Weizmannia coagulans: an ideal probiotic for gut health
Shiqi Zhng,Pingln Li,Suwon Lee,Yu Wng,Chunming Tn,Nn Shng*
a Key Laboratory of Functional Dairy,Ministry of Education,College of Food Science and Nutritional Engineering,China Agricultural University,Beijing 100083,China
b Coree Beijing Co.,Ltd.,Beijing 101312,China
c Key Laboratory of Precision Nutrition and Food Quality,Department of Nutrition and Health,China Agricultural University,Beijing 100083,China
d College of Engineering,China Agricultural University,Beijing 100083,China
Keywords:Weizmannia coagulans Spore former Probiotics Microbiota Gut disorders
ABSTRACT Weizmannia coagulans (formerly Bacillus coagulans) is a spore-forming and lactic acid-producing bacterium.It has recently attracted much attention from researchers and food manufacturers due to its probiotic functions and stability in processing and storage.W. coagulans is capable of improving gut health through the regulation of gut microbiota,modulation of immunity,and improving digestibility and metabolism.Spores,germinated cells and metabolites of W. coagulans modulate the gut micro-environment and further affect other organs.W. coagulans is an environment-friendly probiotic since it can contribute to the host by reconstructing the balance of gut microbiota and only temporarily resides in the intestine after administration.W. coagulans has been generally recognized as safe (GRAS) by the US Food and Drug Administration (FDA),thus it is an ideal probiotic for improving gut health.The merit of its stability in processing and storage provides W. coagulans spores many possibilities for its use in various types of functional foods.This review presents an overview of the characteristics of W. coagulans that make it an ideal probiotic candidate and highlights the proposed health benefits with scientific evidence conferred by the administration of W. coagulans.
1. Introduction
Probiotics is well-known as “live microorganisms which when administered in adequate amounts confer a health benefit on the host”[1],which includes three essential elements: live microorganisms,adequate amounts,and health benefits.In order to provide health benefits,probiotics should survive during production,storage and administration.It is an ideal choice to use spore-forming bacteria as probiotics for functional food and pharmaceutical formulations since spores are relatively invulnerable to negative environment compared to commonly used bacteria such asLactobacillusandBifidobacterium[2,3].Several studies have reported that some strains ofBacillusspp.can be used to prevent and treat gut disorders via producing organic acids and antimicrobial compounds,improving gut barrier integrity,formation of enzymes,and immunomodulation[4-6].Weizmannia coagulans(formerlyBacilluscoagulans) was firstly isolated from spoiled evaporated milk by Hammer in 1915[7].W.coagulanshas characteristics of bothLactobacillus(producing lactic acid) andBacillus(forming spore)[8].Several strains ofW.coagulanshave been shown to play potential roles in the modulation of microbiota composition and activity,enhancement of immunity,and alleviation of metabolic disorders[9-11].
Healthy adults gut microbiota is typically composed of anaerobic members of Bacteroidetes and Firmicutes,which is established in the first two to three years through a process similar to ecological succession[12].Those bacteria initially colonized the infant’s intestine are considered to be pioneer bacteria[13].Many factors including environmental (diet,alcohol,and antibiotics) and genetic determinants can disrupt the balanced microbiota into a state of dysbiosis[14-16].Dysbiosis directly refers to a loss of beneficial bacteria and an increase in harmful microorganisms[17].It can further weaken the gut barrier by disrupting the normal function of intestinal epithelial cells,resulting in immune and metabolic disorders[15].Moreover,the negative feedback from a disordered metabolic system can aggravate dysbiosis[18].Whether dysbiosis is the cause or result,mounting evidence has shown that regulating gut flora can improve or cure numerous dysbiosis-associated diseases[19,20].Dietary interventions involving prebiotics and probiotics,and fecal microbiota transplantation,were widely researchedin vivoand in clinical trials to restore disease-associated dysbiosis[21].The treatment of intestinal microecology can be regarded as secondary succession (Fig.1).W.coagulanscan help the host to reconstruct the balance of gut microbiota[22,23].It can be seen as a pioneer species in the secondary succession of restoring gut microbiota.
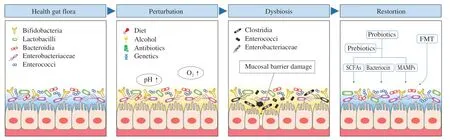
Fig.1 Maintaining intestinal microecology.The healthy gut microbiota is dominated by beneficial bacteria.Environmental and genetic factors can lead to the excessive proliferation of harmful bacteria and disrupt the balanced microbiota into a state of dysbiosis.Intervention of probiotics and/or prebiotics and FMT could be effective in restoring intestinal microecology.FMT,fecal microbiota transplantation;SCFAs,short chain fatty acids;MAMPs,microbe-associated molecular patterns.
Probiotic effects are known to be strain-,condition-,and dose-specific[3,24].Basic screening indexes for probiotics include identification of the microorganism strain,tolerance to the gastrointestinal tract (GIT) conditions,adherence to mucus and/or human epithelial cells,antibiotic susceptibility,antimicrobial activity,and stability during processing and storage[3,25].For a specific effect,in vitrotests of particular properties andin vivotrials in humans or animals are required[26].As a living microorganism,and usually used as food ingredients,the safety of probiotic strains is particularly important.Two major types of side effects that may be theoretically caused by probiotics are systemic infections and gene transfer[27,28].W.coagulanscan be isolated from traditional fermented food such as Ngari,an Indian traditional fermented fish[29],and bean sauce[30],and has been generally recognized as safe (GRAS) by the US Food and Drug Administration (FDA).W.coagulanshas recently attracted much attention from researchers and food manufacturers due to its spore-forming ability and probiotic activity.SeveralW.coagulansstrains have been applied in cereal-based products,fruit juices,and milk wherein the high-temperature processing is involved[31,32].In this review,we summarized the characteristics ofW.coagulansthat make it an ideal probiotic candidate and highlighted the proposed health benefits with scientific evidence conferred by the intervention ofW.coagulans.
2. Weizmannia coagulans
W.coagulanswas first described by Gupta et al.[33]in 2020,formerly recognized asB.coagulans.The genusWeizmanniawas named in honor of Dr.Chaim Weizmann (1874-1952),a noted biochemist,who was one of the pioneers in the field of industrial microbiology.Gupta and colleagues performed comprehensive phylogenomic and comparative analyses on >300Bacillusgenomes and found that this genus can be reliably distinguished from all other Bacillaceae species by two conserved signature indels (CSIs) in the proteins: acetate kinase andO-methyltransferase.The taxonomy of the genusWeizmanniais shown in Fig.2.Three species were classified into this genus:W.coagulans,W.acidiproducens,andW.ginsengihumi[33].Weizmanniastrains were mostly isolated from soil and its type species isW.coagulans.

Fig.2 Taxonomy of genus Weizmannia.
W.coagulansis facultatively anaerobic to microaerobic,Grampositive,rod-shaped,endospore-forming,and lactic acid-producing bacterium.W.coagulansis a moderate thermophile.Its optimum growth temperature ranges from 35 °C to 50 °C.The maximum growth temperature is 57 °C to 61 °C.The spores ofW.coagulanscan resist heat treatment at 90 °C for 30 min and ensure long-term shelf life[34-36].This characteristic allowsW.coagulansto overcome the great challenge of heat resistance in the development of probiotics,especially compared toBifidobacteriumandLactobacillus,which typically have less than 15% survival at the end of shelf life[37].W.coagulanscan be isolated from soil,milk,canned foods,and silage[30,34,38].Up to date,there are 44 genome data ofW.coagulansstrains available in the NCBI genome database,including completely assembled 15 strains.The total length ofW.coagulansgenome is around 3.42 Mbp with a 46.5% GC content.
W.coagulansis usually applied in the form of spores that can assure survivability under the hostile environment of GIT and during production and storage processes.After oral administration,the spores ofW.coagulanscan safely pass through the stomach not killed by gastric acid and germinate in the upper part of the small intestine and then resporulate in the large intestine[39,40].Surface layer proteins,hydrophobicity,and electrostatic forces are three main factors that promote bacteria to colonize in the gut[41,42].In a 2-year follow-up clinical trial,administration ofBifidobacteriumlongumsubsp.infantisin 7-day-old infants for 21 days led to a stable colonization for one year[43].It is believed thatW.coagulansis not a natural inhabitant of the gut and can only temporarily persist in the intestine.A study by Adami and Cavazzoni demonstrated thatW.coagulanswas lost in one week after administration in piglets[23].Another study indicated thatW.coagulansspores obviously declined through passing GIT but highly survived in faecal samples in rats[22].Therefore,achieving beneficial long-term effects may require continuously administration ofW.coagulans.
3. Regulation of gut microbiota
Gut microbiota dysbiosis may possess various compositional and functional attributes in different kinds of diseases[44].Raised intraluminal oxygen concentrations,reduced abundance of anaerobes,and increased proportion of facultative anaerobes were often found in many different diseases[20,44].Studies have shown thatW.coagulanscan induce an anaerobic and acidic intestinal environment that is unfavorable to pathogenic bacteria but beneficial to the proliferation of other short-chain fatty acids (SCFAs) producing bacteria[45].SCFAs including acetate,propionate,and butyrate are essential for the health and well-being of the host[46].Approximately 10% of the human daily caloric requirement is provided by SCFAs[47].SeveralW.coagulansstrains have been found to produce a high yield of phenyllactic acids (PLA),which is an effective antimicrobial compound with broad antimicrobial activity against both bacteria and fungi[48,49].Abhari and colleagues demonstrated that 30-day administration ofW.coagulansspores to healthy rats resulted in a significant decrease in Enterobacteriaceae counts and an obvious increase of total anaerobic bacteria in faecal samples[22].Another animal study in rats investigated the germination ratio ofW.coagulansspores in GIT with the agar plate culture methods[50].This study demonstrated that more than 60% ofW.coagulansspores germinated in GIT and the ratios of spores and vegetative cells in colon and feces were approximately 2:8 and 4:6,respectively.Also,the oral administration ofW.coagulansspores contributed to the elevation of SCFAs concentrations and reduction of pH in faeces[50].In anin vitrohuman colonic microbiota model KUHIMM,W.coagulansSANK 70258 significantly increased the abundance of Lachnospiraceae,thereby enhancing butyrate production and suppressing bacteria related to the Enterobacteriaceae family[51].
DifferentW.coagulansstrains have been reported to produce antimicrobial compounds such as bacteriocins and lipopeptides(Table 1).Bacteriocins are proteins or peptides produced by bacteria extensively used as bio-preservatives and alternatives to traditional antibiotics to overcome the problem of drug-resistant pathogens due to their intolerance to proteases in GIT[52].Most bacteriocins can disturb the cell membrane by forming pores or degrading cell walls,but the producing strains have immunity to their bacteriocins[53,54].W.coagulansI4was found to produce a pediocin-like peptide,firstly named coagulin,which displayed an inhibitory spectrum that includedW.coagulansand unrelated bacteria such asEnterococcus,Leuconostoc,Oenococcus,ListeriaandPediococcus[55].Pediocin,a class II bacteriocin with great values in the food industry after nisin,has a particularly strong inhibitory activity againstListeria[52].W.coagulansL1208 generates coagulin with a broad spectrum of antimicrobial activity against foodborne pathogens including Gram-positive bacterium:Bacillussubtilis,Staphylococcusaureus,andLactiplantibacillusplantarum;Gram-negative bacterium:Escherichia coli,Salmonella enterica,Shewanellaputrefaciens,andPseudomonas aeruginosa[56].W.coagulanshas been also found to produce surfactins,a cyclic lipopeptide produced by a large variety ofBacillusspp.,that possess antibacterial and antifungal activity[57].The type strain ofW.coagulansATCC 7050 was shown to generate a novel antimicrobial substance,named Lactosporin,with activities against the Gram-positive microorganismsMicrococcus luteusandL.monocytogenes[58].

Table 1 Antimicrobial compounds produced by W.coagulans.
Existing research suggests thatW.coagulanscannot colonize in the intestine in the long run,but it can significantly regulate the gut microbiota by increasing the abundance of beneficial bacteria and decreasing pathogenic bacteria.W.coagulansis more likely to balance gut flora toward the population of beneficial native bacteria rather than to permanently colonize the gut.It can be used as a pioneer probiotic for improving gut dysbiosis.
4. Modulation of immunity
Probiotics in the gut microbiota is intimately related to the innate and adaptive immune responses of the GIT[60].Several intestinal diseases,such as inflammatory bowel diseases (IBD)and irritable bowel syndrome (IBS),are significantly correlated with an inappropriate immune response to bacterial antigens[61].Gut microorganisms mediate intestinal immune homeostasis through regulating pattern recognition receptor signaling pathways,improving gut barrier function,preventing pathogen translocation,stimulating immune cell activities,and balancing Th1/Th2 immune response[62,63].There is mounting evidence supporting that administration of probiotics can modulate host immunity.However,this efficacy is dependent on species,strains,and even doses.In an allergic asthma mouse model induced by ovalbumin (OVA),the antiallergic effects of 6 bacterial strains (BifidobacteriumbreveM-16V,B.infantisNumRes251,B.animalisNumRes252 and NumRes253,LactiplantibacillusplantarumNumRes8,andLacticaseibacillus rhamnosusNumRes6) were compared.Results demonstrated that strain M-16V was most effective in reducing the allergic response[64].A meta-analysis showed thatL.rhamnosusGG (LGG) is more likely effective in treating acute gastroenteritis in children with a daily dose not less than 1010CFU/day[65].It is important to note that some strains have been proven functional in certain diseases,but they may not be beneficial under all disease contexts[66].In a clinical trial,LGG was proven to reduce the frequency of atopic dermatitis by 50% in the first two years of life in infants[67].However,a later clinical study demonstrated that LGG had no therapeutic effect against mild to moderate atopic dermatitis in infants[68].
Both the spores and the vegetative cells ofW.coagulansexhibit activities in enhancing immunity in the GIT (Fig.3).Anin vitrostudy found that spores efficiently germinated in the macrophages RAW264.7 and induced the expression of pro-inflammatory cytokines but the immune activation was also delayed[40].In an immunosuppression mice model induced by cyclophosphamide(CTX),W.coagulans13002 significantly restored the CTX-induced injury by improving the abundance of some beneficial bacteria in the gut,including a butyrate-producing bacteriaAnaerotruncus,increasing serum immunoglobulin levels and upregulating IFN-γ and IL-4 in the intestinal mucosa[10].In another CTX-induced immunosuppression and streptomycin-associated diarrhea mice model,administration ofW.coagulansspores significantly decreased diarrhea syndromes,normalized cellular immune function,and prevented weight loss.The authors also found thatW.coagulanswas more effective in restoring intestinal dysbiosis and immunity than the combined use ofLactobacillusacidophilusandBifidobacteriumanimalissubsp.lactis[69].Overall,Microbe-associated molecular patterns (MAMPs)generated in the life cycle ofW.coagulanscan stimulate the normal intestinal immune system to boost the immune response against pathogenic microorganisms,and when the host is in the context of inflammatory disease,it can play an anti-inflammatory role.Inhibition of pathogens and repair of gut barrier may fundamentally solve the problem of inflammation.
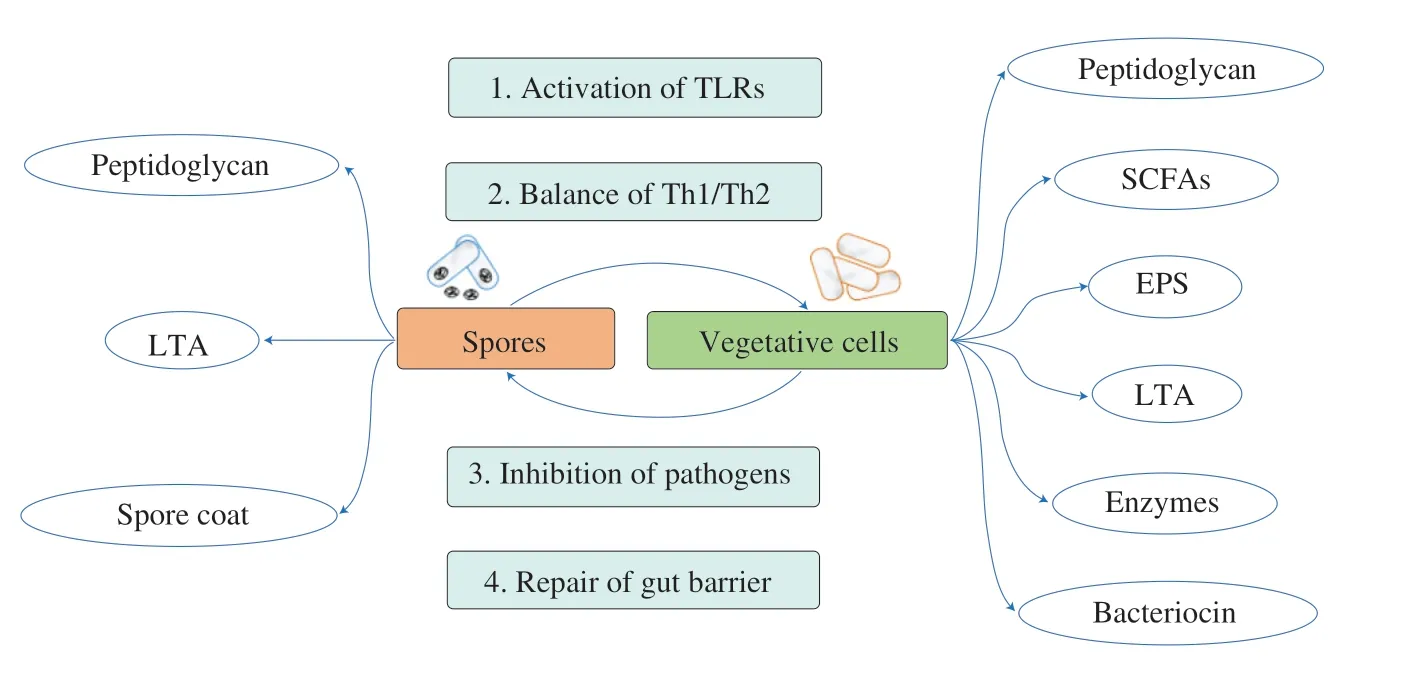
Fig.3 Immunity modulating modes of action by spores and vegetative cells of W. coagulans in the gut.Microbe-associated molecular patterns (MAMPs)generated in the life cycle of W. coagulans mediate intestinal immune homeostasis.LTA,lipoteichoic acid;TLRs,toll-like receptors;Th,T helper cell;SCFAs,short chain fatty acids;EPS,exopolysaccharide.
W.coagulanshas been shown to have an anti-inflammatory effect in several inflammatory diseases such as IBS,Clostridium difficileinduced colitis,and arthritis.A synbiotic combination of green banana resistant starch andW.coagulansMTCC5856 spores ameliorated dextran-sulfate sodium-induced colitis in mice by maintaining the expression of tight junction proteins,decreasing the expression of IL-1β and C-reactive protein,and increasing the production of IL-10 in the serum.The synbiotic combination also increased SCFA levels in the colon[70].In anin vivostudy ofC.difficile-induced colitis in mice,the authors found that treatment ofW.coagulansGBI-30,6086 attenuated the colonic pathology (crypt damage,edema,and leukocyte influx),improved stool consistency,and prolonged survival[71].Further study demonstrated that the administration ofW.coagulansGBI-30,6086 significantly prevented the recurrence ofC.difficileinduced colitis in mice[72].Additionally,in a complete Freund’s adjuvant (CFA) arthritis-induced rat model,pretreatment withW.coagulanssuppressed the fibrinogen,serum amyloid A,and TNF-α production,and also inhibited the development of paw swelling[73].In a lipopolysaccharide (LPS)-induced caecum damage model in rats,Wang et al.assessed the effect and mechanism ofW.coagulansTL3 on inflammatory injury of cecum[74].This study demonstrated that compared to the LPS group,treatment withW.coagulansTL3 significantly downregulated levels of proinflammation related cytokines and chemokines,resulted in a significant improvement in cecal tissue damage through Toll-like receptor 4 (TLR4)/ Myeloid differentiation factor 88 (MyD88)/ nuclear factor kappa B (NF-κB)signaling pathways.W.coagulansTL3 was also found to increase the relative abundance ofAkkermansia muciniphila,a symbiotic bacterium of the mucus layer in the intestines[74].
Previous studies have revealed that cell wall components such as lipoteichoic acids (LTAs) and peptidoglycans,and metabolites such as exopolysaccharides and proteins could be served as immunostimulants[54,75].LTA,the second major component of cell walls,is considered a major microbe-associated molecular pattern(MAMP) of Gram-positive bacteria[76].LTA interacts with TLR2/6,leading to pro-or anti-inflammatory effects through mitogen activated protein kinase (MAPK)/NF-κB signaling pathways[77,78].Jensen and colleagues evaluated the anti-inflammatory and immune-modulating properties of cell wall fragments and spent culture supernatant ofW.coagulansGBI-30,6086 with peripheral blood mononuclear cells and polymorphonuclear cells.Both fractions increased the phytohemagglutinin (PHA)-induced production of IL-6 and decreased the PHA-induced production of TNF-α.Both fractions enhanced the Pokeweed Mitogen (PWM) induced production of TNF-α and IFN-γ.In addition,spent culture supernatant also enhanced both the PHA-and the PWM-induced expression of IL-10[79].These findings suggested that probioticW.coagulanscould be a prophylactic or therapeutic intervention for immune function regulation via activating important immune signaling pathways and balancing the activity of immune cells.A majority of research is focusing on bacteria-derived molecules.It will help us to better understand the exact molecular mechanisms underlying the effect ofW.coagulanson the immune system.
5. Improving digestibility and metabolism
Bacillusspp.are key players in naturally fermented foods such as soy,maize,and rice,mainly due to their abundant enzymes,including carbohydrate degrading enzymes,proteases,lipase,nucleases,phosphatases,and others[2,80].Lactobacillusspp.have been recently found to possess a considerable number and diversity of bile salt hydrolase (BSH)[81].Microbial BSHs in the gut play an important role in the metabolism of bile acids,which is associated with obesity,type-2 diabetes (T2D),and cardiovascular diseases[82].SeveralW.coagulansstrains have been reported to produce a wealth of digestive enzymes and employed to produce fermented foods,lactic acid,and various enzymes[31].W.coagulansRCS3 isolated from a hot-water spring showed significantβ-galactosidase activity,which can be used to alleviate lactose intolerance[83].Microbialβ-galactosidase breaks down lactose into digestible glucose and galactose in the small intestine to avoid abnormal lactose fermentation in the gut[84].W.coagulansMA-13 was found to overproduceα-andβ-galactosidases simultaneously[85].Alkan et al.used aW.coagulansstrain to produce high titers of lipase with melon waste[86].
The capacity ofW.coagulansto improve digestibility has been studiedin vitroandin vivo.Keller et al.[39]assessed the germination,survival,and metabolic activity ofW.coagulansGBI-30,6086 spores within vitromodels of the stomach and small intestine (TIM-1) and the colon (TIM-2).Viable cell counting results demonstrated thatW.coagulansGBI-30,6086 spores tolerated gastric acids and bile salts with 97% and 51% survival,respectively,and most of the survived spores germinated in the mimic colon (97%) after 24 h.Metabolic activity assay found that the levels of amino acids,dipeptides,and citric acid cycle metabolites were increased by the intervention ofW.coagulansGBI-30,6086 spores compared to the control group.Either germinated bacteria or the enzymes released from the dead cells were regarded to aid the digestion of the meal[39].In an animal study,dietary supplementation ofW.coagulansto broilers as an alternative to antibiotics was evaluated[87].This study revealed that dietaryW.coagulans,significantly increased the levels of antioxidative enzymes,immunoglobulins,and anti-inflammatory factors in serum,modulated gut microbiota by decreasingDesulfovibrioandParasutterellaand by increasingAlistipesandOdoribacter,resulting in a significant improvement in body weight and average daily gain compared to antibiotics group[87].
Research increasingly demonstrates the mutual interactions between gut microbiota and the metabolism system[82,88,89].Gut microbiota-derived metabolites modulate intestinal endocrine cells producing gut hormones,including glucagon-like peptides (GLP),peptide YY (PYY),and gastric inhibitory polypeptide (GIP),which are involved in obesity and T2D[90,91].The concept of the gut–liver axis explains the crosstalk between gut microbiota and the hepatic metabolism through several mediators such as trimethylamine (TMA),LPS,ethanol,SCFAs,and secondary bile acids that may contribute to non-alcoholic fatty liver disease (NAFLD)[92].W.coagulanshas been shown to improve metabolic disorders through direct or indirect ways.In a health rat model,daily consumption of yogurt containingW.coagulansGBI-30,6086 spores for 21 days showed the synergistic effect that the levels of glucose and triglycerides in serum were decreased and the abundance of Bacillales and Bifidobacteriales in the gut were increased compared to the control group,yogurt group,and probiotic alone group.It was concluded that yogurt is a suitable food carrier ofW.coagulansspores[93].Moreover,the combination ofW.coagulansGBI-30,6086 spores with apple cider vinegar not only further attenuated weight gain and enhanced glucose tolerance but also improved the serum lipid profile and prevented hepatic steatosis in high-fat diet mice.These effects were mediated by alleviating fat accumulation in the liver and restoring leptin and insulin sensibilities.WhetherW.coagulans-derived SCFAs contribute or not to the vinegar activity was proposed as further research[94].In an animal study,Lee et al[95].evaluated the beneficial effects ofW.coagulanslilac-01 and soya pulp in cholic acid-fed rats.Rats were fed cholic acid to simulate the bile acid conditions in diet-induced obesity and aging.Administration of soya pulp significantly improved gut permeability,plasma adiponectin,and liver index,but also caused the increased levels of secondary bile acids such as deoxycholic acid andω-muricholic acid.However,the combination ofW.coagulanslilac-01 and soya pulp showed a synergistic effect by abolishing the increased production of secondary bile acids and without diminishing the beneficial effects of soya pulp[95].W.coagulanshas shown great potentials for applications in various types of functional foods such as yogurt,fermented beverages,and cereal-based products by enhancing food utilization,promoting the digestion and absorption of nutrients in the intestine,and improving the metabolic balance.
6. Evidence of clinical research on improving gut health
There is accumulating clinical evidence indicating that the administration ofW.coagulanscan have a variety of beneficial effects on different health conditions including gut disorders,T2D,NAFLD,rheumatoid arthritis (RA),and boosting immunity.Some of the important clinical trials ofW.coagulansstrains have been highlighted in Table 2.
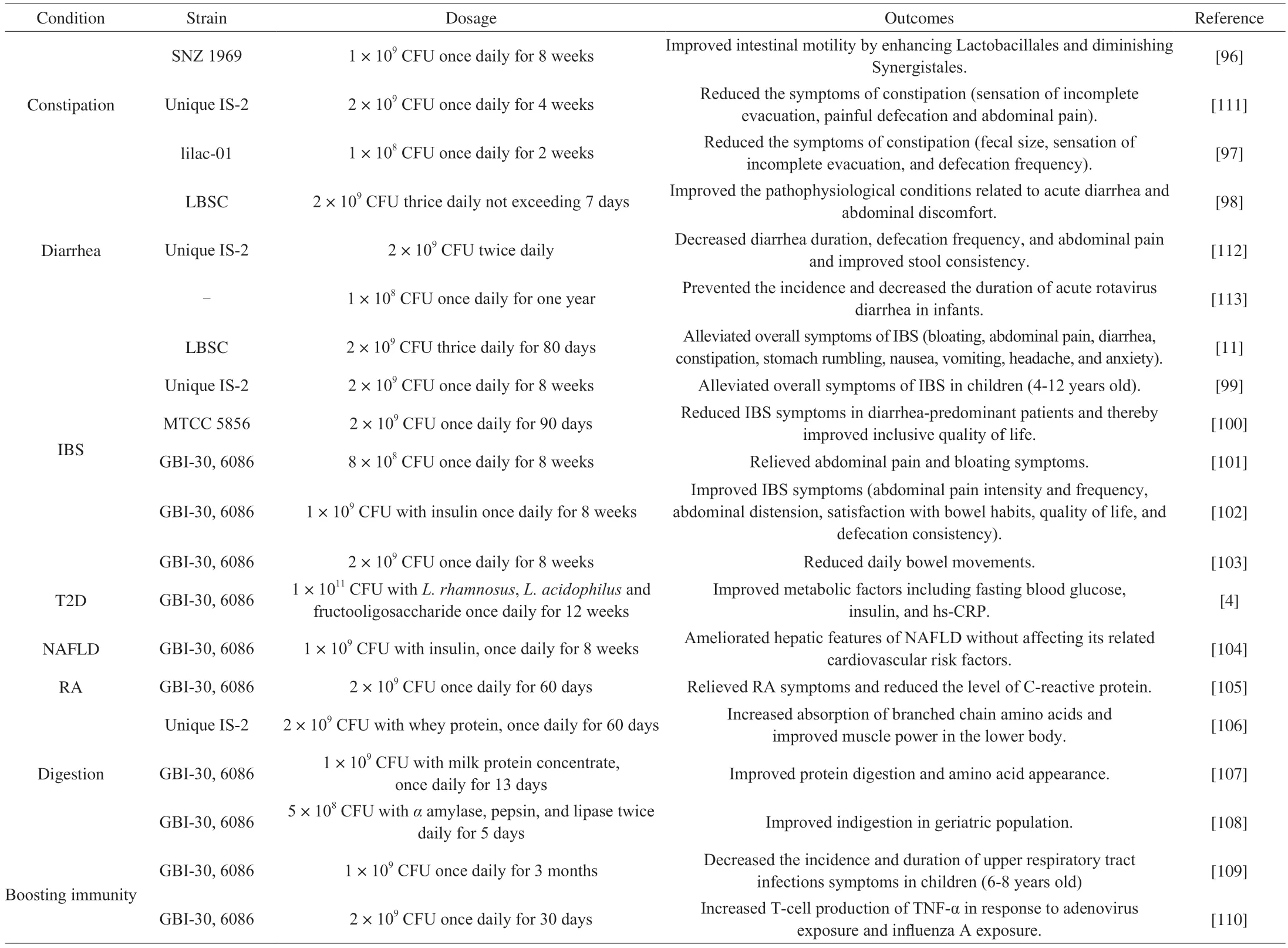
Table 2 Clinical trials of probiotic W.coagulans strains representing health benefits.
Gastrointestinal symptoms,including abdominal pain,diarrhea,and constipation,are frequent reasons for medical consultations.Emerging evidence suggests that the use of probiotic supplements has recently been recognized as a promising option for the prevention and management of gastrointestinal symptoms associated with dysbiosis.Kang and colleagues assessed the impact of 8-week treatment withW.coagulansSNZ 1969 on mild intermittent constipation in healthy adults[96].This study found that,compared with the placebo group,administration ofW.coagulansSNZ 1969 effectively improved intestinal motility by enhancing Lactobacillales and diminishing Synergistales.In a randomized double-blind placebo-controlled trial involving 297 healthy volunteers with a tendency for constipation,2-week treatment withW.coagulanslilac-01 significantly reduced the symptoms of constipation (fecal size,sensation of incomplete evacuation,and defecation frequency)[97].On the other side,Maity and Gupta examined the efficacy ofW.coagulansLBSC on acute diarrhea with abdominal discomfort[98].The strain LBSC was effective in recovering from acute diarrhea with abdominal pain and exhibited improved quality of life.Furthermore,severalW.coagulansstrains showed therapeutic effects on alleviating symptoms of IBS(such as bloating,abdominal pain,diarrhea,constipation,stomach rumbling,nausea,vomiting,headache,and anxiety) in both children and adults[11,99-103].
In a randomized,double-blind,placebo-controlled trial,T2D patients orally received a combination symbiotic supplementation of 1011spores ofW.coagulansGBI-30,6086,1010CFUL.rhamnosus,109CFUL.acidophilusand 500 mg fructooligosaccharide once daily for 12 weeks.The synbiotics significantly improved metabolic factors including fasting blood glucose,insulin,and hs-CRP[4].In another clinical study,administration ofW.coagulansGBI-30,6086 with insulin for 12 weeks in NAFLD patients,significantly decreased alanine amino transferase (ALT),gamma glutamine transaminase(GGT),TNF-α and NF-κB in serum compared to the placebo group,and also reduced hepatic steatosis[104].Additionally,the intervention ofW.coagulansGBI-30,6086 spores effectively relieved clinical symptoms and reduced the level of C-reactive protein in RA patients[105].W.coagulanspossesses anti-inflammatory effects when the host is in the context of inflammatory disease.
There is also evidence thatW.coagulansprompt the digestion of protein in humans.In a double-blind,placebo-control trial,oncedaily administration ofW.coagulansUnique IS-2 with whey protein concentrate (WPC-80) for 60 days significantly increased absorption of branched-chain amino acids in resistance-trained males compared with WPC-80 alone and improved leg press and vertical jump power[106].W.coagulansGBI-30,6086 has been also shown to increase protein digestion and amino acid appearance in healthy adults when administered with milk protein concentrate[107].Co-administration ofW.coagulanswith protein is a good choice to maximize the health benefits associated with protein supplementation.In an open-label,randomized,prospective study,5-day treatment withW.coagulansGBI-30,6086 along with digestive enzymes (amylase,pepsin,and lipase) improved indigestion,abdominal pain,and flatulence in geriatric patients[108].
The effect ofW.coagulanson boosting immunityin vitroand in animal models is well-established[10,40,87].However,evidence for enhancing immunity in humans is relatively limited.In a randomized,double-blind,placebo-controlled trial,the authors evaluated the effect ofW.coagulansGBI-30,6086 against upper respiratory tract infections in 80 healthy school-aged children (6–8 years old)for three months[109].Results showed that daily intake of 109spores significantly decreased the incidence and duration of upper respiratory tract infection symptoms.In another controlled study,Baron assessed the impact of daily administeredW.coagulansGBI-30,6086 for30 days on the immune system when exposed to adenovirus and influenza in healthy adults[110].This study demonstrated that intervention ofW.coagulansGBI-30,6086 significantly increased T-cell production of TNF-αin response to adenovirus exposure and influenza A (H3N2 Texas strain) exposure.W.coagulanscan be used as a stimulant of the normal intestinal immune system to boost the immune response to pathogenic microorganisms.
7. Safety
As live microorganisms,probiotics may theoretically cause systemic infections,metabolites toxication,and antibiotic gene transfer[28,114].In recognition of the importance of assuring the safety,the FAO/WHO guidelines recommended that a novel strain even among a group of bacteria that are Generally Recognized as Safe(GRAS) must be carefully assessed for safety before it can be used as a probiotic[1].Bang et al.evaluated the safety ofW.coagulansIDCC 1201 at the phenotypic or genomic levelsin vitro[34].Wholegenome analysis showed thatW.coagulansIDCC 1201 had no antibiotic resistance or toxigenic genes;the strain was susceptible to the tested nine antibiotics;hemolytic activity was negative;it produced undesirable metabolites (biogenic amines orD-lactate) at a safe level.The authors also tested the acute oral toxicity of this strain in rats.No abnormalities were observed during the treatment of 3.8 × 1010CFU/kg body weight once daily for 14 days.It was concluded thatW.coagulansIDCC 1201 is a safe probiotic for human use[34].In a sub-acute oral toxicity study in rats,W.coagulansSNZ 1969 showed no observed adverse effects at the dose of 5 × 1011CFU/kg body weight once daily for 28 days[115].In a chronic oral toxicity study in rats,no evidence of toxicity was observed in one-year administration of 1.3×1011CFU/kg body weight once daily ofW.coagulansGBI-30,6086[116].It was concluded thatW.coagulansGBI-30,6086 is safe for long-term administration at up to 9.38 × 1010CFUs per day for adults[116].SeveralW.coagulansstrains are available in various probiotic foods in global market (Table 3).
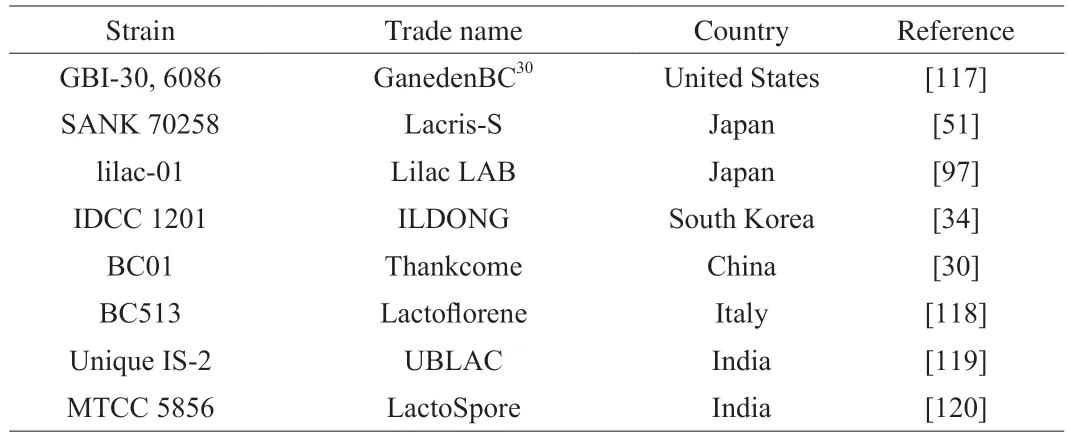
Table 3 Examples of commercialized W. coagulans strains in global food market.
Some clinical trials evaluated the therapeutic effect ofW.coagulansstrains as well as their safety.In a randomized,doubleblind,placebo-controlled trial,IBS patients orally received 6 × 109spores ofW.coagulansLBSC for 80 days.There were no serious adverse events compared to placebo control[11].W.coagulansLBSC was also well-tolerated at the dose of 2 × 109CFU thrice daily in patients with acute diarrhea[98].In another clinical trial on the efficacy and safety of association of simethicone andW.coagulansin patients with IBS,no adverse events were reported during the 4-week study period[121].In an open-label,single-arm pilot study ofHelicobacter pylorieradication,administration of 3 tablets ofW.coagulansfor 8 weeks had no side effects during treatment[122].
On the other hand,W.coagulansis not a natural inhabitant of the gut and can only temporarily persist in the intestine.It can be seen as an environment-friendly probiotic since it is more likely to balance gut flora toward the population of beneficial native bacteria rather than permanently colonize the gut.The transience property ofW.coagulansin GIT reduces the risk of developing opportunistic infections and pathogenicity-related genes transfer compared with other probiotics that adhere to and colonize the gut epithelium.Nevertheless,as FAO/WHO (2002) has recommended,researchers should carry out epidemiological surveillance of adverse incidents of living microorganisms after their large-scale application in food or pharmaceutical fields.
8. Challenges and future perspectives
ProbioticW.coagulansis capable of improving human health through direct or indirect ways (Fig.4).W.coagulanscontributes the host to reconstructing the balance of gut microbiota by increasing the abundance of beneficial bacteria and decreasing pathogenic bacteria.It also can stimulate the normal intestinal immune system to boost the immune response against pathogenic microorganisms.W.coagulanspossesses anti-inflammatory effects when the host is in the context of inflammatory disease.W.coagulanscan improve digestibility and metabolic disorders.W.coagulansspores can germinate in GIT conditions.Spores,germinated cells and metabolites modulate the gut microecological environment and further affect other organs.However,evidence supporting the life cycle ofW.coagulanssporesin vivois limited.No strain-specific studies have been reported to reveal the time,location,and ratio of germination and re-sporulation after oral administration ofW.coagulansspores.From a probiotic perspective,spores must germinate to play their roles better.Employing reverse transcription-PCR to determine the expression of vegetative genes and sporulation-specific genes may be more accurate than culturing methods adopted mostly to understand the life cycle of oral administration ofW.coagulansspores in GIT.The exact molecular mechanisms underlying the effect ofW.coagulansin GIT,especially the key substances for regulating immunity and immune response pathways,also need more in-depth exploration.

Fig.4 Overview of modes of action of probiotic W. coagulans for gut health.After oral administration,the W. coagulans spores can germinate,proliferate,and resporulate in the gut.The life cycle of W. coagulans induces anaerobic and acidic intestinal environment that results in improving commensal microbiota.Spores,LTA,EPS and peptidoglycans serve as microbe-associated molecular patterns (MAMPs) to balance Th1/Th2 immune response and mediate pro-/anti-inflammatory effects.W. coagulans also can promote the digestion of protein and carbohydrates by producing digestive enzymes such as proteases and galactosidases.Furthermore,metabolic disorders can be alleviated by increasing the concentrations of beneficial substances and decreasing the contents of harmful substances in the gut.LTA,lipoteichoic acid;EPS,exopolysaccharide;SCFAs,short chain fatty acids;LPS,lipopolysaccharide;Treg,regulatory T cell;Th,T helper cell;NK cell,natural killer cell.
The property of spore-forming allows using spray-drying as a possible formulation system ofW.coagulans,which can greatly reduce the production cost compared to freeze-drying.However,how to obtain high spore yield is a technical challenge for the fermentation process.The merit of processing and storage stability ofW.coagulansspores provides many possibilities for its combined use with various types of functional food,such as cereal-based products,beverages,and dairy product.Additionally,W.coagulansas a starter for fermented foods may reduce the unique smell generated byBacillusspp.due to its relatively mild enzyme activity[123].It has a vast potential to develop synbiotic fermented foods.
9. Conclusion
The gut microbiota playing one of the key roles in regulating host health is gaining attention.W.coagulansis an ideal probiotic candidate for gut health in view of its advantages of efficacy,safety,processing,and stability.Certainly,more clinical evidence-based proofs are still needed to support strain-specific probiotic efficacy.With the systematic exploration in gut microbiome,the exact molecular mechanisms of probiotics can be further elucidated.Thereby,efficient and precise probioticW.coagulansstrains can be developed.
Conflict of interest
Nan Shang is an associate editor forFood Science and Human Wellnessand was not involved in the editorial review or the decision to publish this article.All authors declare that there are no competing interests.
Acknowledgement
This work was supported by National Natural Science Foundation of China (32172172,32201994) and the Foreign Expert Collaboration Project (G2021108010L).
- 食品科学与人类健康(英文)的其它文章
- GUIDE FOR AUTHORS
- Targeting gut microbiota in osteoporosis: impact of the microbial based functional food ingredients
- Natural sources,refined extraction,biosynthesis,metabolism,and bioactivities of dietary polymethoxyflavones (PMFs)
- A review of salivary composition changes induced by fasting and its impact on health
- Minerals in edible insects: a review of content and potential for sustainable sourcing
- Food nutrition and toxicology targeting on specific organs in the era ofsingle-cell sequencing

