Purification of Sulfuric and Hydriodic Acids Phases in the Iodine-sulfur Process*
BAI Ying (白莹), ZHANG Ping (张平)**, GUO Hanfei (郭翰飞), CHEN Songzhe (陈崧哲), WANG Laijun (王来军) and XU Jingming (徐景明)
Purification of Sulfuric and Hydriodic Acids Phases in the Iodine-sulfur Process*
BAI Ying (白莹), ZHANG Ping (张平)**, GUO Hanfei (郭翰飞), CHEN Songzhe (陈崧哲), WANG Laijun (王来军) and XU Jingming (徐景明)
Institute of Nuclear and New Energy Technology, Tsinghua University, Beijing 100084, China
Iodine-sulfur (IS) thermochemical water-splitting cycle is the most promising massive hydrogen production process. To avoid the undesirable side reactions between hydriodic acid(HI) and sulfuric acid (H2SO4), it is necessary to purify the two phases formed by the Bunsen reaction. The purification process could be achieved by reverse reaction of the Bunsen reaction. In this study, the purification of the H2SO4and HI Phases was studied. The purification proceeded in both batches and the continuous mode, the influences of operational parameters, including the reaction temperature, the flow rate of nitrogen gas, and the composition of the raw material solutions, on the purification effect, were investigated. Results showed that the purification of the H2SO4phase was dominantly affected by the reaction temperature,and iodine ion in the sulfuric acid phase could be removed completely when the temperature was above 130°C; although, the purification effect of the HI phase improved with increasing of both the flow rate of nitrogen gas and temperature.
iodine-sulfur process, H2SO4phase, hydriodic acid phase, purification, reverse Bunsen reaction
1 INTRODUCTION
Hydrogen will become an attractive energy carrier in the future with its storable and transportable characteristics. To achieve a carbon-free energy system, many hydrogen production methods have been studied that manage to produce hydrogen from water. Among these methods, thermochemical water-splitting is a promising one, as both the materials used and the hydrogen production process would be clean when nuclear or solar energy was employed as the heat source [1-4]. Among the many thermo-chemical processes proposed, iodine-sulfur (IS) cycle, which was initially proposed by General Atomics [5], with its apparent advantages, was considered as the best one. It was studied world widely during the past two decades [6-15].
The concept of the IS process is shown in Fig. 1 [16].
Iodine-sulfur cycle is composed of the following three chemical reactions:
Bunsen reaction:

HI decomposition reaction:
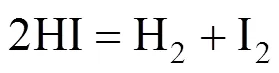
H2SO4decomposition reaction:
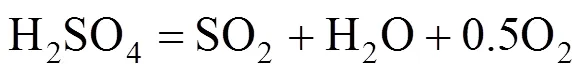
In the Bunsen reaction, sulfuric acid and hydriodic acid are produced with sulfur dioxide, iodine, and water as reactants. In the following decomposition reactions, H2SO4and hydriodic acid (HI) are decomposed, respectively. Except for hydrogen and oxygen, the other decomposition products are returned to the Bunsen reaction. Therefore, the total result of these reactions is that water is decomposed to hydrogen and oxygen.
Figure 1 Concept of the IS process
At high iodine concentration, the H2SO4and HI phases formed can separate spontaneously. However, the two phases contaminate each other, which means that a small amount of HI and H2SO4are contained in the H2SO4phase and the HIphase, respectively. In certain conditions, the operation of the Bunsen reactionand the separation step of the two liquid phases at high temperature are supposed to cause a side reaction that occurs in the mixture of both H2SO4and HI solutions.
The possible side reactions that occur between H2SO4and HI are as follows [17]


According to Ref. [18], the side reactions easily occur with the increase of reaction temperature and acid concentration, whereas, high iodine concentration impedes the side reactions. Sakurai reported that the predominant side reaction was the sulfur formation reaction. These side reactions may unbalance the cycling material, or clog pipes. To avoid these undesired side reactions, any sulfuric acid should be removed from the HI phase solution before its distillation and recycled in the IS process. For the same reason, hydriodic acid should also be removed from the H2SO4solution.
At high temperature, the reverse reaction of the Bunsen reaction also proceeds [7]. Since both sulfur dioxide and iodine produced by the reverse Bunsen reaction can be utilized in the closed-cycle again, this reaction theoretically can be used to purify the two phases without introducing any other materials or impurities. However, little study on the application of the reverse Bunsen reaction in the purification of the two liquid phases has been reported [19-21].
In this article, an experimental study on the purification of both H2SO4and HI phases was carried out in both batches and continuous modes, based on the reverse reaction of the Bunsen reaction. The influences of operational parameters, such as, operation temperatures, flow rates of nitrogen gas, and solution compositions on the purification effect, were studied.
2 EXPERIMENTAL
2.1 Batch mode operation
Figure 2 shows the simplified scheme of an experimental setup of the batch mode operation. A mixed solution of H2SO4and HI was heated in the reactor by a temperature controlled heater, nitrogen gas was bubbling in the reactor. Vapor and iodine gas was cooled down by a condenser, and sulfur dioxide was absorbed by NaOH solution.
2.2 Continuous mode operation
Figure 3 is the simplified scheme of the experiment setup of continuous mode operation. The experiment in continuous mode was carried out in a packed column, which was 34 cm in length and 4 cm in diameter. The column was packed with small glass-ring fillings; the packed length was 26 cm. The mixed solution of H2SO4and HI was pumped into the top and nitrogen gas was flowing from the bottom of the column, the countercurrent of both liquid and gas was heated by the heating belt wound on the wall of the column. Vapor was cooled down by a condenser, and sulfur dioxide produced was absorbed by NaOH solution. The effluent was collected from the production exit.
2.3 Analytical method

Figure 2 Simplified scheme of the batch reactor
Figure 3 Simplified scheme of the continuous reactor
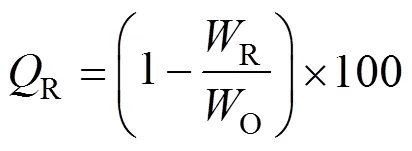

3 RESULTS AND DISCUSSION
The reverse reaction of the Bunsen reaction is expressed in the following formula:
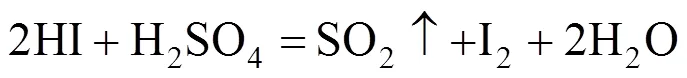
To improve the purification efficiency, the reaction should be facilitating forward. Some effects may affect the equilibrium. First, as the Bunsen reaction is exothermic, an increase in reaction temperature will naturally promote reverse reaction, therefore, purification should be carried out at a higher temperature. Second, as a gaseous product (SO2) forms in the reverse reaction, to remove SO2will be beneficial, hence, nitrogen was used for carrying the produced SO2, and the suitable gas flow rate was chosen. Third, the feed rate in the continuous mode not only influences the removal efficiency but also the operational stability; therefore, the best feed rate should be ascertained. In addition, the composition of raw material may vary in closed cycle operation. With these considerations, the purification process was studied.
3.1 Purification of sulfuric acid phase
3.1.1
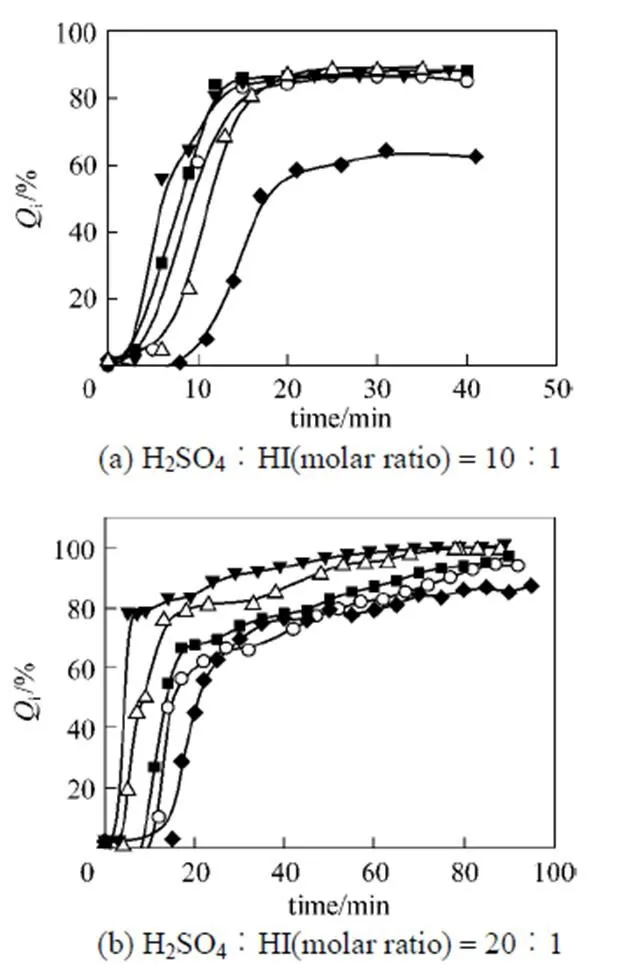
Figure 4 Effects ofiwith different flow rates of nitrogen gas
N2flow rate/ml·min-1:○ 50;■ 100;△ 150;▼ 200;◆ 0

The trend of the purification effects with different feeding materials [Fig.4 (b)] was similar to those with prior composition. Although the gap was not that long between the utilization of nitrogen, the results were similar, by using different flow rates.iremained constant when the operation temperature was stable. It could also be observed that the finalireached a higher level when the amount of HI was smaller in the mixed solution. After one and a half hours, it could reach almost 100%, denoting that the HI impurity could be totally removed.
3.1.2
With the results obtained from the batch mode, the reaction order and reaction rate constant of the reverse Bunsen reaction could be acquired by an integral (concentration to time curve), that is, the concentration of one or more products is measured at different times by substituting the data into the integral formula of a rate equation and solving it by linear fitting, following which a suitable equation would be obtained.
Generally, a rate equation would be expressed as follows:
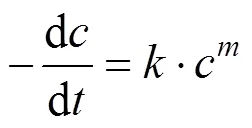
Integrating the equation, at= 0,=0:
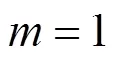
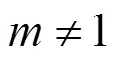

Suppose that the rate equation of the reverse Bunsen reaction is as follows:
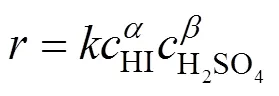



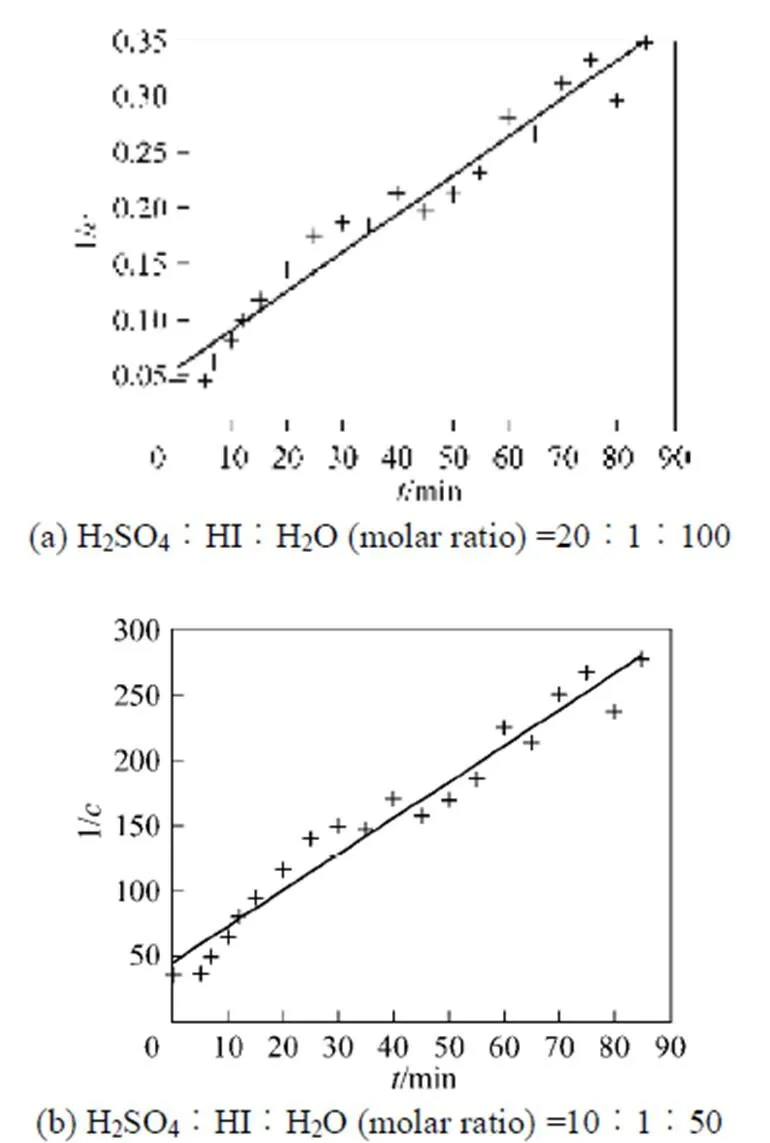
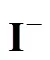

3.1.3
To make the IS process become a closed cycle, each unit operation, including the purification of the two liquid phases, should be able to operate continuously. In the continuous operating mode, the operation temperature was considered as the most important parameter affecting the purification effect.
The continuous purification process was carried out in a packed column. The composition of the feeding material was H2SO4︰HI︰H2O (molar ratio) = 10︰1︰50, the flow rate of the carrier gas was 50 ml·min-1, and the feed rate was 1.6 ml·min-1. The operation temperatures were controlled stably at various temperatures. Preliminary experiment results showed that the removal efficiency of I-was kept stable within the experiment period (about 40 min). The results are shown in Fig. 6, it shows thatiincreased with increasing temperatures, and HI was totally removed when temperature was above 130°C.
3.2 Purification of hydriodic acid phase
In the batch mode experiment, the influence of operational parameters, including composition of feeding material and flow rate of nitrogen, on the HI phase purification was studied. In the continuous mode, besides temperature and flow rates of nitrogen, the effect of the feed rate was studied.

Figure 6 Effects ofiwith various temperatures
3.2.1
The compositions of feeding materials are H2SO4︰HI =1︰20 and H2SO4︰HI =1︰10 (molar ratio). The reactor was heated and the temperature remained at a stable value of about 108°C. Fig. 7 shows the dependency ofsaon the flow rate of nitrogen.
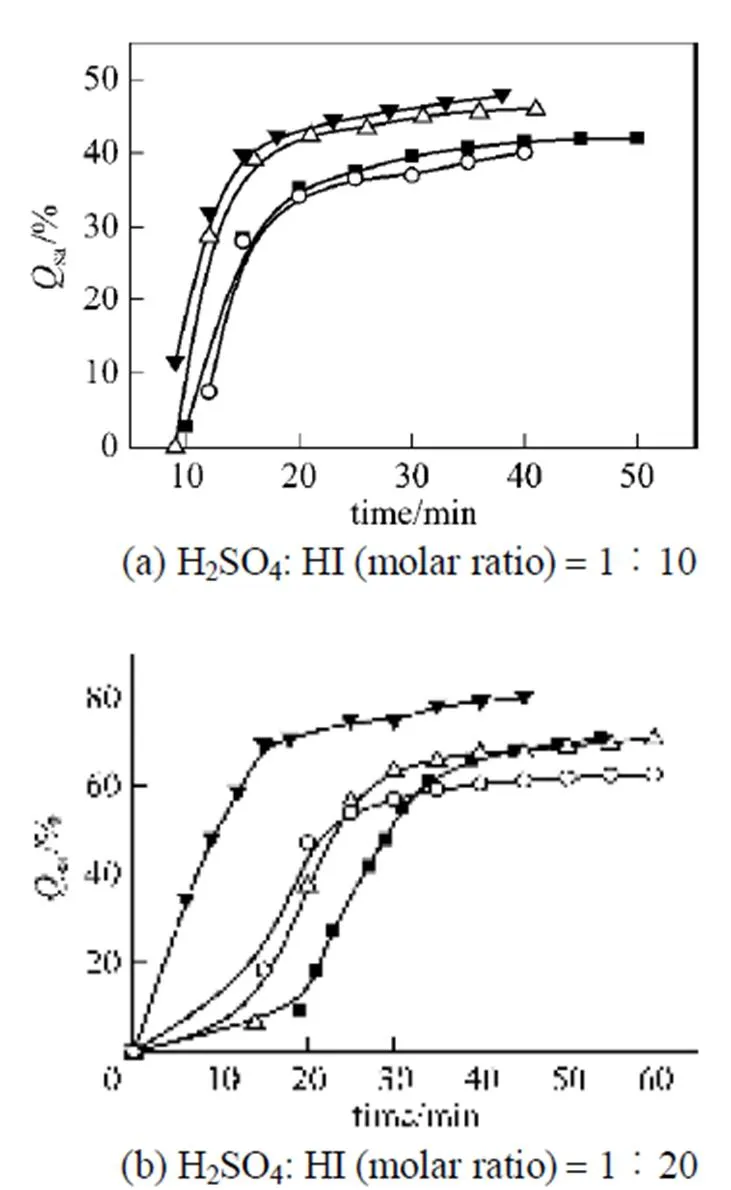
Figure 7 Effects of flow rates of nitrogen gas onsa
N2flow rate/ml·min-1:○ 50;■ 100;△ 150;▼ 200
Figure 7 shows thatsaincreases with an increase in the flow rate of nitrogen gas, because SO2produced from the reverse Bunsen reaction is carried away by the bubbling nitrogen. The reverse Bunsen reaction is greatly promoted and purification will be more effective with a higher flow rate of nitrogen.
Similar to the purification of the sulfuric acid phase, by the comparison of (a) and (b) in Fig. 7, it can be seen that the finalsaincreases when the content of the H2SO4impurity is smaller in the feed.
3.2.2
Similar to the analysis of sulfuric acid purification, the reaction order and rate constant for reverse Bunsen reaction for excessive HI can also be calculated.

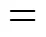


Therefore, the overall reverse Bunsen reaction can be considered as a fourth order one, the reaction rate equation may be expressed as follows:

3.2.3
Due to the complexity of influences of the operation conditions (including the temperature, flow rate of nitrogen gas, and the feed rate of raw material) on the purification effects, the average and maximumsaof the HI phase purification experiments were presented in the continuous operation mode experiments.
(1) Effect of operation temperature onsa
In this experiment, the composition of the feeding material was of H2SO4︰HI = 1︰20 (molar ratio), flow rate of N2was 200 ml·min-1, and the feed rate was 0.48 ml·min-1. The results are shown in Table 1.

Table 1 Average and maximum Qsa with different temperature ranges
(2) Effect of flow rate of nitrogen gas onsa
The composition of the feeding material was H2SO4︰HI = 1︰20 (molar ratio), operation temperature was between 100 and 120°C, the feed rate was 0.48 ml·min-1, the results are shown in Table 2.

Table 2 Average and maximum Qsa with different flow rates of nitrogen gas
(3) Effect of the liquid flow rate of the mixed solution onsa
The composition of the feeding material was H2SO4︰HI =1︰20 (molar ratio). The operation temperature was between 100 and 120°C, the flow rate of N2was 150 ml·min-1, and the results are shown in Table 3.
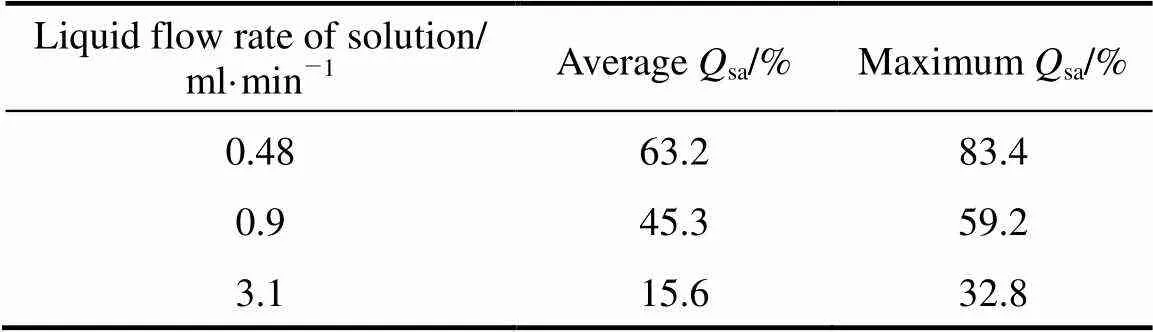
Table 3 Average and maximum Qsa with different flow rates of the mixed solution
4 CONCLUSIONS
Regarding the condition of purification of H2SO4and HI phases in the IS process, experimental study was conducted and the concluding remarks are as follows.

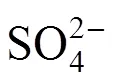
(3) Based on the experiment results, the operation conditions of the efficient purification of both H2SO4and HI phases in a closed-cycle IS process was proposed as follows: the purification process should be carried out in a continuous operation mode; lower nitrogen flow rate (such as 50 ml·min-1) and higher temperature (above 130°C) should be adopted for the sulfuric acid phase purification process; and higher temperature and flow rate of nitrogen gas would be beneficial for the hydriodic acid phase purification process.
1 International Atomic Energy Agency, “Hydrogen as an energy carrier and its production by nuclear power”, IAEA TECDOC 1085, Vienna, Austria (1999).
2 Roger, H.H., Scott, D.S., “Building sustainable energy system: the role of nuclear-derived hydrogen”, In: Proceedings of 1st Information Exchange Meeting, Paris, France (2000).
3 Funk, J.E., “Thermochemical hydrogen production: Past and present”,.., 26, 185-190 (2001).
4 Terada, A., Kubo, S., “Development of hydrogen production technology by thermo-chemical water splitting IS process”, In: Proceeding of Global 2005, Tsukuba, Japan (2005).
5 Norman, J.H., Besenbruch, L.C., Brown, L.C., “Thermo-chemical water-splitting cycle, bench-scale investigations and process engineering”, General Atomics Report, GA-A16713 (1982).
6 Nakajima, H., Ikenoya, K., Onuki, K., Shimizu, S., “Closed-cycle continuous hydrogen production test by thermochemical IS process”,...., 24, 352-355 (1998).
7 Nomura, M., Kasahara, S., Okuda, H., Nakao, S., “Evaluation of the IS process featuring membrane techniques by total thermal efficiency”,..y, 30, 1465-1473 (2005).
8 Vitart, X., Duigou, A.L., Carles, P., “Hydrogen production using the sulfur-iodine cycle coupled to VHTR: An overview”,.., 47, 2740-2747 (2006).
9 Sakaba, N., Kasahara, S., Onuki, K., Kunitomi, K., “Conceptual design of hydrogen production system with thermochemical water-splitting iodine-sulphur process utilizing heat from the high temperature gas-cooled reactor HTTR”,.., 32, 4160-4169 (2007).
10 Vitart, X., Carles, P., Anzieu, P.A., “General survey of the potential and the main issues associated with the sulfur-iodine thermochemical cycle for hydrogen production using nuclear heat”,..y, 50, 402-410 (2008).
11 Goldstein, S., Borgard, J.M., Vitart, X., “Upper bound and best estimate of the efficiency of the iodine sulphur cycle”,..y, 30, 619-626 (2005).
12 Kasahara, S., Kubo, S., Hino, R., Onuki, K., “Flowsheet study of the thermochemical water-splitting iodine-sulfur process for effective hydrogen production”,.., 32, 489-496 (2007).
13 Roth, M., Knoche, K.F., “Thermochemical water splitting through direct HI-decomposition from H2O /HI/I2solutions”,..y, 14 (8), 545-549 (1989).
14 Kubo, S., Nakajima, H., Kasahara, S., Higashi, S., “A demonstration study on a closed-cycle hydrogen production by the thermochemical water-splitting iodine-sulfur process”,..., 233, 347-354 (2004).
15 Kasahara, S., Hwang, G.J., Nakajima, H., Choi, H.S., “Effects of process parameters of the IS process on total thermal efficiency to produce hydrogen from water”,...., 36, 887-899 (2003).
16 Zhang, P., Yu, B., Chen, J., Xu, J.M., “Study on the hydrogen production by thermochemical water splitting”,.., 17 (4), 643-650 (2005). (in Chinese)
17 Sakurai, M., Nakajima, H., Amir, R., Onuki, K., Shimuzu, S., “Experimental study on side reaction occurrence condition in the iodine-sulfur thermochemical hydrogen production process”,..y, 25, 613-619 (2000).
18 Sakurai, M., Nakajima, H., Onuki, K., Ikenoya, K., Shimizu, S., “Preliminary process analysis for the closed cycle operation of the iodine-sulfur thermochemical hydrogen production process”,..y, 24, 603-612 (1999).
19 Kasahara, S., Kubo, S., Onuki, K., Nomura, M., “Thermal efficiency evaluation of HI synthesis/concentration procedures in the thermochemical water splitting IS process”,.., 29 (6), 579-587 (2004).
20 Bae, K.K., Park, C.S., Kim, C.H., Kang, K.S., Lee S.H., “Hydrogen production by thermochemical water-splitting IS process”, In: Proceedings of WHEC 16, Lyon, France (2006).
21 Kubo, S., Kasahara, S., Okuda, H., Terada, A., “A pilot test plan of the thermochemical water-splitting iodine-sulfur process”,..., 233, 355-362 (2004).
22 Wei, Y.J., Liu, C.G., Mo, L.P., “Ultraviolet absorption spectra of iodine, iodine ion and triiodide ion”,..., 25 (1), 86-88 (2005). (in Chinese)
2008-02-25,
2008-10-09.
the National Defense Fundamental Research Fund (A1420080145).
** To whom correspondence should be addressed. E-mail: zhangping77@tsinghua.edu.cn
 Chinese Journal of Chemical Engineering2009年1期
Chinese Journal of Chemical Engineering2009年1期
- Chinese Journal of Chemical Engineering的其它文章
- Modeling and Optimization for Scheduling of Chemical Batch Processes*
- Simulation of Droplet-gas Flow in the Effervescent Atomization Spray with an Impinging Plate*
- Numerical Investigation of Constructal Distributors with Different Configurations*
- The Kinetics of the Esterification of Free Fatty Acids in Waste Cooking Oil Using Fe2(SO4)3/C Catalyst
- Multiple Model Soft Sensor Based on Affinity Propagation, Gaussian Process and Bayesian Committee Machine*
- Measurement and Correlation of Solid-Liquid Equilibria of Phenyl Salicylate with C4 Alcohols
