Coumarin and eugenol ameliorate LPS-induced inflammation in RAW 264.7 cells via modulating the NLRP3 inflammasome pathway
Jyotsana Bakshi,Somnath Singh,KP Mishra
DRDO Defence Institute of Physiology and Allied Sciences, Delhi, India
ABSTRACT Objective:To investigate the underlying mechanism of antiinflammatory action of coumarin and eugenol in lipopolysaccharide(LPS)-stimulated RAW 264.7 cells.Methods:RAW 264.7 cells were treated with 2.5 µg/mL of LPS,50 µM of coumarin,and 50 µM eugenol for 24 h.The viability of the cells was assessed using MTT assay.The production of nitric oxide was determined using Griess reagent and DCFH-DA was used to measure the production of reactive oxygen species.The protein expression of NLRP3,IL-1β,NF-κB,and cyclooxygenase 2 was assessed using Western blot analysis.Results:Coumarin and eugenol showed anti-inflammatory effects against LPS-induced inflammatory response by ameliorating the expression of NLRP3 inflammasome and NF-κB,which further led to a subsequent reduction in IL-1β,nitric oxide,and reactive oxygen species.Conclusions:Coumarin and eugenol exert their anti-inflammatory activities by modulating the NLRP3 inflammasome pathway and NF-κB.These compounds may have promising therapeutic applications for the treatment of various inflammatory diseases.
KEYWORDS: Inflammation;Cytokines;Coumarin;Eugenol;NLRP3;LPS
1.Introduction
Inflammation is the body’s natural defense mechanism against various infections due to invasion of viruses,bacteria,toxins,and parasites.Sometimes,due to various environmental and biological factors,the inflammatory response persists for longer than required and develops into chronic inflammation which is considered detrimental to human health.Prolonged and unregulated inflammation within the body can result in the development of various inflammatory conditions such as asthma[1],diabetes[2],rheumatoid arthritis[3],and atherosclerosis[4].These chronic inflammatory responses can significantly impact an individual’s overall health and well-being.
Chronic inflammation is associated with the accumulation of monocyte-derived macrophages which increases the production of pro-inflammatory mediators like interleukin-6 (IL-6),IL-1β,tumor necrosis factor-alpha (TNF-α),and nitric oxide (NO).A constant increase in NO production also leads to tumor progression and mutagenesis[5].Various plant-derived immunomodulatory compounds are used in traditional medicine to treat multiple inflammatory diseases.Recent studies published by Guptaet al.reported the anti-arthritic effect of an immunomodulatory compound andrographolidein vitroas well asex vivo[6,7].A recently discovered multiprotein complex known as the inflammasome regulates the inflammatory response by caspase-mediated activation of various pro-inflammatory cytokines like IL-1β and IL-18[8].One such inflammasome multiprotein complex known as the nucleotidebinding domain-like receptor protein 3 (NLRP3) is an important component of the host innate immune response against fungal,bacterial as well as viral infections[9,10].The NLRP3 complex consists of a pro-caspase-1,NLRP3 protein,NOD-like receptor,and an apoptosis-associated speck-like protein[11].The assembled NLRP3 inflammasome has emerged as a crucial regulator of inflammation,orchestrating the maturation and release of proinflammatory cytokines like IL-1β and IL-18,which further leads to the induction of cell death by pyroptosis[12].High levels of proinflammatory cytokine release resulting from NLRP3 inflammasome activation contribute to the development and progression of chronic inflammatory diseases[13,14].Therefore,it requires potential plantderived compounds that can modulate the expression of NLRP3 inflammasome activation.The lipopolysaccharide (LPS)-stimulated macrophages are known to be a key activator of the NLRP3 inflammasome.The fine balance between the activities of various anti-inflammatory and pro-inflammatory cytokines is crucial for the proper functioning of the immune system.The transcription factor NF-κB is known to induce the expression of multiple proinflammatory cytokines to elicit an inflammatory response[15].Recent studies have also suggested the possible role of NF-κB in inflammasome activation[16].In addition to NF-κB,the signaling molecules like NO and reactive oxygen species (ROS) as well as enzymes like cyclooxygenase-2 (COX-2) in its inducible form are also highly expressed during the inflammatory response[17,18].The overexpression of COX-2 directly leads to an increase in prostaglandin production,which in turn decreases the expression of certain apoptotic proteins which contributes to uncontrolled cell proliferation,metastasis,and angiogenesis[19].
Plant-derived immunomodulatory compounds are known to possess anti-inflammatory properties and are considered potent therapeutic agents.Benzopyrones,commonly known as coumarins,are phenolic compounds isolated first from tonka beans in 1820[20].Coumarins are secondary metabolites of many higher plants which can be easily synthesized chemically[21].Coumarin and its derivates are known to possess anti-cancer,anti-viral,anti-bacterial,and antioxidant properties[22].Kontogiorgiset al.reported the anti-inflammatory properties of coumarin and its derivatives in an adjuvant-induced arthritis model[23].Another study reported that coumarin derivatives showed antioxidant properties by inhibiting lipid peroxidation[24].
Clove oil or eugenol is an aromatic oil that is actively found in a variety of herbal plants like tulsi,cinnamon,clove,and nutmeg.Eugenol is known to possess both pro-oxidant as well as antioxidant properties.Similar to coumarin,eugenol is known to show various pharmacological properties like anti-cancer[25],anti-fungal[26],antibacterial[27],and anti-inflammatory.At lower concentrations,eugenol acts as an antioxidant by minimizing ROS-mediated oxidative stress,and at higher concentrations,it behaves as a pro-oxidant by increasing ROS production[28,29].Even though multiple studies report the anti-inflammatory activity of coumarin and eugenol,the precise mechanism through which they exert the anti-inflammatory effect remains to be investigated.Hence,the present study aimed to investigate the effect of coumarin and eugenol on LPS-stimulated RAW 264.7 macrophages and to elucidate the regulatory pathways involving NLRP3 inflammasome.
2.Materials and methods
2.1. Chemical and reagents
Coumarin (C4261,purity ≥99%),eugenol (E51791,purity 99%),and LPS (from Escherichia coli strain) were obtained from Sigma-Aldrich,USA.Griess reagent,2,7-dichlorofluorescein dictate(DCFH-DA) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2Htetrazolium bromide (MTT) were purchased from Sigma (St.Louis,USA).Fetal bovine serum (FBS),Dulbecco’s Modified Eagle Media(DMEM) low glucose,penicillin,streptomycin,and trypsin-EDTA were purchased from Gibco (Grand Island,New York,USA).
2.2. Cell culture
Murine macrophage cells RAW 264.7 were procured from the American Type Culture Collection (Manassas,VA,USA).They were cultured in DMEM low glucose media containing penicillin(100 units/mL),streptomycin (100 µg/mL),and 10% FBS.Cells were incubated in a humidified incubator maintained at 37 ℃ with 5% CO2.
2.3. Cell viability using MTT assay
RAW 264.7 macrophage cells were plated in a 96-well plate at a cell density of 1.5 × 105cells per well.The cells were then treated with 2.5 µg/mL of LPS and 50 µM of coumarin and eugenol alone and in combination with LPS for 24 h.To assess the viability of cells,MTT dye was used.The dye was added to the 96 well plate and incubated further for 4 h at 37 ℃ at 5% CO2.The purple formazan crystals formed in the live cells were solubilized using dimethyl sulfoxide (DMSO) and the absorbance was measured at 570 nm using a spectrophotometer (BioTek Synergy HTX Multimode Reader).
2.4. NO estimation
NO is an essential signaling molecule that plays a crucial role in mediating inflammatory response[30].In this study,NO level was quantified by using Griess reagent.The principle behind the assay involves the formation of an azo compound when diazonium compounds react withN-(1-naphthyl) ethylenediamine.The formation of the azo derivative can be quantified spectrophotometrically at 548 nm.In brief,RAW 264.7 cells were incubated in a 96-well plate at a total concentration of 1.5 × 105cells/mL.The cells were treated with LPS (2.5 µg/mL),coumarin(50 µM),and eugenol (50 µM).The plate was incubated at 37 ℃ in a humidified chamber for 48 h.After incubation,the supernatant was collected from each well and was incubated with freshly prepared Griess reagent for 30 min at 37 ℃.The OD was measured at 548 nm and a sodium nitrite standard plot was used to calculate the concentration of NO in the sample.
2.5. Estimation of intracellular ROS using DCFH-DA dye
The intracellular ROS was estimated by plating the cells with DCFH-DA.This dye readily enters the cells and cellular enzymes deacetylate the DCFH,after which the ROS converts the DCFH into fluorescent by-product DCF which can be visualized using a fluorescence microscope.The 10 mM stock of DCFH-DA was prepared by dissolving 4.86 mg of DCFH-DA in DMSO and diluted to 1 mM concentration for use.Briefly,the RAW 264.7 cells treated with LPS,coumarin,and eugenol were washed twice with PBS and incubated with 20 µM of DCFH-DA dye for 30 min in the incubator.The cells were then observed under a fluorescence microscope(Nikon Eclipse Ti2 microscope) and the relative fluorescence was calculated using ImageJ software.
2.6. Hemolytic assay
The human ethical clearance was obtained from the Institutional Human Ethical Committee (IHEC),DIPAS approved on 18th May 2022 (IHEC/DIPAS/VI-C-17/2022).The purpose of the study was informed to the participants before sample collection.Six mL venous blood was collected from healthy human volunteers (n=3) in 15-mL Vacutainer tubes containing heparin and washed three times with sterile saline solution (0.15 M NaCl) by centrifugation at 1 500 rpm for 5 min.The pellet was resuspended in 0.5% saline solution.A volume of 0.5 mL of the cell suspension was mixed with 50 µM and 100 µM of coumarin and eugenol.The mixtures were placed in an incubator at 37 ℃ for 30 min.Following incubation,the samples were subjected to centrifugation at 2 000 rpm for 10 min.The resulting supernatants were then analyzed for free hemoglobin content using a spectrophotometer(BioTek Synergy HTX Multimode Reader) at a wavelength of 540 nm.Negative and positive hemolytic controls were prepared using saline and distilled water,respectively.
2.7. Western blot assay
The RAW 264.7 cells treated with LPS,coumarin,and eugenol were lysed using radioimmunoprecipitation assay buffer (1 mM EDTA,1%,10 mM Tris HCL,0.1% SDS,140 mM NaCl,Triton X-100,10 μL protease inhibitor cocktail,and 1 mM PMSF).The cells were incubated with RIPA buffer on ice for 30 min and then briefly vortexed.The lysate was centrifuged at 14 000 rpm for 20 min at 4 ℃.The supernatant was collected and the total protein content was estimated using a BCA reagent.Briefly,30 µg of protein was loaded onto 10%SDS-PAGE and transferred on a PVDF membrane.Primary antibodies specific for NF-κB (ab16502),NLRP3 (MA5-16274),IL-1β (M421B),COX-2 (ab179800),and beta-actin (PA1-183) (dilution 1∶500) in blocking buffer,were incubated with the membrane for binding to target proteins.The membrane was then washed thrice with TBST and secondary antibody conjugated to HRP was added.The expression of the protein was estimated using a chemiluminescence substrate.The Western blots were quantitated using the ImageJ software available online.
2.8. Statistical analysis
Data were statistically analyzed using one-way ANOVA test using GraphPad Prism version 7 software.Statistical significance was set atP<0.05.
3.Results
3.1. Effect of coumarin and eugenol on cell viability
To test whether coumarin and eugenol possess any cytotoxic effect on RAW 264.7 macrophage cells,we assessed their effects on cell viability using an MTT assay.As shown in Figure 1,coumarin and eugenol exerted no cytotoxic effect on cells at the given concentration.Hence all the experiments were performed using the abovementioned concentrations.

Figure 1.Effects of coumarin and eugenol on the viability of RAW 264.7 cells by MTT assay.Cells were treated with 2.5 μg/mL of LPS and 50 μM of coumarin and eugenol for 24 h.Results are presented as mean±SE.LPS:lipopolysaccharide.
3.2. Effect of coumarin and eugenol on LPS-stimulated NO production
To estimate the amount of NO produced by cells,we performed a Griess assay.LPS-treated cells showed a significant increase in NO levels which was significantly reversed by coumarin and eugenol at 50 µM (Figure 2).

Figure 2. Effects of coumarin and eugenol on LPS-stimulated nitric oxide production in RAW 264.7 cells by Greiss assay.Results are expressed as mean±SE.###P<0.001 compared with the control group.**P <0.01,***P<0.001 compared with LPS-treated group.
3.3. Analysis of hemolytic activity
This assay was performed to analyze the hemolytic activity of coumarin and eugenol in human blood.Coumarin and eugenol did not exhibit hemolytic activity at any of the doses tested in comparison to distilled water-treated cells (Figure 3).

Figure 3.The hemolytic effect of coumarin and eugenol on human red blood cells.Results are expressed as mean±SE.
3.4. Coumarin and eugenol inhibit the intracellular production of ROS in LPS-stimulated RAW 264.7 macrophages
A considerable increase in intracellular ROS production was observed after LPS stimulation.However,LPS-stimulated cells treated with coumarin and eugenol showed decreased ROS generation in comparison to the LPS group (Figure 4).The results suggested the role of both coumarin and eugenol in suppressing excessive production of ROS in stimulated RAW 264.7 macrophage cells.
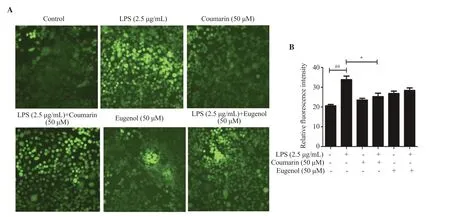
Figure 4.Effects of coumarin and eugenol on intracellular ROS production in LPS-stimulated RAW 264.7 cells (magnification: 20×).(A) DCFH-DA dye was used to observe intracellular ROS production by immunoflourescence microscopy.(B) The relative fluorescence intensity was quantified using ImageJ software.Results are expressed as mean±SE.##P<0.01 compared with the control group.*P <0.05 compared with LPS-treated group.
3.5. Coumarin and eugenol inhibit the expression of NLRP3,IL-1β, COX-2 and NF-κB in LPS-stimulated RAW 264.7 cells
To further explore the anti-inflammatory properties of coumarin and eugenol in LPS-stimulated RAW 246.7 cells,we examined the protein expression of NLRP3 which is known to mediate the production of IL-1β from pro IL-1β.A significant rise in the protein expressions of NLRP3,IL-1β,NF-κB,and COX-2 was observed in LPS-stimulated cells.Coumarin and eugenol could diminish LPSinduced increased expressions of these proteins (Figure 5).Taken together,these results suggest that coumarin and eugenol exert their anti-inflammatory response by regulating the expressions of NLRP3,IL-1β,NF-κB,and COX-2.
4.Discussion
As the field of anti-inflammatory drug discovery continues to expand,an increasing number of studies have highlighted the potential of plant-derived immunomodulatory compounds as a significant source of anti-inflammatory drugs.Among these compounds,coumarin and eugenol,two naturally occurring compounds found in various plants have garnered great attention for their potent anti-inflammatory properties.While the anti-cancer[31,32],antioxidant[33,34] and anti-inflammatory properties[35,36] of coumarin and eugenol have been reported,the exact mechanism through which they exert anti-inflammatory effects remains unclear.In this study,we investigated the mechanism through which coumarin and eugenol exert their anti-inflammatory effects.
A timely and controlled inflammatory response is required by the host to combat against infectious agents.However,an uncontrolled and dysregulated inflammatory response can contribute to tissue damage and can become a chronic inflammatory disease[37].During inflammatory events,macrophages are known to produce various pro-inflammatory mediators upon stimulation with LPS.It is a component of the cell wall of Gram-negative bacteria which is known to promote inflammatory response,therefore LPS-stimulated RAW 264.7 macrophages have been commonly used as an essential model for investigating anti-inflammatory agents.Therefore,in the present study,the mechanism through which both coumarin and eugenol exert their anti-inflammatory effects was evaluated in LPSstimulated macrophages.
Cell viability study indicated that 50 µM of coumarin and eugenol did not cause any cytotoxic effect on the cells.This was confirmed by the lack of significant changes in cell viability observed during treatment of cells with these compounds.Additionally,both coumarin and eugenol did not exhibit any significant hemolytic activity compared to negative control,suggesting that they do not cause lysis of red blood cells.
An increase in the production of NO in LPS-stimulated RAW 264.7 cells is indicative of an exaggerated inflammatory response.The excessive production of NO leads to the translocation of NFκB to the nucleus which enhances the expression of various proinflammatory cytokines and chemokines.Coumarin and eugenol significantly reduced NO production in LPS-stimulated cells.During inflammation,in addition to NO,the levels of ROS are also increased.ROS act as important signaling molecules during inflammatory events but overproduction of ROS during chronic inflammation leads to detrimental effects like tissue injury[38,39].It was observed that coumarin and eugenol suppressed ROS production in LPS-stimulated cells which might indicate towards the property of both the compounds in scavenging free radicals.In a manner similar to NO,prostaglandins E2is also known to act as a pro-inflammatory mediator which is produced by the action of COX-2 enzyme.The inducible form of cyclooxygenase enzyme is known to play a key role in inflammatory response.In the study,coumarin and eugenol suppressed the expression of COX-2 in macrophage cells stimulated by LPS.
A member of the NOD-like receptor family,NLRP3 is also known to play a crucial role in inflammation.When NLRP3 is activated,it oligomerizes and forms a complex called the inflammasome,which then activates caspase-1,an enzyme that cleaves and activates proinflammatory cytokines such as IL-1β and IL-18.The increased expression of the NLRP3 inflammasome as well as IL-1β in LPSstimulated RAW 264.7 cells was ameliorated by coumarin and eugenol.The property of coumarin and eugenol to suppress the activation of inflammasome is associated with reduced production of IL-1β,which can further aid in resolving local inflammation.
The transcription factor NF-κB is known as the master regulator of inflammation as it induces the transcription of many proinflammatory cytokines as well as mediates the activation of T cells[40,41].It has been reported in multiple studies that a change in the expression of NF-κB is associated with various inflammatory diseases[42-44].The activation of NF-κB requires the proteolytic degradation of the inhibitory protein called IκB-α,after which the activated NF-κB translocates to the nucleus.Once inside the nucleus NF-κB regulates the expression of various genes involved in the process of inflammation,including cytokines.In this study,treatment with coumarin and eugenol also inhibited NF-κB activation.
In summary,our findings suggest that coumarin and eugenol inhibit the overproduction of important inflammatory mediators like NO and ROS in LPS-stimulated macrophages.In addition to this,both these plant-derived immunomodulatory compounds exert their antiinflammatory effects by suppressing the increased expression of NLRP3 and its downstream pro-inflammatory cytokine IL-1β in LPS-exposed macrophage cells.Most importantly,the expression of the major transcription factor which regulates the inflammatory response is also ameliorated by both compounds.Coumarin and eugenol may be potential therapeutic agents against various inflammatory diseases.While this study provides valuable insights into the anti-inflammatory mechanism of coumarin and eugenol in LPS-stimulated macrophage cells,furtherin vivostudies are necessary to fully understand their effects.Given the involvement of NLRP3 in various inflammatory diseases,targeting this inflammasome has attracted significant interest.
Conflict of interest statement
The authors declare no competing interests.
Acknowledgments
The authors would like to thank Ms Gitika Sharma for her support during the study.JB thanks DRDO for financial support in the form of a Junior Research Fellowship.
Funding
This work was supported by the Defence Institute of Physiology and Allied Sciences.
Data availability statement
The data supporting the findings of this study are available from the corresponding authors upon request.
Authors’contributions
KPM designed the study;JB performed the experiments and collected the data;JB and KPM analyzed the data;JB and KPM drafted the manuscript;KPM and SS provided the resources and materials;JB,SS and KPM edited the manuscript.All authors have read and agreed to the published version of the manuscript.
 Asian Pacific Journal of Tropical Biomedicine2024年1期
Asian Pacific Journal of Tropical Biomedicine2024年1期
- Asian Pacific Journal of Tropical Biomedicine的其它文章
- CRX-527 as a candidate adjuvant in a recombinant BCG-based malaria vaccine
- Agmatine ameliorates diabetes type 2-induced nephropathy in rats
- Ethanol extract of Abelmoschus manihot suppresses endoplasmic reticulum stress in contrast-induced nephropathy
- Foeniculum vulgare Mill.inhibits lipopolysaccharide-induced microglia activation and ameliorates neuroinflammation-mediated behavioral deficits in mice
