These excellent performance metrics demonstrate the promise of MXene derivatives in advanced gas sensors.
2.Experimental section
2.1.Preparation of Ti3C2Tx MXene
First,3.2 g of LiF was added to a solution of 20 mL of 9 M HCl and sonicated uniformly for 30 min,followed by the addition of 2.0 g Ti3AlC2powder,and stirred at 50°C.After 36 h of reaction,the precipitated bulk Ti3C2Txwas collected by centrifugation at 3500 rpm for 10 min.The product was collectedviarepeated centrifugations for further purification until the pH of the solution became neutral.After strong agitation using a VORTEX mixer at 1000 rpm and centrifuging for 30 min,the stable dark green supernatant of delaminated Ti3C2Txwas collected and freeze-dried to obtain Ti3C2Txaerogel.
2.2.Preparation of ZnO@MXene
Different amounts of MXene (20 mg,10 mg,and 5 mg) were slowly introduced into 5 mL of Zn(NO3)2•6H2O aqueous solution(7 mg mL-1).Following ultrasonic treatment for 10 min,5 mL of NaOH aqueous solution(10 mg mL-1)was added dropwise into the above solution.After reaction for 2 h,the mixture was collected by centrifugation and vacuum drying for 12 h.
2.3.Preparation of ZnO@M-TiO2
The ZnO@MXene was placed in a tubular furnace and annealed under atmospheric air at 500°C for 36 h with a ramp rate of 1°C min-1.The resulting nanocomposites label ZnO@M-TiO2-1,ZnO@M-TiO2-2,and ZnO@M-TiO2-3 represent the wt% of ZnO with values of 50%,67%,and 80%,respectively.For comparison,a ZnO/M-TiO2nanocomposite(2:1,wt%) was also prepared by physically mixing and without additional processing.
2.4.Characterization
The morphology and structure were characterized using a fieldemission scanning electron microscope (SEM,Hitachi SU-8010).Transmission electron microscopy (TEM) and high-resolution transmission electron microscopy(HRTEM)images were obtained using a JEM-2100F transmission electron microscope.X-ray diffraction(XRD)patterns of the samples were collected with a Rigaku D/max 2200 pc diffractometer equipped with Cu Kα radiation at a scanning rate of 2°min-1.X-ray photoelectron spectroscopy (XPS) measurements were conducted on a Thermo ESCALAB 250Xi spectrometer with monochromatic Al Kα radiation (hγ=1486.6 eV).All XPS spectra were calibrated with respect to the C 1s peak at 284.8 eV and fitted with Avantage 5.97 software.The nitrogen adsorption-desorption analysis was conducted using an automated N2adsorption-desorption instrument (ASAP TriStar II 3020,Micromeritics Inc.) at -196°C.The specific surface areas and the pore size distribution plots of ZnO@M-TiO2-2 were obtained using the Brunauer-Emmett-Teller (BET) method and the Barrett-Joyner-Halenda method,respectively.Atomic force microscopy (AFM) images were obtained using an OmegaScope 1000 system from AISTNT.
2.5.Gas sensing measurements
Gas sensing measurements were performed at RT(25±2°C)using a gas sensor testing system (Wuhan Huachuang Ruike Technology Co.,Ltd.).The obtained sample powder was first mildly ultrasonically dispersed in deionized water to obtain a uniform slurry.The gas sensors were fabricated by drip-coating the slurry on the cleaned ceramic substrates attached by Pt interdigital electrodes and dried at 60°C in a vacuum oven for 12 h.The humidity interference test was carried out by introducing gas analytes into saturated salt solution.Commercial humidity sensors were installed to monitor the relative humidity.
3.Results and discussion
The synthetic process of the ZnO@M-TiO2nanocomposite-based sensor is schematically illustrated in Fig.1.The fabrication involved four steps.First,Ti3C2TxMXene nanosheets were typically obtained by selectively etching the bulk Ti3AlC2powder(MAX phase) with HCl and LiF and exfoliation,followed by the formation of the MXene aerogelviafreeze drying.Second,ZnO nanoparticles were self-assembled on the surface of MXene with the presence of hydrophilic oxygen-containing functional groups by adding MXene into a mixed solution of Zn(NO3)2and NaOH[26].Third,the ZnO@MXene nanocomposite was annealed in air at 500°C for complete conversion to the ZnO@M-TiO2nanocomposite.Finally,a droplet of ZnO@M-TiO2suspension was pipetted on the surface of the platinum inter-digitated electrode to construct the sensor (Fig.S1).For comparison,a nanocomposite comprising physically mixed ZnO nanoparticles and M-TiO2nanosheets(ZnO/M-TiO2) was also prepared without additional processing.

Fig.1.Schematic illustration of the preparation of the ZnO@M-TiO2 gas sensors.
To elucidate the structure and morphology of the ZnO@M-TiO2nanocomposite,various characterizations were performed.As shown in the TEM image in Fig.2a,transparent and flat features of wrinkled thin Ti3C2TxMXene nanosheets were observed.According to the related selected area electron diffraction (SAED) pattern in Fig.2b,the MXene nanosheets exhibit a well-defined hexagonal crystal symmetry that is consistent with earlier published findings [32].The lateral dimension and thickness of MXene nanosheet were~500 nm and 1.5 nm,respectively,according to the AFM image in Fig.2c,confirming that the nanosheets were delaminated.The TEM images of ZnO@MXene nanocomposite shown in Fig.2d–f demonstrate that the ZnO nanocrystals were uniformly attached to the surface of the MXene nanosheet with an average size of~10 nm.During the assembly of the ZnO@MXene nanocomposite,no large ZnO nanoparticles were formed,probably due to the spatial restrictions between adjacent MXene nanosheets,which limited the nanoparticle growth [33].In addition,the suitable nanoparticle size provided good stability and homogeneity of the mixed colloidal dispersion,which effectively limited the re-stacking MXene nanosheet[34].The high angle annular dark field scanning transmission electron microscopy (HAADF-STEM) and corresponding elemental mappings in Fig.S2 clearly indicate the even distribution of Ti,C,O,F,and Zn elements throughout the ZnO@MXene nanocomposite.The morphology of the ZnO@M-TiO2(Fig.2g) including the 2D flake structure was retained during the annealing process without obvious damage.The HRTEM image shown in Fig.2h exhibits lattice fringes with spacings of 0.35 nm and 0.28 nm,which are attributed to the(101)plane of the anatase phase of TiO2and the (100) crystal planes of hexagonal ZnO [35,36],respectively.In addition,as shown in Fig.2i,the energy dispersive spectrometry mapping scanning of Ti,C,Zn,and O indicate that ZnO nanoparticles were evenly distributed on the 2D M-TiO2nanosheets,which facilitated the transmission of electron and diffusion of target gas molecules inside the electrode[37].

Fig.2.(a)TEM image,(b)SEAD image,and(c)AFM image of MXene.(d,e)TEM image and(f)HRTEM image of the ZnO@MXene nanocomposite.(g)TEM image,(h)HRTEM image,and (i) Elemental mappings of the ZnO@M-TiO2 nanocomposite.
The structural and crystal integrity of the as-prepared MXene,ZnO@MXene nanocomposite,and the ZnO@M-TiO2nanocomposites were investigated by XRD(Fig.3a).An intense(002)diffraction peak at around 6.9°was observed in the XRD pattern of the pure MXene.After decorating with ZnO nanoparticles,the(002)diffraction peak of MXene exhibited a clear shift downward to~5.9°,while the diffraction planes of(101),(002),(101),(102),(110),and (103) detected at 31.8°,34.4°,36.3°,47.5°,56.6°,and 62.9°,respectively,associated to anatase ZnO(JCPDS No.#36–1451) [36].In the XRD pattern of the ZnO@M-TiO2nanocomposites,the (101) crystal face of anatase TiO2was observed at 25.3°[38],and the(101),(002),(101),(102),(110)and(103)planes of ZnO were also detected,demonstrating the formation of TiO2after annealing in air at 500°C.

Fig.3.(a)XRD patterns of MXene,ZnO@MXene,and ZnO@M-TiO2 nanocomposites.XPS spectra of Ti 2p(b)and Zn 2p(c)of the ZnO@M-TiO2 nanocomposite.(d)N2 adsorption/desorption isotherm and corresponding pore size distribution curve of the ZnO@M-TiO2 nanocomposite.
XPS characterization was also carried out to further confirm the surface chemical state of the nanocomposite.The XPS survey spectrum presented in Fig.S3 strongly establishes the coexistence of Ti,O,and Zn.Fig.3b shows the Ti 2p core level XPS spectrum of the ZnO@M-TiO2nanocomposite,and the two characteristic peaks located at 464.8 and 458.8 eV are attributed to the Ti4+–O bonds with the binding energy of Ti 2p1/2and Ti 2p3/2[39,40],respectively.Fig.3c shows the Zn 2p core level XPS spectrum of the ZnO@M-TiO2nanocomposite,and the two characteristic peaks located at 1043.98 and 1020.88 eV correspond to the binding energy of Zn 2p1/2and Zn 2p3/2,respectively.The binding energy separation was 23.1 eV,which indicates that the valence of Zn was in the 2+oxidation state[41].
The N2adsorption-desorption measurement was utilized to validate the decrease of restacking structures in the nanocomposite.As shown in Fig.3d,the N2adsorption/desorption isotherm of the ZnO@M-TiO2nanocomposite demonstrates reversible type IV characteristics with an H3 hysteresis loop in the relative pressure range of 0.45–1.0,indicating the presence of mesoporosity [42].The BET specific surface area of the ZnO@M-TiO2nanocomposite was calculated at 95 m2g-1,higher than that of other MXene-derived oxide obtained by thermal annealing of exfoliated Ti3C2TxMXene reported in the literature(Table S1)[13,35,37,43].The pore size distribution displayed unique mesopore structures ranging from 2 to 10 nm.The resulting high specific surface area and mesoporous structure of the ZnO@M-TiO2nanocomposite may not only facilitate target gas molecule adsorption,but also promote gas diffusion and transport[15].
Following the characterization of morphology and microstructure,the gas sensing properties of the ZnO@M-TiO2nanocomposite-based sensors were systematically analysed using a dynamic test system at RT.Considering the normal atmospheric environment in daily life,the sensing performances of the gas sensor were measured at a relative humidity(RH)of 40%.Here,the gas response is defined as Ra/Rg,where Raand Rgdenote the resistance of the sensors in the air of 40% RH and after exposure to the target gas [44,45],respectively.The dynamic sensing response,an important index used to evaluate the performance of the gas sensor upon exposure to gas analytes,was detected by continuously changing the NO2concentration over a wide range of concentrations from 0.5 to 4 ppm.As shown in Fig.4a,the gas responses of all samples increased with increasing concentration of NO2gas.Obviously,the sensors based on pure M-TiO2show minimal response to NO2,while the NO2response of the ZnO@M-TiO2nanocomposites-based sensors is significantly higher at the same concentration.This enhancement may be due to the formation of heterojunctions between ZnO and M-TiO2,which not only generate rapid electron transport but also serves as an efficient catalyst due to its appropriate chemical potential[46].To obtain further insight into the effect of composite material,the response of the sensors based on ZnO/M-TiO2nanocomposite exposed to 0.5–4 ppm of NO2is presented in Fig.S4.The significant aggregation of the ZnO nanoparticles in the ZnO/M-TiO2nanocomposite (Fig.S5) adversely affects the sensing performance.In addition,it was observed that the response of the ZnO@M-TiO2nanocomposite-based sensor to NO2increased first and then decreased with the increase of ZnO load from 50 wt% to 80 wt%.The highest response was obtained when the ZnO load was 67 wt%(ZnO@M-TiO2-2).This is due to the amount and size of ZnO nanoparticles have increased accordingly,as evidenced by TEM images in Fig.S6.Excessive ZnO nanoparticles will agglomerate and reduce the active specific surface area for the reaction between gas molecules and metal oxides [47],thus negatively affecting the gas sensitivity.The responses of the sensors with concentration variations of NO2are shown in Fig.4b.The sensing response values of the ZnO@M-TiO2-2 sensors to 0.5,1,2,3,and 4 ppm NO2were calculated to be 2.1,3.5,14.2,30.5,and 42.5,respectively.The limit of detection,described as the lowest analyte concentration that can be reasonably differentiated from noise[48],was calculated as 0.2 ppm.The excellent sensing response of the ZnO@M-TiO2-2 nanocomposite-based sensor was superior to that of a majority of the NO2sensors based on TiO2,ZnO,and other sensitive materials recently reported,as shown in Fig.4c and Table S2[49–59].

Fig.4.Gas sensing properties of the ZnO@M-TiO2 sensor.(a) Response-recovery curves in the presence of different concentrations of NO2 at 40% RH.(b) Correlations of the sensor response and NO2 concentration.(c) Gas sensing performance compared with other state-of-the-art NO2 sensors.(d) Repeated sensing tests following exposure to 0.5 ppm,1 ppm,and 2 ppm NO2.(e)Response and recovery times in the presence of 1 ppm NO2.(f)Response and recovery times compared with the reported values in the literature.(g)Long-term stability of the ZnO@M-TiO2 sensor at 1 ppm NO2 in 16 days.(h)Sensing selectivity towards NO2 and interfering gases.(i) Responses of the ZnO@M-TiO2 sensor in the presence of 1 ppm NO2 under different RH conditions.
To further investigate the repeatability of the ZnO@M-TiO2-2 nanocomposite-based sensor,the sensor was exposed to 0.5,1,and 2 ppm NO2gas for five consecutive cycles,respectively.As displayed in Fig.4d,the response curves were basically the same except for some slight changes,indicating repeatable gas response and recovery.Fig.4e shows typical response and recovery curves of the ZnO@M-TiO2-2 sensor to 1 ppm of NO2.The response and recovery times are defined as the time taken to reach 90% of the maximum gas response after exposure to the target gas analyte and the time taken to return to 10% of the initial gas response output signal after the removal of the gas analyte,respectively[38].The ZnO@M-TiO2-2 sensor showed a response time of 128 s and a recovery time of 175 s.Compared with other NO2sensors reported previously (Fig.4f) [58–63],the response and recovery processes are accelerated.In addition,the long-term stability of the ZnO@M-TiO2-2 sensor was evaluated upon exposure to 1 ppm NO2for 16 days.The response magnitude during the test was relatively stable.As presented in Fig.4g,the ZnO@M-TiO2-2 sensor still retained~94% of the initial response value after 16 days,indicating outstanding long-term stability.
In addition to enhanced sensitivity,the ZnO@M-TiO2-2 sensor also showed remarkable selectivity for NO2compared with other gases.As an important parameter for evaluating the practical application of gas sensing ability,the selectivity of ZnO@M-TiO2-2 nanocomposite,refers to the ability of a sensor to identify the target gas from other interfering gases[64],was determined by comparing the cross sensitivity with other gases at a given concentration.As depicted in Fig.4h,the selectivity of the ZnO@M-TiO2-2 sensor was tested in the presence of NO2(1 ppm),SO2(1 ppm),methanol (1 ppm),ethanol (1 ppm),glycerol (1 ppm),methane (10 ppm),and CO2(10 ppm) at RT.It can be seen that the response of the ZnO@M-TiO2-2 sensor to NO2was at least twice as high as any other interfering gases.Meanwhile,the ZnO@M-TiO2-2 sensor also process superior selectivity and response than that of the M-TiO2sensor (Fig.S7).Moreover,the effect of humidity on the gas sensing performance of the ZnO@M-TiO2-2 sensor was also evaluated by measuring its response to 1 ppm of NO2at different RH levels from 0% to 80%.
As shown in Fig.4i,due to the partial occupation of the sensing sites by water molecules[13,45],the response of the ZnO@M-TiO2-2 sensor changed from 3.3 to 1.8 as the RH increased from 0% to 80%,indicating that the ZnO@M-TiO2-2 sensor can still work normally under high humidity.As shown in Fig.4i,due to the partial occupation of the sensing sites on the sensing material surface by water molecules [13,46],the response of the ZnO@M-TiO2-2 sensor changed from 3.3 to 2.2 as the RH increased from 0% to 20%.Nevertheless,since water molecules could promote the adsorption of water-soluble NO2to adsorb onto the surface of the sensing material in a humid environment[13],the response value of the ZnO@M-TiO2-2 sensor was relatively stable in the range of 20%–80% RH,which indicates that it can still work normally under high humidity.
The enhanced gas sensing properties of the ZnO@M-TiO2sensors can be attributed to the synergistic effects of individual components.To gain a better understanding of the charge transfer at the ZnO/M-TiO2heterojunction,the energy bands of the ZnO/M-TiO2heterojunction before and after the Fermi level equilibrium are displayed in Fig.5a and b.Electrons are transported across the interfaces between ZnO and M-TiO2due to the difference in their conduction bands.As a result,a depletion layer is formed on the surface of M-TiO2with higher-energy conduction band due to the loss of electrons [29].At the same time,due to the accumulation of electrons,an accumulation layer is formed on the surface of ZnO with lower-energy conduction band.The diffusion of electrons and holes continues until the Fermi level of the system reaches equilibrium.When the sensor is exposed to NO2,as shown in Fig.5c,NO2molecules extract electrons from ZnO nanoparticles and create another hole accumulation layer inside ZnO [65],resulting in a decrease in the width of the heterojunction barrier and improved response value.
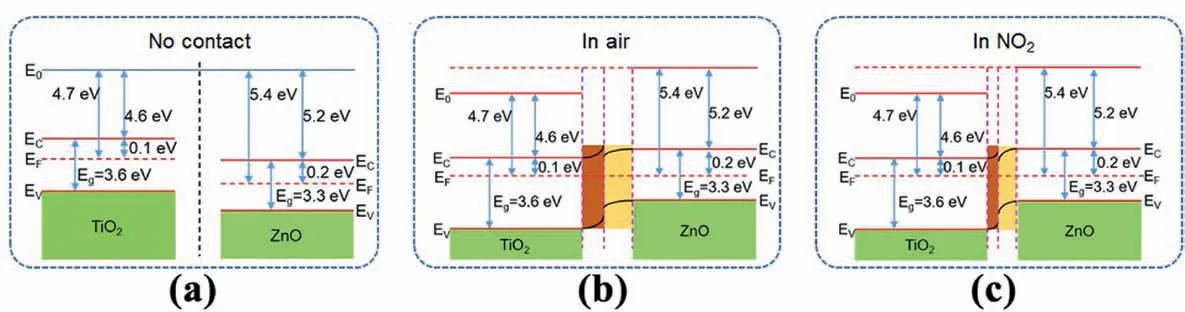
Fig.5.Schematic diagrams showing the energy band structures of the ZnO@M-TiO2 nanocomposite before (a) and after (b) the Fermi level equilibrium in air.(c)Energy band structure of the ZnO@M-TiO2 nanocomposite in NO2 atmosphere.
4.Conclusions
In summary,highly sensitive NO2detection at RT was achieved based on the synergistic coupling of Ti3C2Tx-derived TiO2nanosheets with ZnO nanoparticles.The 0D ZnO nanoparticles can effectively reduce the selfstacking of adjacent M-TiO2nanosheets,thereby generating efficient diffusion paths and accessible surfaces for target gas molecules.At the same time,2D M-TiO2nanosheets with high surface-to-volume ensure the availability of robust active sites for enhanced adsorption.In addition,multiple heterojunction interfaces between ZnO nanoparticles and M-TiO2nanosheets contribute to enhanced detection sensitivity.By integrating the properties and synergistic effects of the individual components,the resulting ZnO@M-TiO2-based gas sensor exhibits outstanding sensitivity to NO2.This work may provide a new avenue for the fabrication of high-performance MXene derivatives-based gas sensors.
Declaration of competing interest
There are no conflicts to declare.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China(No.52103308),the Natural Science Foundation of Jiangsu Province of China (No.BK20210826),Outstanding Youth Foundation of Jiangsu Province of China (No.BK20211548),National Key Research and Development Program of China (No.2017YFE0115900),Innovative Science and Technology Platform Project of Cooperation between Yangzhou City and Yangzhou University (No.YZ2020266),Lvyang Jinfeng Plan for Excellent Doctor of Yangzhou City,Special Funds for Self-Made Experimental Equipment of Yangzhou University,and the Doctor of Suzhou University Scientific Research Foundation Project(No.2022BSK003).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nanoms.2023.02.001.
- Namo Materials Science的其它文章
- Advancing the pressure sensing performance of conductive CNT/PDMS composite film by constructing a hierarchical-structured surface
- In situ confined vertical growth of Co2.5Ni0.5Si2O5(OH)4 nanoarrays on rGO for an efficient oxygen evolution reaction
- Water-based synthesis of nanoscale hierarchical metal-organic frameworks:Boosting adsorption and catalytic performance
- An overview of recent progress in the development of flexible electrochromic devices
- Wearable and stretchable conductive polymer composites for strain sensors:How to design a superior one?
- Addressing cation mixing in layered structured cathodes for lithium-ion batteries: A critical review