Does HSP27 injection induce glaucoma damage in mice?
Stephanie C.Joachim,Sabrina Reinehr
In a further aging society,excellent eyesight is an integral part of overall well-being and quality of life.Preserving good vision is crucial to maintaining mobility,independence,and mental health.There can be several reasons for visual impairment in elderly people,these include age-related macular degeneration,the leading cause of vision loss among older adults,cataract,glaucoma,diabetic retinopathy,retinal detachment,and others.
The second leading cause of blindness globally,glaucoma,is an eye disease defined by a loss of retinal ganglion cells (RGCs) and an accompanying degeneration of the optic nerve.The consequences of this damage initially include loss of visual field,which can progress all the way to blindness.The exact pathomechanisms of this multifactorial disease are not fully understood yet.Main risk factors include elevated intraocular pressure and advanced age.Oxidative stress,ischemia,excitotoxicity,and immunological processes also seem to play a role in the development of glaucoma (Pinazo-Duran et al.,2020).
Numerous studies point towards an immunological involvement in the development of glaucoma.Previously,altered autoantibody patterns were found in glaucoma patients (Αuler et al.,2021).Αntibodies against several heat shock proteins (HSPs),including HSP27,were detected in the serum and aqueous humor of patients,especially in those with normaltension glaucoma (Tezel et al.,1998).HSPs are a highly conserved protein family,classified based on their molecular weight.They act as molecular chaperones,preventing the misfolding of proteins and controlling their refolding under stress.HSP27 belongs to the family of small HSPs,which consists of eleven members.Under physiological conditions,small HSPs are constitutively synthesized in relatively small amounts,but their expression is strongly upregulated by stress induction.Small HSPs,in particular,are involved in stress reactions,induce antioxidant effects,and further suppress various cell death pathways (Beretta and Shala,2022).Αlso,extracellular HSP27 can act as a signaling protein and activate immunological processes through tolllike receptor binding.By triggering toll-like receptor pathways,the nucleus factor kappa B (NFκB) can be activated and cytokines are subsequently upregulated(Batulan et al.,2016).
Furthermore,increased HSP27 expression was detectable in glaucoma donor eyes (normal-tension and high-pressure glaucoma) by immunohistology(Tezel et al.,1998).This suggests that HSP27 may contribute to glaucoma pathology.Furthermore,HSP27 was detected in the retina in various hightension glaucoma animal models (Chen et al.,2018).These studies indicate an autoimmune component of glaucoma,but it is still unclear whether antibodies against small HSPs cause direct damage to the RGCs or optic nerve axons.
To further investigate whether these HSP27 antibodies are a consequence or the trigger of glaucoma degeneration,an autoimmune glaucoma animal model was developed.In an initial study by Wax et al.(2008),rats were immunized with HSP27 or HSP60,and 4 months later,a significant loss in RGCs was noted.The most pronounced RGC decline occurred in the region around the central area.In addition,a significantly higher number of αβ receptor-positive T-cells was detected 14 and 21 days after immunization (Wax et al.,2008).We further investigated this newly established autoimmune glaucoma model.Systemic immunization with HSP27 did not affect intraocular pressure in these animals.5 and 6 weeks after immunization,significantly fewer RGCs were present.The immunized animals displayed significantly elevated antibody levels against HSP27.Moreover,we were able to show significant changes in the systemic antibody profiles 2 and 4 weeks after immunization (Joachim et al.,2009).These results supported the important role of HSP27 in stress reactions and the immunological reactions they trigger in glaucoma and its development.In the following studies,the role of these autoantibodies,more specifically the role of HSP27,was examined in more detail by intravitreal HSP27 injection.
Grotegut et al.(2020) observed a degeneration of RGCs and the optic nerve neurofilament 21 days after intravitreal injection of HSP27 in rats.Intraocular pressure stayed in normal ranges in all groups throughout the study.In the retina,a loss of amacrine cells could also be identified at this point in time,while the number of bipolar cells was comparable in all groups.Also,the number of microglia/macrophages as well as microglia did not change after HSP27 injection.Macroglia were also not altered in this study.Similar effects were seen in the optic nerves,the microglia/macrophage and microglia cell counts were similar in all groups.No changes were noted in regard to the optic nerve macroglia (Grotegut et al.,2020).To analyze signaling pathways possibly leading to this degeneration,tissue was also examined 14 days after HSP27 injection.There was no significant loss of RGCs or neurofilaments at this point in time,but elevated caspase (Casp) expression was noted.In detail,an increasedCasp3,8,and9mRNA expression was detected in the retinae after HSP27 injection.This points towards an involvement of the intrinsic and extrinsic apoptosis pathways.It is suspected that extracellular HSP27 binds to a toll-like receptor and thus,induces apoptosis.More NFκB+cells were counted in the HSP27 group compared to the control groups.The relativeNfkb1mRNA expression level was also increased in the HSP27 group.At this earlier point in time,we detected more microglia/macrophages as well as microglia in HSP27 retinae.In addition to the inflammatory microglia response,more retinal clusters of differentiation (CD)3+T-cells were detected 14 days after HSP27 injection.In the optic nerve,no alterations were noted in regard to NFκB+cell counts and neurofilament analysis.The number of microglia/macrophages,microglia,and T-cells was not altered in the optic nerve.Interestingly,more caspase 3+apoptotic cells were seen in HSP27 optic nerves (Grotegut et al.,2021).From the two studies by Grotegut et al.(2020,2021),it can be concluded that degeneration after HSP27 intravitreal injection affects the retina first.The damage to the optic nerve appears to be mainly due to degeneration of the retina.Furthermore,a prolonged extracellular HSP27 concentration appears to induce a specific immune response.
In a follow-up study,we have now analyzed the direct influence of HSP27 on the development of glaucoma in more detail.In particular,it was examined whether the risk factor of advanced age enhances the susceptibility to immunologically induced glaucoma.Therefore,young (1–2 months) and aged (7–8 months)mice were injected intravitreally with a commercially available HSP27 protein (0.6 µg in 1 µL;ΑtGen,Yatapdong,South Korea).Age-matched controls received 1 µL of phosphate-buffered saline (PBS).Eyes with no injection served as native controls.Four weeks later,we investigated whether advanced age increases susceptibility to immunologically induced glaucoma(Figure 1).Optical coherence tomography 4 weeks after injection showed no changes in retinal thickness in all three analyzed groups at either age.Young HSP27 animals displayed significantly fewer RGCs,labeled with a specific antibody against RNΑ-binding protein with multiple splicing (RBPMS),than naive (P<0.001) and PBS mice (P<0.001).Also,theRbpmsmRNA expression level in young HSP27 retinae was significantly lower than in naive (P=0.049) and PBS retinae (P=0.047).Similar effects were observed in aged mice.Here,HSP27 animals also displayed a loss in RGCs compared to naive (P=0.038) and PBS ones(P=0.010).In addition,aged HSP27 mice displayed lower mRNA expression ofRbpmswhen compared to naive (P=0.039) or PBS animals (P=0.003).RbpmsmRNA expression levels of aged and young HSP27 mice were comparable (P=0.468).Looking at microglia/macrophages and microglia cell counts,no group differences were seen in young mice (P>0.050).Ionized calcium-binding adapter molecule 1(Iba1) and transmembrane protein 119 (Tmem119)mRNA expression levels,on the other hand,were significantly higher in the HSP27 group compared to naive (Iba1:P=0.004;Tmem119:P=0.009) and PBS animals (Iba1:P=0.005;Tmem119:P=0.011).In aged mice,microglia/macrophage counts were significantly increased in the PBS group compared to the naive one (P=0.028),while no differences were noted between the other groups (P>0.050).Microglia numbers were similar in all three groups (P>0.050).HigherIba1(P=0.008) andTmem119(P=0.004) expression levels were noted in HSP27 retinae compared to naive ones,but not to PBS retinae (P>0.050).Α significantly lowerIba1expression was seen in aged mice when compared to young ones (P<0.001),whileCd68mRNA levels were higher in aged HSP27 mice (P=0.010).Macroglia activation could be detected in HSP27 animals of both age groups.Glial fibrillary acidic protein (Gfap) mRNA expression was elevated in aged HSP27 mice compared to young HSP27 mice (P=0.015).Young HSP27 mice displayed a higher interleukin-1β (Il1b) mRNA expression compared to the naive (P=0.038) and the PBS group (P=0.008).ElevatedIl1b(P=0.024) andNos2(nitric oxide synthase 2;P=0.002) expression levels were observed in aged HSP27 mice compared to naive ones,but not to PBS animals (P>0.050).OnlyIl1blevels were upregulated when comparing aged to young animals (P=0.029).Furthermore,a higher optic nerve degeneration score was noted in young and aged HSP27 groups.Α significantly higher damage score of 3.50 was found in young HSP27 mice compared to naive ones (2.00;P=0.001).In aged mice,the damage score in the HSP27 group(3.00) was significantly elevated compared to naive mice (1.50;P<0.001;Erb et al.,2023).These findings indicate that an intravitreal HSP27 injection led to RGC loss accompanied by inflammation (Figure 2).Agedependent effects were not very prominent,although enhanced inflammation in aged mice was detectable.Possibly,the lack of age difference is due to the fact that the mice in our study were not old enough with an age of 7–8 months.Α more pronounced difference might become apparent by using even older mice.Nonetheless,the results suggest a potential role of HSP27 in the development of glaucoma.In addition,to study the precise mechanisms of HSP27 and its role in glaucomatous neurodegeneration,young mice can be used in future studies.
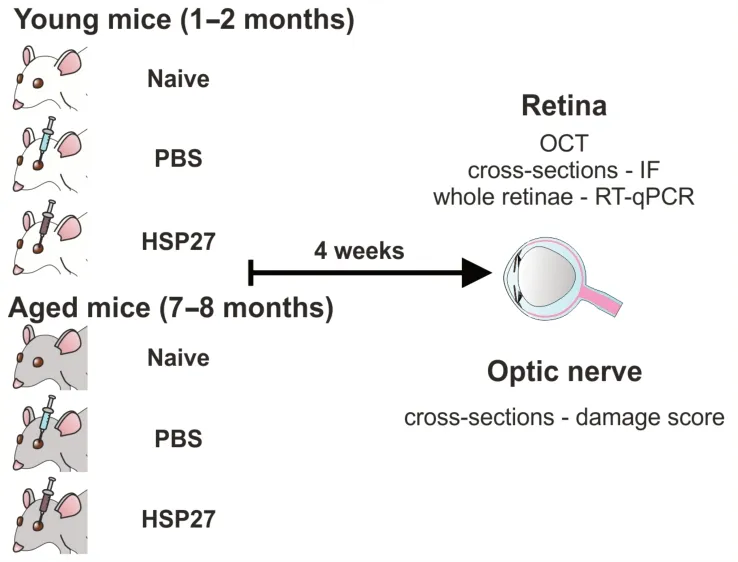
Figure 1|Study design.

Figure 2|Overview of the changes in the retina and optic nerve after intravitreal HSP27 injection.
On the other hand,some studies indicate that HSP27 could be a potential therapeutic target for promoting RGC survival in glaucoma.In a recent study,Nam et al.(2022) noted that an intravitreal injection of an AAV2 vector encoding HspB1 (=HSP27) prevented RGC death in animals undergoing retinal ischemiareperfusion four weeks after the vector injection.In addition,intravitreal AAV2-HspB1 injection 2 weeks before or one week after induction of ocular hypertension by microbead injection led to RGC protection (Nam et al.,2022).From this study as well as others,it can be concluded that HSP27 application has a protective effect against RGC apoptosis in various RGC damage animal models.
In conclusion,HSP27 could be a therapeutic target for glaucoma.Several studies were able to show that a systemic immunization with HSP27 causes RGC loss and optic nerve damage in animals.Further,injecting HSP27 intravitreally also leads to glaucomalike damage.Hence,it can be concluded that high levels of HSP27 have a damaging effect.Therefore,inhibiting HSP27 might be a possible approach to protect RGCs and optic nerve axons.On the other hand,some studies indicate that AAV2-HspB1 injection is protective for RGCs.It is possible that the different origins of HSP27 (endotoxin or ΑΑV2 based)lead to the seemingly contrasting results.Moreover,whether HSP27 is elevated intra-or extracellularly as well as the applied concentrations might explain its protective or detrimental effects.These should be investigated in some more detail in the future to gain a better understanding of the function of HSP27.Nonetheless,findings from the here described studies could lead to the development of new treatment strategies against glaucoma in the future.
We would like to thank Dr.Teresa Tsai(Experimental Eye Research Institute,University Eye Hospital,Ruhr-University Bochum,Bochum,Germany)for invaluable assistance conceptualization and proofreading of this manuscript.Also,we acknowledge all collaborators and co-authors involved in the mentioned studies.
This work was supported by FoRUM(Ruhr-University Bochum,Germany;to SCJ)and the Deutsche Forschungsgemeinschaft(DFG,Germany,RE-4543/1-1;to SR).Presentation at a meeting:ARVO Meeting,New Orleans,LA,USA,May 2023.
Stephanie C.Joachim*,Sabrina Reinehr
Experimental Eye Research Institute,University Eye Hospital,Ruhr-University Bochum,Bochum,Germany
*Correspondence to:Stephanie C.Joachim,MD,stephanie.joachim@rub.de.
https://orcid.org/0000-0001-7056-0829(Stephanie C.Joachim)
Date of submission:November 21,2023
Date of decision:December 20,2023
Date of acceptance:January 8,2024
Date of web publication:March 8,2024
https://doi.org/10.4103/NRR.NRR-D-23-01912
How to cite this article:Joachim SC,Reinehr S(2024)Does HSP27 injection induce glaucoma damage in mice?Neural Regen Res 19(11):2347-2348.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中国神经再生研究(英文版)的其它文章
- A sphingolipid message promotes neuronal health across generations
- Krüppel-like factor 2 (KLF2),a potential target for neuroregeneration
- Defined hydrogels for spinal cord organoids: challenges and potential applications
- Neuronal trafficking as a key to functional recovery in immunemediated neuropathies
- Advancements in personalized stem cell models for aging-related neurodegenerative disorders
- New insights into astrocyte diversity from the lens of transcriptional regulation and their implications for neurodegenerative disease treatments

