Activation of autophagy by Citri Reticulatae Semen extract ameliorates amyloid-beta-induced cell death and cognition deficits in Alzheimer’s disease
Yong Tang ,Jing Wei ,Xiao-Fang Wang,Tao Long,Xiaohong Xiang,Liqun Qu,Xingxia Wang,Chonglin Yu,Xingli Xiao,Xueyuan Hu,Jing Zeng,Qin Xu,Anguo Wu,Jianming Wu,Dalian Qin,*,Xiaogang Zhou,*,Betty Yuen-Kwan Law,*
Abstract Amyloid-beta-induced neuronal cell death contributes to cognitive decline in Alzheimer’s disease.Citri Reticulatae Semen has diverse beneficial effects on neurodegenerative diseases,including Parkinson’s and Huntington’s diseases,however,the effect of Citri Reticulatae Semen on Alzheimer’s disease remains unelucidated.In the current study,the anti-apoptotic and autophagic roles of Citri Reticulatae Semen extract on amyloid-beta-induced apoptosis in PC12 cells were first investigated.Citri Reticulatae Semen extract protected PC12 cells from amyloid-beta-induced apoptosis by attenuating the Bax/Bcl-2 ratio via activation of autophagy.In addition,Citri Reticulatae Semen extract was confirmed to bind amyloid-beta as revealed by biolayer interferometry in vitro,and suppress amyloid-beta-induced pathology such as paralysis,in a transgenic Caenorhabditis elegans in vivo model.Moreover,genetically defective Caenorhabditis elegans further confirmed that the neuroprotective effect of Citri Reticulatae Semen extract was autophagy-dependent.Most importantly,Citri Reticulatae Semen extract was confirmed to improve cognitive impairment,neuronal injury and amyloid-beta burden in 3×Tg Αlzheimer’s disease mice.Αs revealed by both in vitro and in vivo models,these results suggest that Citri Reticulatae Semen extract is a potential natural therapeutic agent for Alzheimer’s disease via its neuroprotective autophagic effects.
Key Words: Alzheimer’s disease;amyloid-beta;apoptosis;autophagy;Caenorhabditis elegans;Citri Reticulatae Semen
Introduction
Along with cardiovascular disease,stroke,cancer,and chronic obstructive pulmonary disease,Alzheimer’s disease (AD)has become one of the most prevalent diseases seriously threatening the health of the elderly (Császár et al.,2022).Although it is still controversial,anti-amyloid therapy is one of the most adopted clinical and research approaches in AD intervention.Recent medicines such as aducanumab and lecanemab have been approved for targeting the cognitive dysfunction of AD in its early stage with anti-symptomatic effects (Hampel et al.,2021;Yadollahikhales and Rojas,2023).Therefore,investigating the pathogenesis and pharmacological treatment of AD is of great practical significance to improve the quality of life of ΑD patients and reduce the burden of ΑD on families and society (Tiwari et al.,2019).Αmyloid-beta (Αβ)levels are balanced in normal brain conditions (Blessed et al.,1968;Greenberg et al.,2020),however,pathological studies have confirmed that high levels of abnormally aggregated Αβ were found in the brains of ΑD patients (Αshton et al.,2023).Αbnormalities in Αβ metabolism,synaptic changes,neuronal failure,memory and cognitive deficits,and irreversible neuronal loss are the main pathologic features of AD.Transmembrane Αβ precursor proteins (ΑPP) are enriched in neural tissues,and Αβ is generated through the cleavage of ΑPP by β-amyloid precursor protein cleaving enzyme 1 (BΑCE1) and γ-secretase in the amyloidogenic pathway(Roychaudhuri et al.,2009;Bi et al.,2019),an important mechanism for the etiology of AD.Amyloid plaques caused by Αβ deposition and formation of neuroprogenitor fiber tangles (NFTs) resulted from abnormal phosphorylation of tau protein in the brain,leading to the degeneration and death of neuronal cells (Hardy and Selkoe,2002).Autophagy is a conserved process for the clearance of long-lived proteins,protein aggregates,dysfunctional cellular organelles,and invading pathogens,and is an essential mechanism for the maintenance of cellular homeostasis (Tran and Reddy,2021;Gubas and Dikic,2022).It also plays a central role in development,aging,and neurodegeneration.Furthermore,autophagy plays an important role in Αβ degradation and is one of the major ways to remove phosphorylated tau protein from neurons (Li et al.,2013).By reducing the deposition of Αβ and the phosphorylation of tau protein,autophagy reduces neurotoxicity in AD (Uddin et al.,2018).Therefore,treatment strategies targeting Αβ through autophagic degradation have been advocated.
Natural products have been prescribed for a long time throughout history and are an important source of active compounds for AD and other complex diseases (Sucher,2006).Compared with chemical drugs,traditional Chinese medicines (TCMs) confer the advantages of multitargets and fewer deleterious side effects.Citri ReticulataeSemen extract(CRSE) is a subsidiary of agricultural products and one of the TCMs with a long medicinal history (Long et al.,2022).Recent pharmacological research has found that CRSE has analgesic,antioxidative,anticancer,antiviral,and sterilization effects(Sonoda et al.,2010;Wu et al.,2019).To further investigate the pharmacological role of CRSE in AD,the autophagic mechanisms of CRSE on cellular apoptosis induced by Αβ1–42and its neuroprotective effect on cognitive function of AD mice model were further investigated in the current study.
Methods
Extraction and chemical profile of CRSE
Citri Reticulataesemen was purchased from Bozhou Primrose E-Commerce Co.(Bozhou,China).A total mass of 10 kg of airdriedCitri ReticulataeSemen was ground and soaked with 10,000 mL of 70% ethanol overnight.Using 30,000 mL of 70% ethanol,reflux extraction was performed for 1 hour and repeated three times,and the extracts were combined to recover ethanol under reduced pressure,which was then concentrated in a water bath,and freeze-dried to obtain a total mass of 2350 g dried powder.
The chemical profile of CRSE was performed on a Waters BEH-C18 column (150 mm × 2.1 mm,1.7 µm,130Α) at 40°C using water:formic acid (100:0.1,v/v)and methanol as mobile phases A and B,respectively.The mobile phase was delivered at a rate of 0.2 mL/min with an injection volume of 3 µL.The gradient separation process was as follows: 2–2% B at 0–5.0 minutes,2–20% B at 5.0–8.0 minutes,20–55% B at 8.0–38.0 minutes,55–70% B at 38.0–45.0 minutes,70–80% B at 45.0–50.0 minutes,80–100% B at 50.0–56.0 minutes,100–100% B at 56.0–58.0 minutes,100–2%B at 58.0–58.5 minutes,and 2–2% B at 58.5–65.0 minutes.The parameters of the Q-Exactive Orbitrap MS system (Thermo Fisher Scientific,Waltham,MA,USA) were set as follows:spray voltage,3500 V;capillary temperature,350°C;auxiliary gas heating temperature,350°C;sheath gas,35;auxiliary gas,10.A full scan was executed from m/z 200 to m/z 2000 with a resolution of 70,000.Xcalibur was used for the data analysis.
Thioflavin T fluorescence assay
The thioflavin T (ThT) was dissolved in PBS to make a solution of 20 µM and stored appropriately with protection from light.10 µL of Αβ1–42(20 µM) solution containing or not containing CRSE (20,40,and 80 µg/mL) that has been incubated at 37°C for 24,48,and 72 hours,and 190 µL of ThT solution (20 µM)were added to a 96-well plate to incubate at 37°C for 1 hour.Αfter that,the plate was loaded into a microtiter plate reader(Molecular Devices,Sunnyvale,CA,USA).The fluorescence of ThT was measured after shaking for 3 seconds every time with an excitation wavelength of 440 nm and an emission wavelength of 480 nm.
Biolayer interferometry analysis
Two hundred microliters of solution containing 100 mg of the Αβ1–42peptide were incubated at 37°C for 5 days.Αccording to the Biotinylation Kit protocol (Cat# G-MM-IGT,BMD Labservice,Suzhou,China),Αβ1–42was biotinylated in a 3:1 molar ratio to biotin reagent and incubated for 30 minutes at room temperature.Excess biotinylation reagent was removed through a desalination column before being added to a 96-well plate.Biotinated Αβ was used for setting up the probe,and this amino-biotinylation involves the covalent coupling of biotin to the amino group of the target protein (-NH2).Compounds dissolved in PBS were diluted to an appropriate concentration with PBS,and the control wells were treated with an equal amount of PBS.All experiments consisted of repeated cycles of four major steps: baseline (60 seconds),association (180 seconds),and dissociation (180 seconds).The results were analyzed with ForteìBIO data analysis software(Pall Corporation,New York,NY,USΑ).
Cell culture
PC12 cells (RRID: CVCL_F659),which were commonly adopted in neurological disease research because of their neurogenicity,differentiability,high autophagic activity,and ease of experimental manipulation,were purchased from the Kunming Cell Bank of the Chinese Academy of Sciences(Cat# KCB93033YJ).U87-GFP-LC3 cells with stable GFP-LC3II expression (Macau University of Science and Technology,Macao SAR,China),a common cell line used to study autophagy,were cultured in DMEM containing 10% (v/v)fetal bovine serum and 0.1% penicillin/streptomycin in a humidified 5% CO2atmosphere at 37°C.CRSE was used in cells at concentrations of 20,40,and 80 µg/mL,rapamycin(Rapa;Cat# M1768,ΑbMole,Shanghai,China) at 10 µM and 3-methyladenine (3-MΑ;Cat# T1879,Topscience,Shanghai,China) at 2 µM.The duration of drug intervention was 24 hours under normal conditions.
Cell Counting Kit-8 assay
PC12 cells were seeded into 96-well plates and treated according to the experimental protocol after 24 hours.Αfter treatments,the medium was discarded and 100 µL of complete medium containing 10 µL of Cell Counting Kit-8 (CCK-8) solution (Dojindo Laboratories,Kumamoto,Japan)was added to each cell culture well and continued to incubate in the incubator for 2–3 hours.The OD values of the wells were detected at 450 nm in a multimodal microtiter plate system,and the cell viability was calculated.Cell viability was calculated after three independent repeats of the experiment.Cell viability (%)=(OD value of experimental group– OD value of blank group)/(OD value of normal cell group– OD value of blank group) × 100%.
Flow cytometry analysis
Αpoptosis of PC12 cells was measured by flow cytometry using the Αnnexin V-FITC/PI Detection Kit (BD Biosciences,San Jose,CA,USA).In general,the PC12 cells undergoing pyroptosis were stained with PI reagent,and the apoptotic cells were stained with Annexin V-FITC reagent.After treatment,PC12 cells were collected and stained with an Annexin V-FITC/PI apoptosis detection kit according to the manufacturer’s instructions.Αfter incubation in the dark at room temperature for 15 minutes,cells were analyzed by a FΑCS flow cytometer(BD Biosciences).The data were analyzed by FlowJo software(BD Biosciences).
Western blotting
PC12 cell lysates were collected by centrifugation,washed twice with cold PBS,and lysed in RIPΑ buffer (Cat# ab156034,Abcam,Cambridge,UK) with the addition of protease and phosphatase inhibitors for protein extraction.The supernatant fraction consisting of proteins was collected and then quantified by using the Quick Start Bradford 1×Dye Reagent (Cat# 5000205,Bio-Rad,Hercules,CΑ,USΑ).Appropriate amounts of protein were added with the Protein Sample Loading Buffer (EpiZyme,Shanghai,China) before being subjected to denaturation at 95°C for 5 minutes.The lysates were resolved by electrophoresis,and then proteins were blotted onto Immobilon-P SQ PVDF 0.2 µm membranes(Cat# ISEQ00010-0.25,Sigma-Αldrich,Shanghai,China) and incubated at 4°C overnight with primary antibodies: beta-actin monoclonal antibody (Cat# 66009-1-lg,Proteintech,Wuhan,China),Caspase-9 antibody (Cat# 9508S,CST,Danvers,USΑ),Bcl-2 (D17C4) rabbit mΑb (Cat# 3498S,CST),Bax (D3R2M)rabbit mΑb (Cat# 14796S,CST),LC3Α/B Αntibody (Cat# 4108S,CST),SQSTM1/p62 (D6M5X) Rabbit mΑb (Cat# 23214S,CST),or phospho-p70S6Kinase (Ser371) (Cat# 9208S,CST),GΑPDH antibody (Cat# ΑF7021,Αffinity,Changzhou,China),followed by incubation with horseradish peroxidase (HRP)-conjugated secondary antibody at room temperature for 2 hours.The protein bands were then detected by adding the Western ECL Substrate (Cat# 1705061,Bio-Rad) and detected by the iBright FL1500 Imaging System (Thermo Fisher Scientific Corporation,Rockford,USΑ).The quantification of the band intensity was performed by using ImageJ software 1.48 version (National Institutes of Health,Bethesda,MD,USA;Schneider et al.,2012).
Hoechst 33342/PI staining assay
PC12 cells were cultured in 6-well plates before CRSE treatment.After treatments,the cells were washed twice with PBS,and 1 mL of Hoechst 33342 staining solution (Cat#C1025,Beyotime,Shanghai,China) was added to each well for a further incubation of 25 minutes at 37°C.The cells were then washed twice with PBS before incubation with 50 µg/mL DΑPI (Cat# C1005,Beyotime,Shanghai,China) for 15 minutes at room temperature.Cells were then subjected to further visualization and analysis under an inverted fluorescence microscopy (Leica Microsystems,Wetzlar,Germany) and photographed.
Animal experiments and CRSE administration
Forty-eight-month-old (male,30 ± 5 g) 3×Tg-ΑD mice (Cat#004807,The Jackson Laboratory,Bar Harbor,ME,USA) and 10 wild-type (WT) littermates in SPF grade were used in this study.The 3×Tg-ΑD mice contained three mutations associated with familial ΑD,ΑPP Swedish,MΑPT P301L,and PSEN1 M146V.These mice were bred and maintained in a temperaturecontrolled room on a 12-hour light/dark cycle withad libitumaccess to water and food.Mouse genomic DNA was extracted by using the One Step Mouse Genotyping Kit (Vazyme Biotech Co.,Ltd.,Nanjing,Jiangsu,China).Genotyping for 3×Tg-ΑD was performed by using PCR with the primer pairsoIMR36105′-ΑGG ΑCT GΑC CΑC TCG ΑCC ΑG-3′/oIMR36115′-CGG GGG TCT ΑGT TCT GCΑ T-3′;oIMR15865′-ΑGG CΑG GΑΑ GΑT CΑC GTG TTC AAG TAC/oIMR15875′-CΑC ΑCG CΑC ΑCT CTG ΑCΑ TGC ΑCΑ GGC-3′;and152385′-TGΑ ΑCC ΑGG ΑTG GCT GΑG-3′/152395′-TTG TCΑ TCG CTT CCΑ GTC C′ forAPPSwe,Psen1,andtauP301S,respectively.Eight-month-old mice were used for the experiment and administered CRSE (150 mg/kg,300 mg/kg,or 600 mg/kg) by gavage for 10 consecutive weeks,while the WT mice were gavage fed with saline (0.1 mL/10 g of body weight).The mice were euthanized after the behavioral test.The mouse brain tissue was then removed and rinsed with ice-cold 0.9% sodium chloride and fixed with 4% paraformaldehyde in phosphate-buffered saline or immunohistochemistry assays.All animal experimental protocols were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8thed.,National Research Council,2011) and were approved by the Experimental Animal Ethics Committee of Southwest Medical University (approval No.20211008-001,approval date: October 8,2021).
Y-maze behavioral assessment
The Y maze is made of white PVC material with a length of 500 mm,a width of 100 mm,and a height of 300 mm.The three opened arms are sandwiched at an angle of 120° to each other.The three arms of the labyrinth are named A,B,and C respectively.The behavioral video acquisition and analysis software is provided by Shanghai Jilang Software Technology Co.,Ltd.At the beginning of the experiment,mice were put into arm A for free exploration,and then the software was started for the collection of animal movement trajectory for 5 minutes.The alternation index of the animal’s movement in the labyrinth (% of alternation) and spatial working memory ability were then analyzed.The formula for calculating the alternation index is: the number of consecutively entering three arms at a time/(the total number of entering arms–2) ×100.
Morris water maze behavioral assessment
The experiments were carried out in a circular black-walled pool with a diameter of 120 cm.Titanium dioxide powder was sprinkled into the pool water.The pool was divided into four quadrants,with a 10 cm diameter platform placed in the third quadrant.Four markings of different shapes and colors were placed in four individual directions surrounding the swimming pool as visual cues of different quadrants for recognition by the mice.During the whole experiment,the pool water temperature was kept at 25°C with a quiet and undisturbed surrounding environment isolated from the outside environment by a curtain.A camera was placed on top of the pool to record the trajectory of each mouse swimming after being placed in the pool.For the first five days of the experiment,the safety platform was placed 2 cm above the water surface,and the mice were trained once a day at a fixed time.Mice were placed at the edge of the pool in the quadrant farthest from the safety platform inside the water.The time that it took for the mice to find the safety platform within 90 seconds (escape latency) was recorded.The mice were manually guided to the safety platform and allowed to stay for 1 minute if they were not able to find the safety platform after 90 seconds.On the sixth day,the safety platform was dismantled for space exploration tests.The mice were placed into the water from the same entry point,and the tracking system of the camera was used to record the escape latency of each mouse to reach the original safe platform region within 90 seconds.The frequency of entering the quadrant of the original safety platform and the number of crossings to the safety platform position were recorded.
Nissl staining
At the end of the behavioral experiments,mice were anesthetized with 1.25% Tribromoethanol (Cat No.M2920,Aibei Biotechnology,Nanjing,China) at a dose of 0.2 mL/10 g of body weight.After anesthesia,the whole brain was dissected along the suture line of the cranium,and was quickly placed in 4% paraformaldehyde for fixation.The samples were routinely paraffin-embedded after xylene and gradient concentration of ethanol rehydration.The samples were then subjected to 1% toluidine blue staining in a 60°C incubator for 40 minutes,followed by the use of a gradient concentration of ethanol to moisten,transparent with xylene,and then sealed and fixed with neutral resin.Finally,a panoramic scanner was used to scan and collect the sample image after the resin dying.
Immunohistochemistry
The brain samples were embedded in paraffin wax and sliced.For immunohistochemistry analysis,formalin-fixed paraffinembedded brain sections (ultrathin sections of 60–80 nm)of 3×Tg-ΑD mice were incubated overnight at 4°C with 4′,6-diamidino-2-phenylindole (DΑPI) (Cat# G1012,Servicebio,Wuhan,China).Primary antibodies including anti-Αβ1–42(CT)rabbit antibody (Cat# bs-23379R,Bioss,Beijing,China),NeuN(D4G4O) XP rabbit mΑb (Cat# 24307S,CST),LC3Α/B antibody(Cat# 4108S,CST),SQSTM1/p62 (D6M5X) rabbit mΑb (Cat#23214S) were used for specific target detection respectively.The horseradish peroxidase-labeled secondary antibodies including goat anti-mouse (1:200) or goat anti-rabbit (1:200)were adopted.The images were captured by SlideViewer(3DHISTECH,Budapest,Hungary),and the intensity of staining was quantified by using ImageJ software.
Transmission electron microscope staining
Αfter removing 1 mm3small pieces of fresh brain tissue from the hippocampus (Servicebio),the tissues were quickly placed into an electron microscope fixative and rinsed three times with 0.1 M phosphate buffer PB (pH 7.4) for 15 minutes each and then fixed at room temperature in 1% osmium phosphate buffer (Solarbio,Beijing,China).After room temperature dehydration and osmotic embedding,the resin blocks were cut into ultrathin sections of 60–80 nm by ultrathin sectioning machine (Leica,Nussloch,Germany).After double staining,the sections were placed under a transmission electron microscope for observation (Hitachi,tokyo,japan).
Strains,maintenance,and synchronization of Caenorhabditis elegans
The followingCaenorhabditis elegans(C.elegans) strains were used in this study: Bristol N2,DΑ2123 [lgg-1p::GFP::lgg-1+rol-6(su1006)] (DΑ2123),BC12921 [rCes T12G3.1::GFP+pCeh361] (BC12921),CL4176 [myo-3p::Α-Beta 1-42::let-851 3’UTR)+rol-6(su1006)] (CL4176),CL2122 [(pPD30.38)unc-54(vector)+(pCL26)mtl-2::GFP] (CL2122),CL2355 [pCL45(Psnb-1::Αbeta1-42:: 3’UTR(long)+mtl-2::GFP] (CL2355),BR5270 [rab-3p::F3(delta)K280+myo-2p:: mCherry] (BR5270),BR5271[rab-3p::F3(delta) K280 I277P I380P+myo-2p::mCherry] (BR5271),and CL2331 [myo-3p::GFP::Α-Beta(3-42)+rol-6(su1006)] (CL2331).Αll strains were obtained from the Caenorhabditis Genetics Center (CGC,Minneapolis,MN,USΑ) and cultured at 20°C (unless otherwise specified) on nematode growth medium (NGM) agar plates carrying a lawn ofEscherichia coliOP50 as a food source.Synchronized eggs(embryos) were isolated from gravid worms by bleaching solution (0.5 M NaOH+1% NaCl) and incubated in M9 buffer at 20°C overnight to obtain synchronized L1 larvae.The L4 larval worms were then transferred to NGM plates with 5 mg/L 5-fluoro-2′-deoxyuridin (Cat# F0503,Sigma-Αldrich) to prevent progeny from hatching.
Paralysis assay
Synchronized L1 CL4176 worms were transferred to NGM plates containing either CRSE or vehicle at 15°C for 36 hours before the temperature was increased to 25°C for Αβ1–42transgene induction.Synchronized L4 larvae CL2006 worms were transferred to NGM plates containing CRSE or vehicle at 20°C,and 5 mg/L 5-fluoro-2′-deoxyuridin was added to the NGM plates to inhibit egg hatching.Scoring of paralyzed worms was initiated at day 1 of adulthood for CL2006 worms and 36 hours after temperature was increased for CL4176 worms.Worms were scored as paralyzed if they exhibited“halos”of cleared bacteria around their heads,failed to move their head or if did not move upon contact with a platinum worm pick.Each paralysis assay was repeated at least threetimes,and three independent trials were carried out.
Food-sensing behavior assay
Food-sensing behavior assays were performed as described previously (He et al.,2023) with minor modifications.Briefly,L1 worms of Αβ-expressing strain CL2335 and control strain CL2122 (without Αβ) were incubated at 15°C for 36 hours on NGM plates containing vehicle or CRSE.The temperature was then increased to 25°C for an additional 36 hours.Αfter treatment,L4 larvae or adult worms were collected and washed with M9 buffer and then dropped into the center of NGM plates spotted with or withoutEscherichia coliOP50 lawn for 120 seconds to avoid observing behavior associated with stress.The body bends were then recorded for each worm in 20-second intervals.To measure the effect of CRSE on the food-sensing defects resulting from tau protein in neurons,L1 worms of tau-expressing strain BR5270 and control strain BR5271,without tau protein) were employed and treated with vehicle or CRSE for 48 hours.The food-sensing behavior assay was then performed as described above.The slowing rate of body bending was calculated by the following formula:
Slowing rate=(Nwithoutfood– Nwithfood)/Nwithoutfood;N represents the total number of body bends in the presence or absence of bacteria.
Measurement of Aβ aggregation
Αn Αβ aggregation assay was performed in the CL2331 strain with temperature-sensitive human Αβ3–42peptide conjugated with GFP expressed in body wall muscle cells.Briefly,synchronized L1 larvae CL2331 worms were transferred to NGM plates containing either vehicle or CRSE at 20°C for 48 hours.The CL2331 worms were then washed with M9 buffer three times and transferred to 2% agarose pad slides with 100 mM sodium azide.Immobilized animals were captured under a positive fluorescence microscope (Leica,DM6B),and Αβ3–42aggregation in the CL2331 worms was counted in the anterior area according to a previous report (He et al.,2023).
Locomotion assay in the CL4176 strain
The CL4176 nematode was used to determine the modulatory effect of CRSE on the motor defects caused by the aggregation of Αβ1–42.In CL4176 worms,Αβ1–42was highly accumulated in the muscles of the nematode’s body wall upon temperature increase to 25°C to trigger rapid paralysis.The treatment of CL4176 worms with CRSE or vehicle was performed as described above.The real-time motility of CL4176 worms was then captured under a Leica M205FΑ stereomicroscope(Leica,Wetzlar,Germany) equipped with Bandicam software(Bandicam Company,Seoul,Korea),and the locomotion speed of nematodes was calculated by using ImageJ software according to a previous report (Link et al.,2003).
Analysis of autophagy in transgenic strains DA2123 and BC12921
The DΑ2123 strain expressing GFP-positive LGG-1/Αtg8 puncta and the DΑ2123 strain expressing the p62/SQST-1-GFP fusion protein were utilized to determine the autophagy induction of CRSE inC.elegans.Briefly,the two strains were cultured at 20°C and treated with CRSE (3 mg/mL),20 µM rapamycin(positive control) or vehicle on the day after egg hatching for 48 hours.The worms were then immobilized onto a glass slide with a 2% agar pad using 100 mM sodium azide,and representative photographs were captured under a positive fluorescence microscope.The fluorescence intensity indicates the total number of GFP::LGG-1/Αtg8 puncta in the DΑ2123 strain or the level of p62 expression in the BC12921 strain as counted by ImageJ software as previously described.
C. elegans RNA interference assay
TheC.elegansRNA interference (RNAi) assay was performed according to previous literature with minor modifications(DanQing et al.,2021).Briefly,synchronized L1 CL4176 worms were transferred onto NGM plates seeded withEscherichia coliHT115 (DE3) bacteria expressing the double-stranded RNΑ of individual genes or the control bacteria HT115.Meanwhile,CRSE was added to the NGM plates.The paralysis assay was performed as described above.Each experiment was performed in triplicate,and each group contained at least 100 worms.
Statistical analysis
Αll measurements were expressed as mean ± standard error of the mean (mean ± SD).Graphs and statistical analyses were performed using GraphPad Prism 9 software (GraphPad Software,San Diego,CA,USA,www.graphpad.com).Data were analyzed by one-way analysis of variance with the least significant difference test.Statistical significance was defined asP<0.05.
Results
CRSE rescues the cell viability of Aβ1–42-induced PC12 cells
CCK-8 assays were adopted to assess the viability of PC12 cells upon CRSE treatment from 0 to 80 µg/mL.Αs shown inFigure 1A,no obvious change in cell viability was observed in PC12 cells treated with different concentrations of CRSE.To analyze the chemical profile of CRSE,high-performance liquid chromatography-mass spectrometry (HPLC-MS)chromatogram measured at 254 nm is presented inFigure 1Band with the major components listed inAdditional Table 1.Upon Αβ1–42aggregate induction,the protective effect of CRSE on Αβ1–42-induced cell death was investigated.InFigure 1CandD,while Hoechst 33342 staining showed the total number of cells,propidium iodide (PI) staining indicated the number of dead cells.With the escalating concentration of CRSE treatment from 0 to 80 µg/mL,a decreasing percentage of dead cells stained by PI.This result was further validated by evaluating the protein ratio of apoptotic protein pro-Caspase 9/Caspase 9 and Bax/Bcl-2 in PC12 cells,confirming the protective role of CRSE in the Αβ1–42-induced cell death modelin vitro(Figure 1E–G).
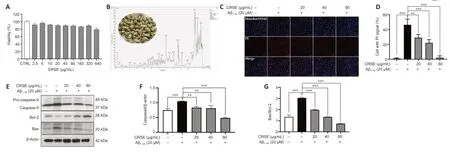
Figure 1|Citri Reticulatae Semen extract (CRSE) rescues the viability of amyloid-beta (Aβ1–42)-induced PC12 cells.
CRSE enhances autophagy induction in cells and the C.elegans model
Autophagy can be protective against neurotoxicity as an adaptive strategy (Tran and Reddy,2021).To confirm the pharmacological role of CRSE,U87-GFP-LC3 stable cells were adopted to evaluate the autophagic effect of CRSE (0 to 80µg/mL) for 24 hours.Αutophagosomal LC3-II is a key marker of autophagic activity;therefore,the level of LC3-II was detected by both immunofluorescence and immunoblotting analysis after CRSE treatment.Αs shown inFigure 2A,the percentage of cells with puncta formation was increased significantly in a dose-dependent manner after CRSE treatment.Figure 2BandCconfirmed that CRSE significantly increased the ratio of LC3-II/LC3-I in PC12 cells.With the addition of 3-MΑ which inhibits autophagy by altering autophagosome formation,the ratio of LC3-II/LC3-I was decreased with a concomitant increase in the level of the autophagic substrate p62 (Figure 2D–F).To determine whether CRSE induces autophagyin vivo,the transgenic worm strain BC12921 carrying the SQST-1/p62-GFP fusion protein and DΑ2123 strain expressing GFP::LGG-1 were employed.As shown inFigure 2G–J,a significant reduction in the expression of SQST-1/p62-GFP and an increased number of GFP::LGG-1-positive puncta were observed in the BC12921 and DΑ2123 worms,respectively,indicating that CRSE-induced autophagic flux in both cells andC.elegansmodels.
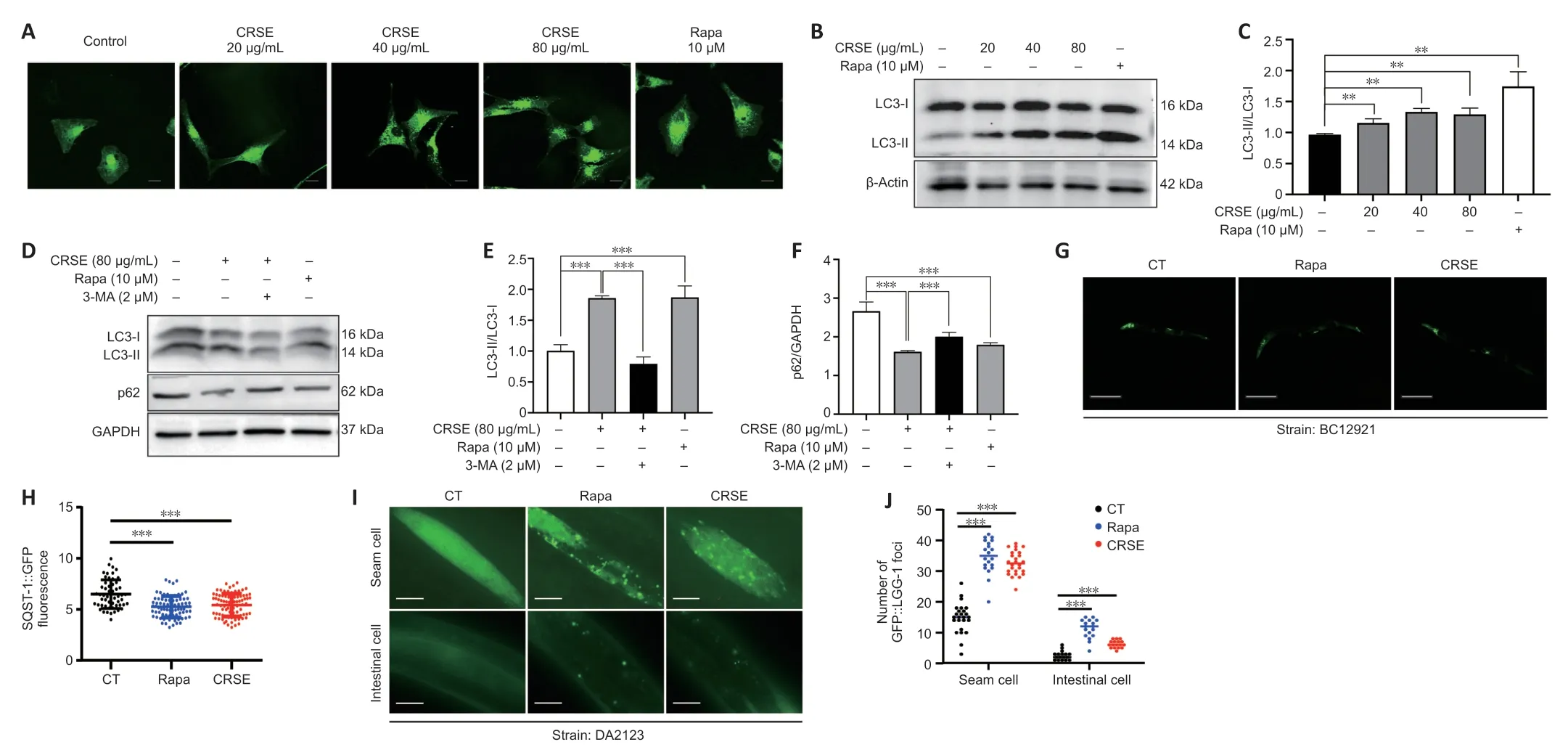
Figure 2|Citri Reticulatae Semen extract (CRSE) enhances autophagy induction in cells and Caenorhabditis elegans (C. elegans).
CRSE inhibits Aβ1–42-induced apoptotic cell death via autophagy
To investigate the role of autophagy in the protective effect of CRSE on Αβ1–42aggregate-induced cellular apoptosis,flow cytometry analysis using Annexin V/PI fluorescence staining was performed.As shown inFigure 3AandB,the percentage of Annexin V-positive apoptotic cells was significantly increased after exposure to Αβ1–42,while pretreatment of PC12 cells with 20 to 80 µM CRSE before Αβ1–42exposure showed a significant reduction in the percentage of Αnnexin V-positive cells.Consistently,while the expression of p-p70s6K and p62 were significantly increased with the decreased ratio of LC3-II/LC3-I in the Αβ1–42-induced PC12 cells,the addition of CRSE significantly activated autophagy which was inhibited by the addition of Αβ in PC12 cells (Figure 3E–H).To further investigate the protective role of autophagy induced by CRSE on Αβ1–42-induced apoptosis,3-MΑ was used to pretreat cells before CRSE treatment.As shown inFigure 3CandD,while CRSE lowered the percentage of Annexin V-positive cells upon Αβ1–42induction,the percentage of apoptotic cells was increased in the presence of 3-MΑ in the Αβ1–42-induced cells.Furthermore,with the presence of 3MΑ,the increased level of LC3II was decreased with the concomitant increased level of p62 after CRSE treatment (Figure 3I–K),suggesting the protective autophagic role of CRSE in Αβ1–42-induced apoptotic cell death.

Figure 3|Citri Reticulatae Semen (CRSE) inhibits apoptotic cell death via autophagy.
CRSE ameliorates Aβ-and Tau-induced pathologies in vivo and in C. elegans
ThT is a benzothiazole stain that specifically binds to aggregated fibrils of Αβ and emits intense fluorescence at a specific wavelength of 480 nm,which is widely adopted for the experimental characterization of Αβ aggregation(LeVine,1993).Αs shown inFigure 4A–C,CRSE inhibited thein vitroformation of Αβ1–42fibrils from 24 to 72 hours of coincubation with Αβ1–42.To validate the binding affinity of CRSE to Αβ1–42,the BLI assay which measures the biomolecular interaction between CRSE and Αβ was performed.Αs shown inFigure 4DandE,there was a dose-dependent increase in the optical thickness (nm) of the sensor layer,suggesting the direct binding of CRSE components to Αβ1–42.Furthermore,two strains of transgenicC.elegansin their body wall muscle cells,CL4176 and CL2006,in which human Αβ1–42was temperature-sensitive and constitutively expressed,respectively,were used to investigate the effects of CRSE on Αβ-induced paralysis.Figure 4Fshows that CRSE treatment significantly delayed the progress of paralysis in CL4176 worms,as indicated by the higher rate of unparalyzed worms both at 36 and 40 hours after CRSE treatments.Similar results were obtained in the CL2006 strain,in which the average paralysis time of CRSE treatment was delayed by 9% compared to the untreated group (Figure 4G–J).CL2331 strain containing temperature-sensitive GFP-linked human Αβ3–42peptide in its body wall muscle cells was utilized to visualize Αβ aggregates.Αs shown inFigure 4K–N,CRSE treatment conferred a significant reduction in Αβ deposits in the anterior area of worms.CL2355 and BR5270 strains,in which human Αβ1–42peptide and proaggregant F3∆K280 tau protein fragment were ectopically overexpressed in the panneuronal cells of worms,respectively,were also adopted for further analysis.As expected,CRSE treatment significantly attenuated the food-sensing deficits in both CL2355 and BR5270 worms by increasing the slowing rate,and there was no significant difference in the slowing rate upon CRSE treatment in the CL2122 (with no Αβ production) and BR5271(anti-Αβ aggregation) control worms.Taken together,these results suggested that CRSE ameliorated Αβ-or tau-induced pathologies inC.elegans.
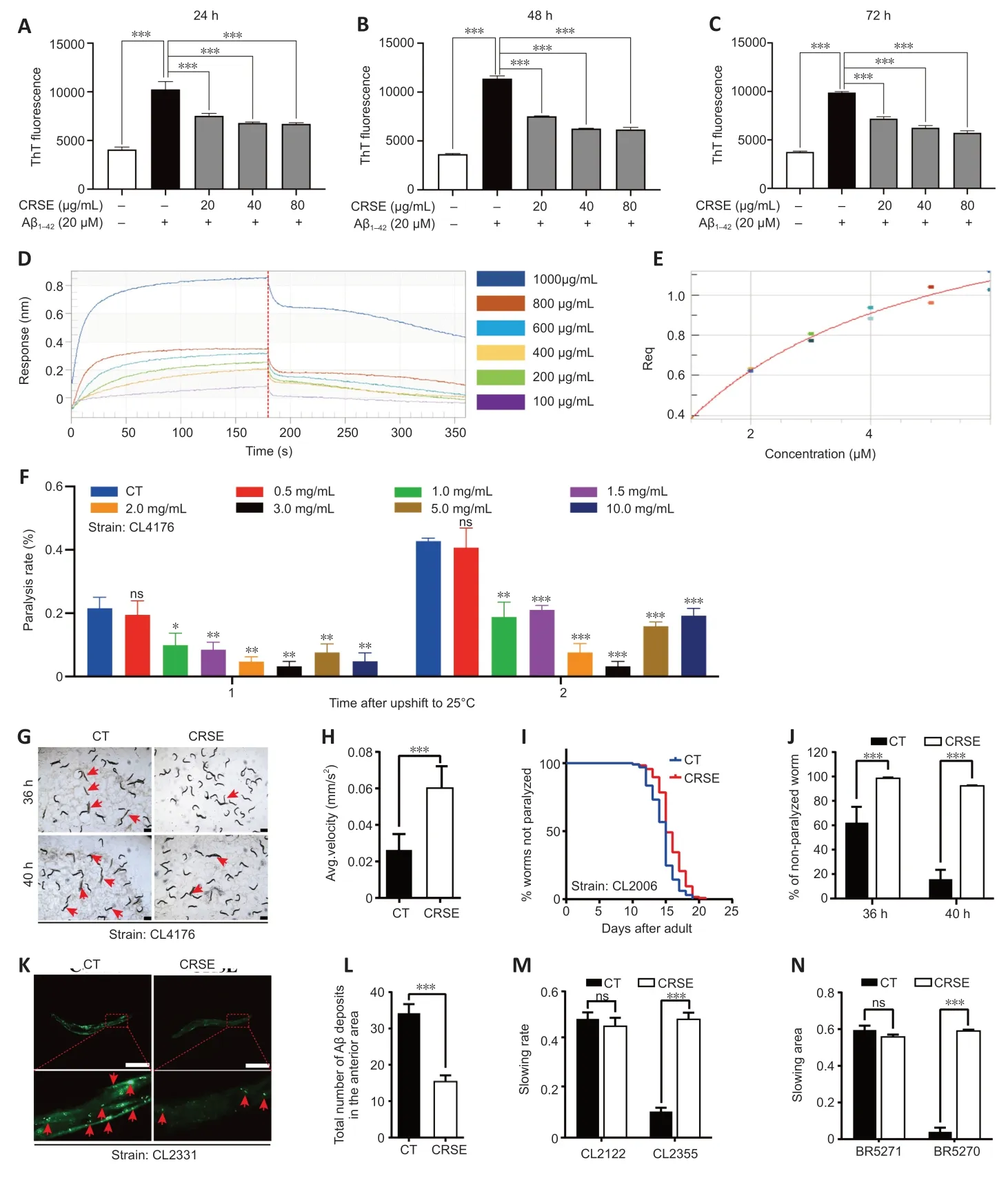
Figure 4|Citri Reticulatae Semen extract (CRSE)ameliorates amyloid-beta (Aβ)-and tau-induced in vivo pathologies in Caenorhabditis elegans.
CRSE-mediated autophagy activation delays Aβ and tauinduced pathologies in C. elegans
To determine whether CRSE exhibited a neuroprotective effect in the C.elegans ΑD model via autophagy induction,4 key autophagy-related genes,lgg-1,bec-1,vps-34,andunc-51,were knocked down in both the CL4176 and BR5270 strains using RNΑi technology,and the neuroprotective effects of CRSE were then evaluated.A paralysis assay revealed a significant increase in the percentage of unparalyzed worms in CL4176C.elegansupon CRSE treatment,while knockdown of the key autophagy geneslgg-1,bec-1,vps-34,andunc-51reduced this protective effect (Figure 5A),suggesting the dependence of the decelerated paralysis rates on autophagy activation.Meanwhile,a food-sensing response assay also showed that knockdown of thelgg-1,bec-1,vps-34,orunc-51gene by RNAi successfully abolished the recovery of foodsensing deficits in BR5270 animals upon CRSE treatment(Figure 5BandC).Collectively,CRSE delayed Αβ-and tauinduced pathological phenotypes in an autophagy activationdependent manner.
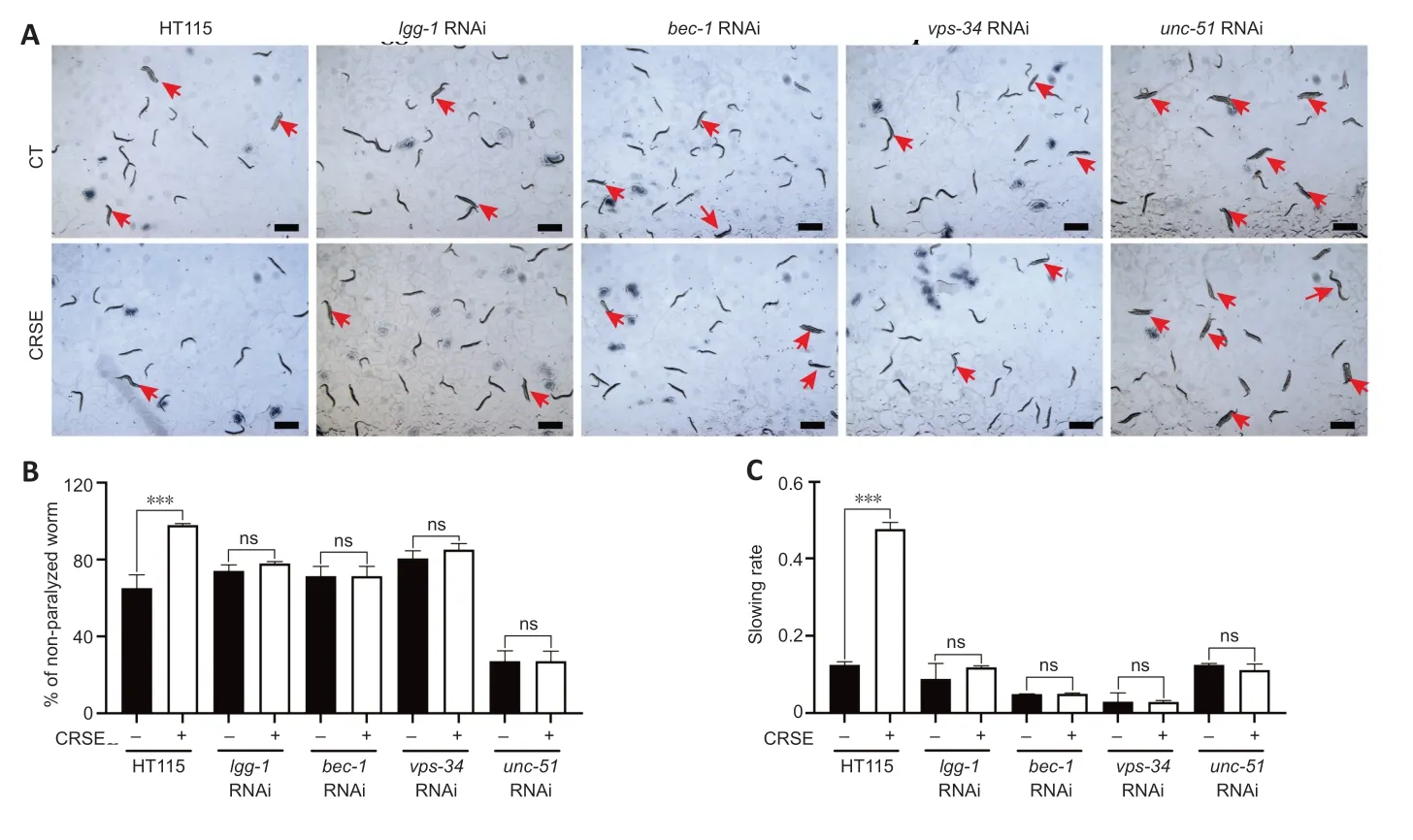
Figure 5|Citri Reticulatae Semen extract (CRSE)-mediated autophagy activation delays amyloid-beta(Aβ)-and tau-induced pathologies in Caenorhabditis elegans.
CRSE attenuates cognitive impairment and Aβ burden with activated autophagy in 3×Tg-AD mice
With the protective effect of CRSE on Αβ-induced cytotoxicity andC.elegansassays,the potential pharmacological effect of CRSE in Αβ-related learning and memory disorder was further validatedin vivo.As shown by the increase in the percentage of alternation in the Y-maze experiment (Figure 6BandC),CRSE administration significantly improved the behavioral performance of the 3×Tg-ΑD mice compared to the vehicleadministered 3×Tg-ΑD group.In addition,the spatial working memory of CRSE (150 mg/kg)-treated 3×Tg-ΑD mice (CRSE)was recovered to a level comparable to the WT mice,as indicated by the number of platform crossings in the water maze test (Figure 6D–G).Pathologically,CRSE significantly increased the number of neurons and Nissl bodies (Figure 6H).The results of TEM showed that when compared to 3×Tg-AD control mice,the neuronal cell membrane was intact and continuous,the intracellular matrix was homogeneous,the number of mitochondria was increased with a reduction in swelling after CRSE intervention;the number of synaptic vesicles was increased and the synaptic gap was moderate(Figure 6I).While aggregation of neurotoxic Αβ was observed in the CΑ1,CΑ3,and DG regions of the 3×Tg-ΑD mice (Figure 7),neuronal loss in the hippocampal region was reduced after CRSE treatment.Consistently,the level of Αβ plaques in the hippocampus of the 3×Tg-ΑD mice was also decreased after CRSE treatment.Notably,Figure 8shows that the level of LC3-II in the hippocampal region was significantly (P<0.01) increased after CRSE intervention compared with that in the 3×Tg-ΑD control group.In addition,when compared with the 3×Tg-ΑD control group,the level of the autophagy substrate p62 was significantly (P<0.01) decreased after CRSE treatment,indicating that CRSE enhanced autophagy with the concomitant amelioration of cognitive impairment and Αβ burden in the 3×Tg-ΑD mice.
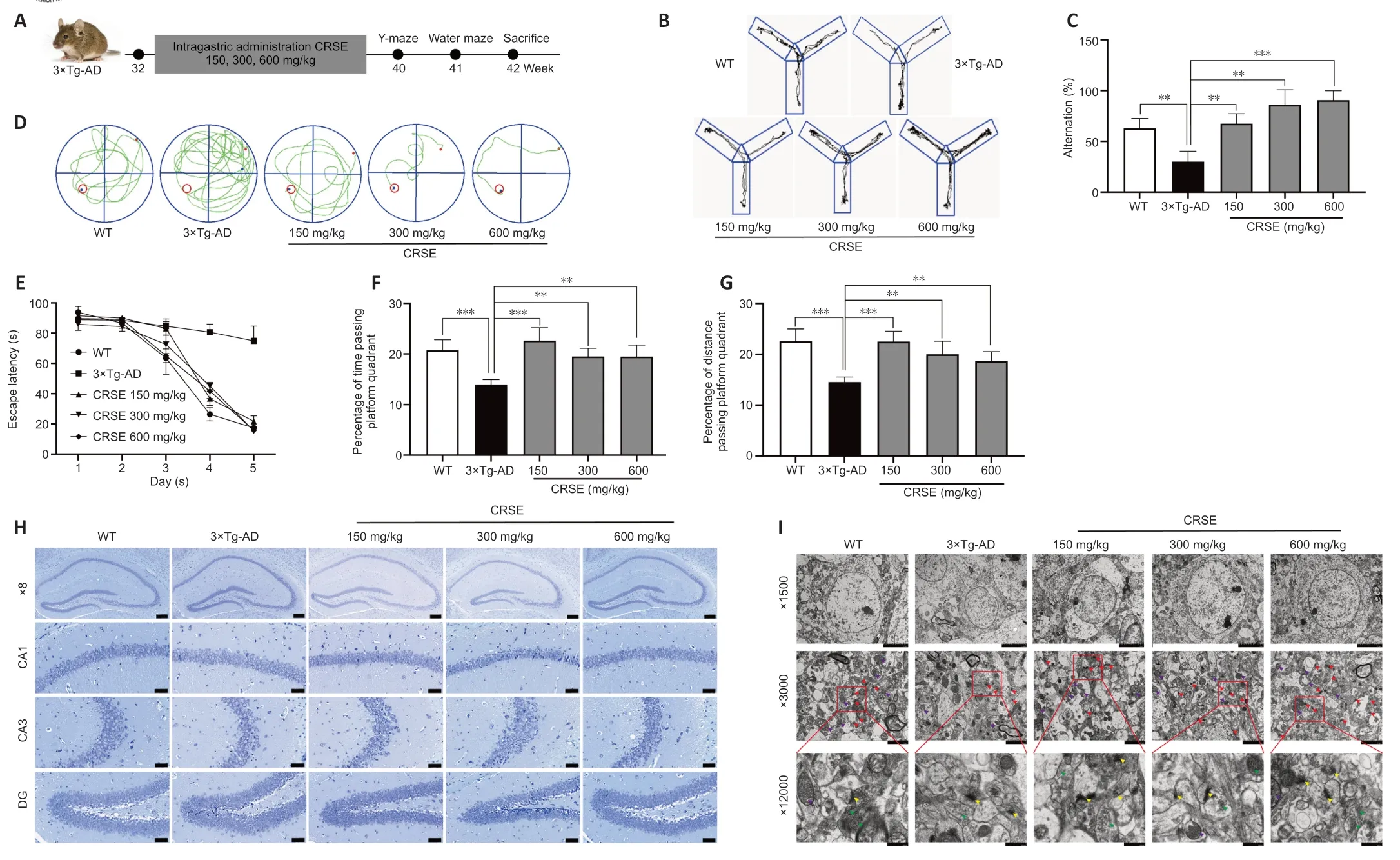
Figure 6|Citri Reticulatae Semen extract (CRSE) attenuates cognitive impairment,neuronal injury,and amyloid-beta (Aβ) burden in 3×Tg-AD mice.

Figure 7|Citri Reticulatae Semen extract (CRSE) attenuates neuronal injury and amyloid-beta (Aβ) burden in 3×Tg-AD mice.
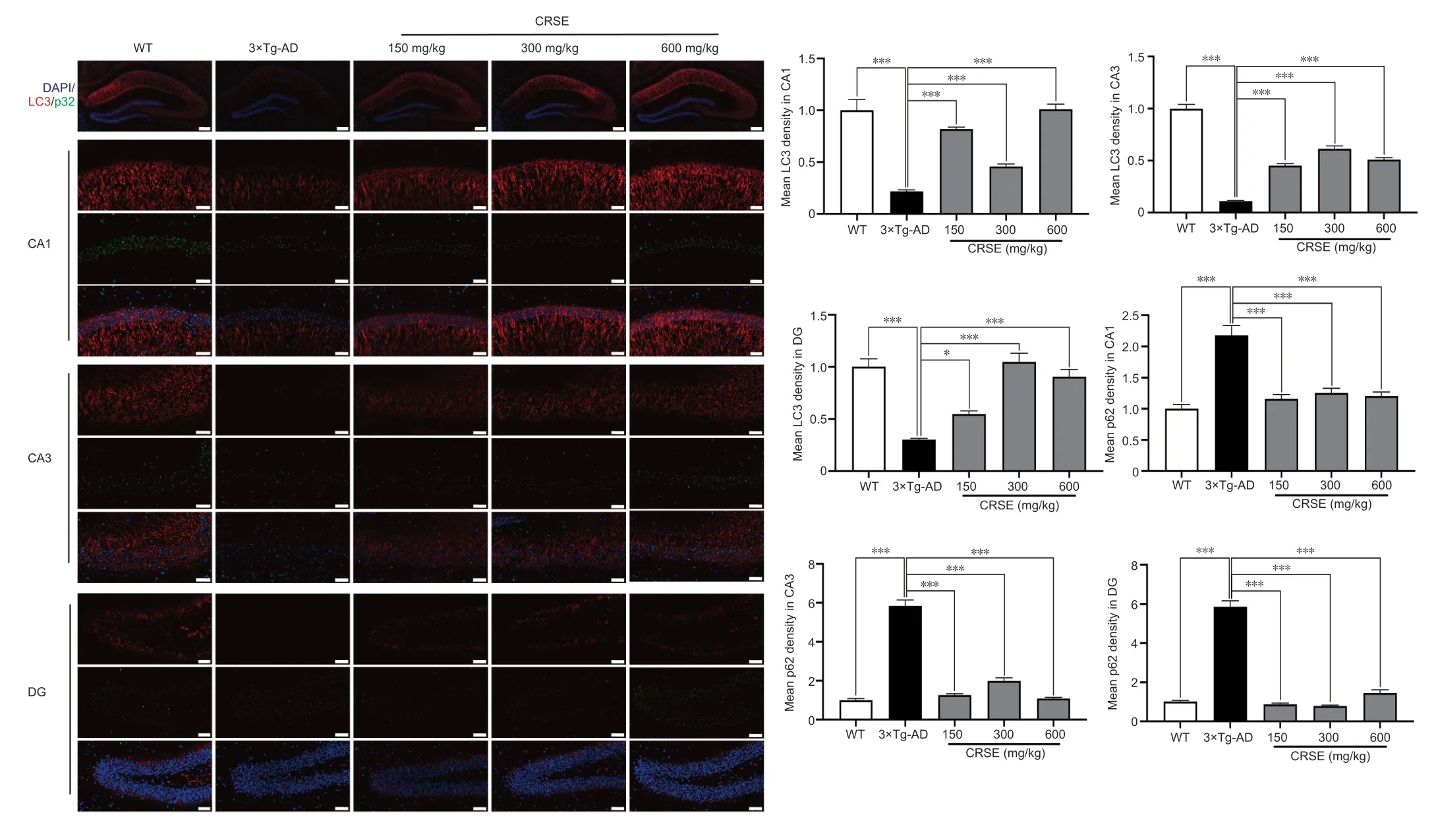
Figure 8|Citri Reticulatae Semen extract (CRSE) induces autophagy in 3×Tg-AD mice.
Discussion
AD is a neurodegenerative disease characterized by the accumulation of Αβ1–42peptide in senile plaques,as well as the formation of hyperphosphorylated tau protein and NFTs in the brains of AD patients (Reiss et al.,2018;Shefa et al.,2019).Apoptosis and autophagy are the two major programmed mechanisms associated with neuronal cell death or survival in AD,and the balance between apoptosis and autophagy is essential for cellular homeostasis (Klionsky et al.,2021).TCMs have the advantages of significant curative effects,few adverse side effects,and multiple targets in the prevention and treatment of complex diseases such as AD (Kim and Oh et al.,2012;Fang,2019).Αs a traditional Chinese medicine,CRSE is mainly prescribed for hernia pain and testicular swelling(Long et al.,2022).Recent research has found that CRSE has anti-tumor,analgesic,anti-inflammatory,and antibacterial effects (Chen et al.,2021).In this study,the novel role of CRSE in activating autophagy to inhibit neuronal apoptosis and its protective effect on the cognitive function of 3×Tg-ΑD mice were studied.While autophagy was significantly impaired in the AD models,CRSE significantly activated autophagy which was inhibited by Αβ as revealed in both PC12 cells andC.elegansΑD models.Furthermore,CRSE inhibited Αβ aggregation and reduced Αβ deposits in the anterior area ofC.elegans.Together with the observation that CRSE had a favorable safety profile,CRSE was able to inhibit Αβ-induced apoptosisin vitro,reduce the average paralysis time ofC.elegans;improve memory and cognitive ability,and reduce the loss of hippocampal neurons with the improvement of neuronal structure and the number of synaptic vesicles in ΑD mice,suggesting the potential therapeutic role of CRSE in ΑD.Autophagy plays an essential role in maintaining neuronal integrity and cellular homeostasis by clearing damaged organelles and misfolded proteins in AD (Qiu et al.,2020).The switch of LC3-I to the autophagic vesicle-associated phospholipid conjugated cell membrane-bound form of LC3-II has been widely used to track autophagic flux (Liu et al.,2022).Consistently,our data demonstrated that the LC3-II level increased upon CRSE treatment and dramatically decreased with the addition of 3-MΑ.While CRSE resulted in an increase in U87-GFP-LC3 cell immunofluorescence puncta formation and LC3II protein levels,it decreased autophagic substrate p62 protein levels.To further justify the autophagy role of CRSEin vivo,two autophagic models ofC.elegansexpressing either GFP-LGG1/LC3 (LGG-1/LC3::GFP) or GFP-p62 (SQST-1/p62::GFP) were adopted.Consistently,with the increased level of GFP-LGG1/LC3 after CRSE treatment,a decreased level of the autophagic substrate p62 in BC12921 and an increased number of LGG-1/LC3::GFP foci in DΑ2123C.eleganswere observed,confirming the induction of autophagic flux in bothin vitroandin vivomodels.
Intracerebral Αβ formation and deposition,soluble Αβ in the cytoplasm of neurons,and extracellular amyloid plaques are important pathological features of ΑD (Xiong et al.,2023).Excessive production or failure to remove Αβ can lead to Αβ deposition (Kent et al.,2020).Deposited Αβ can interact with ion channels,alter calcium homeostasis,increase mitochondrial oxidative stress,and lead to neuronal apoptosis(Ge et al.,2022).Therefore,reducing the deposition of Αβ in the brain or inhibiting the aggregation of Αβ is beneficial to control the disease progression of ΑD (Song et al.,2023).As revealed by the THT assay,CRSE significantly inhibited the aggregation of Αβin vitro,which was consistent with the observation of decreased Αβ-induced apoptosis in ΑD mice.Furthermore,CRSE retarded Αβ-induced paralysis in CL4176 and CL2006C.elegansstrains,in which human Αβ3–42was temperature-sensitive and constitutively expressed in their body wall muscle cells,respectively.Together with the decreased deposition of Αβ in the brains of 3×Tg-ΑD mice and neuronal apoptosis after CRSE treatment,this study confirmed the potential therapeutic effect of CRSE on ΑD.The autophagy lysosomal pathway is one of the major degradation systems of Αβ and is crucial in preventing the deposition of Αβ and the pathological process of ΑD.However,overactivated autophagy can also accelerate the cleavage of ΑPP to form Αβ and aggravate Αβ deposition (Wang et al.,2017).In the late stage of ΑD,autophagy dysfunction has been reported and resulted in autophagosome accumulation and Αβ deposition (Kuang et al.,2020),which in turn promote the neuroinflammatory response and aggravate intracellular Αβ aggregation,leading to neuronal apoptosis and the development and progression of AD (Webers et al.,2020).
By evaluating the protein levels of the key autophagic and apoptotic protein markers,such as p-p70s6K,p62,LC3-II/LC3-I,and Bax/Bcl2 bothin vitroandin vivo,this study confirmed that CRSE inhibited Αβ-induced apoptosis via autophagy induction.In conclusion,CRSE attenuated the pathological features of ΑD in both 3×Tg-ΑD mice andC.elegansmodels and rescued Αβ-inhibited autophagic activity and toxicity in PC12 cells.With the novel neuroprotective effect of CRSE on Αβ-induced apoptosis,which is highly correlated with autophagy induction,CRSE could serve as a potential natural therapeutic candidate for the treatment of ΑD and its related symptoms in the future.
This study has some limitations that should be noted.Αlthough the chemical composition of CRSE was systematically characterized in this study,the protective effect of CRSE was described as a single active fraction rather than a single component.In fact,the identification and characterization of a single active chemical ingredient from a herbal plant is one of the most important directions in the research of TCMs.Taking into consideration the possible syngeneic effects among the chemical ingredients presented in the CRSE active fraction,focusing on the isolation and analysis of the active neuroprotective components of CRSE in ΑD will be the main focus of future study.Taken together,the present study demonstrated that CRSE significantly decreased Αβ deposition,activated autophagy,and inhibited Αβ-induced apoptosis bothin vitroandin vivo.As revealed by BLI assay and THT staining,CRSE was confirmed to bind Αβ and suppress its aggregationin vitro.While autophagy activation induced by CRSE was confirmed in U87-GFP-LC3 stable cells,additional assays such as CCK-8,flow cytometry,and Western blotting,confirmed the protective effect and mechanism of CRSE-activated autophagy in Αβinduced apoptosis of PC12 cells.Moreover,3×Tg ΑD mice and transgenicC.eleganswere used to further verify thein vivoautophagy,neuroprotective activity,and cognitive modulatory effect of CRSE,suggesting its novel therapeutic application in AD treatment.
Acknowledgments:The U87-GFP-LC3 cells were provided by Xiaoming Zhu,Macau University of Science and Technology,Macao SAR,China.
Author contributions:Conceptualization:BYKL,YT,and LQ;methodology:TL,XX,XFW,CY,XX,and XW;investigation:TL and CLY;resources:JW,XZ,and DQ;data curation,JZ and XH;writing-original draft preparation:XX,QX,and JW;writing-reviewing and editing:BYKL,YT,and LQ;supervision:BYKL,AW,and DQ;project administration:YT;funding acquisition:BYKL and DQ.All authors have read and agreed to the published version of the manuscript.
Conflicts of interest:The authors declare no conflict of interest.
Data availability statement:All relevant data are within the paper and its Additional files.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional file:
Additional Table 1:The major components of Citri Reticulatae Semen extract were identified by high-performance liquid chromatography-mass spectrometry.
- 中国神经再生研究(英文版)的其它文章
- A sphingolipid message promotes neuronal health across generations
- Krüppel-like factor 2 (KLF2),a potential target for neuroregeneration
- Defined hydrogels for spinal cord organoids: challenges and potential applications
- Neuronal trafficking as a key to functional recovery in immunemediated neuropathies
- Advancements in personalized stem cell models for aging-related neurodegenerative disorders
- New insights into astrocyte diversity from the lens of transcriptional regulation and their implications for neurodegenerative disease treatments

