Mechanism Study of Rice Straw Pyrolysis by Fourier Transform Infrared Technique*
FU Peng (付鹏), HU Song (胡松), XIANG Jun(向军), SUN Lushi (孙路石), YANG Tao (杨涛), ZHANG Anchao (张安超) and ZHANG Junying (张军营)
Mechanism Study of Rice Straw Pyrolysis by Fourier Transform Infrared Technique*
FU Peng (付鹏), HU Song (胡松)**, XIANG Jun(向军), SUN Lushi (孙路石), YANG Tao (杨涛), ZHANG Anchao (张安超) and ZHANG Junying (张军营)
State Key Lab of Coal Combustion, Huazhong University of Science and Technology, Wuhan 430074, China

rice straw, pyrolysis, mechanism, Fourier transform infrared
1 INTRODUCTION
With the excessive use of fossil fuels and the concerns over environmental protection, the utilization of biomass resources has attracted increasing worldwide interest. Biomass including agricultural residues is one of the main renewable energy resources available especially in an agricultural country such as China. Biomass can be converted to energy and clean fuelsthermochemical and biochemical processes. Pyrolysis, as one of the promising thermochemical conversion routes, plays a vital role in biomass conversion [1]. Pyrolysis is also a vital process in biomass combustion and gasification [2]. However, pyrolysis is an extremely complex process, which generally goes through a series of reactions and can be influenced by many factors [3-5]. Thus, it is necessary to analyze the pyrolysis characteristics of agriculture residues and build comprehensive pyrolysis models that can predict product specification and yields.
So far, numerous studies were focused on developing kinetics models for predicting behavior of biomass pyrolysis [3, 5-7]. The structure property of biomass was found to influence greatly the pyrolysis characteristics. However, the releasing characteristics of gas products, their relationships with the chemical structure of biomass, and the chemical structure changes during pyrolysis were not discussed in-depth in the previous studies. The lack of data, combining with the large variety and complexity of agricultural residues, leads to difficulties in understanding emission behavior of agricultural residues during the thermal treatment process.
Rice straw is one of the China’s main agricultural residue resources. Rice straw is not only a potential source of energy but also a value-added by-product. Various technologies for utilization of rice straw through thermochemical conversion requires knowledge of relevant aspects concerning pyrolysis. In the present study, we focused on the gas formation behaviors during pyrolysis of rice straw. The changes in the surface chemistry during pyrolysis were also studied by FTIR analysis. From these measurements, the pyrolysis mechanism of rice straw was examined in details. It is favorable for the development of advanced biomass pyrolysis.
2 EXPERIMENTAL
2.1 Experiment materials
Rice straws (RS) were used in this study as the representatives of agricultural residues. RS were first crushed and sieved, the fraction of particle sizes less than 0.295 mm being chosen for subsequent studies. Elemental analysis and proximate analysis were carried out in a Euro-EA 3000 (HEKAtech, Italy) and TGA-2000 (Navas Instruments, Spain). The respective data are given in Table 1.

Table 1 Proximate and ultimate analysis of rice straw
① Dry and ash-free basis.
②Calculated by difference.
③As-received basis.
2.2 Experiment method
2.2.1
Thermogravimetric experiments were carried out on a computerized thermobalance (NETZSCH STA 409C, Germany). Thermobalance configuration gives a sensitivity of 0.1 mg. In order to establish an inert atmosphere, a controlled nitrogen flow (fixed at 300 ml·min-1) sweeps the measurement cell that is purged during 30 min. During experiments, the nitrogen flow is fixed at 100 ml·min-1. The initial mass of samples is about 10 mg. The experiments were carried out at a constant heating rate of 5, 10, 20 and 50°C·min-1from the ambient to 900°C, at a steady nitrogen flow of 100 ml·min-1.
2.2.2
The experimental apparatus mainly consists of a tubular reactor and a Fourier transform infrared spectrometer. The portable FTIR gas analyzer, GASMET Dx-4000 (Temet Instrument Oy, Finland) FTIR spectrometer, is designed for analysis of multicomponent gases. After the background calibration by N2, test gases were conducted into the sampler and heated up to 180°C during the entire sampling procedure. In the FTIR spectrometer, specific molecular components and structures were specified by the corresponding infrared absorption bands, which allowed the computerized data to be searched against the reference libraries. The concentrations of the gases were further confirmed by classical least squares (CLS) algorithm.
Pyrolysis experiments were carried out as follows. About a (1.0±0.05) g of virgin biomass particles was loaded into the sample holder and the sample holder assembly was inserted into the cylindrical quartz tube reactor. Then the sample was heated up to 900°C at a constant heating rate of 10°C·min-1, and held for 5 min. Purified nitrogen (≥99.995%) at a flow rate of 2.0 L·min-1was used as the carrier gas to provide an inert atmosphere for pyrolysis. The gases released out were swept immediately to a gas cell, followed by the Gasmet FTIR Dx4000. The transfer line and gas cell were heated to an internal temperature of 180°C in order to avoid cold spots and thus prevent the condensation of the gaseous products. The evolving rates of gaseous products were estimated from the measurements.
2.2.3
To investigate the changes in the surface chemistry during pyrolysis, information on the surface chemistry of the samples was provided by FTIR spectroscopy. The raw sample was heated at a constant heating rate of 10°C·min-1to a fixed pyrolysis temperature varied from 200-900°C. The pyrolysis temperatures investigated were 200, 300, 350, 400, 500, 700 and 900°C. The residence time at the maximum temperature was 10 min. This holding time made sure that no significant decomposition occurred during the cooling of the sample. All the IR spectra of rice straw and the chars prepared at different pyrolysis temperatures were measured at 4 cm-1resolution on the VERTEX 70 FTIR spectrometer. The samples were first powdered in an agate mortar and then mixed with KBr at a ratio of 1︰100 to prepare transparent disks. The disks were oven-dried at 110°C for at least 48h to remove water. Eventually, the spectra were plotted with the same scale on the transmittance axis. Abbreviations used in the study of the FTIR results are, stretching;, in-plane bending;, out-of-place bending; as, asymmetric; and s, symmetric.
3 RESULTS AND DISCUSSION
3.1 Thermal decomposition characteristics
The thermogravimetric (TG) and differential thermogravimetric (DTG) curves at a heating rate of 10°C·min-1for rice straw are shown in Fig. 1. The pyrolysis process with the increase of temperature from TG can be divided into three stages, including drying stage(<200°C), main pyrolysis stage (200-400°C), and carbonization stage(>400°C). At the temperature lower than 200°C, the small change of mass is attributed to vaporization of moisture attached on the surface of the sample. The major decomposition occurs between 220 and 380°C. The maximum pyrolysis peak (7.3%·min-1) at 313°C is attributed to the decomposition of cellulose and hemicellulose [8]. Lignin is known to decompose slowly and is responsible for the tailing at high temperatures. As can be seen from Fig. 2, with increasing the heating rate, the maximum pyrolysis rate increases and the corresponding temperature also increases from 304°C at 5°C·min-1to 327°C at 50°C·min-1.
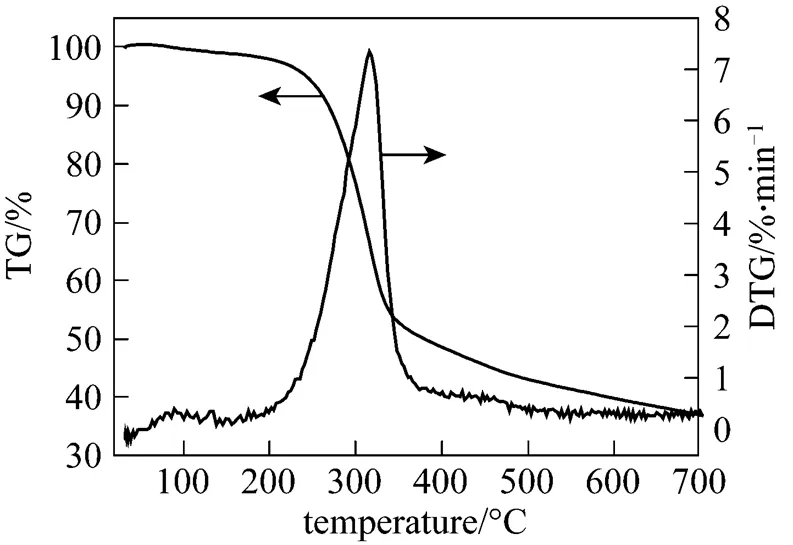
Figure 1 TG and DTG curves of rice straw pyrolysis
Figure 2 Comparison of pyrolysis DTG of rice straw at the different heating rates heating rate/°C·min-1:■ 5;○ 10;△ 20; ★ 50
3.2 Kinetic modeling
A kinetic study of rice straw pyrolysis is necessary to achieve an efficient production of fuel gases, chemicals and energy. The information is also important for the design of large-scale pyrolysis reactors. In this study, the three-pseudocomponent model (TPM) proposed in the Ref. [6] is used for the evaluation of the kinetics. The TPM model assumes that the biomass consists of three pseudocomponents and the pyrolysis rate is then described by

The variableis the degree of transformation. The subscriptsrepresents the different pseudocomponent of the biomass. Parametersc,andare the coefficient which expresses the contribution of the partial processes to the overall mass loss, the activation energy and the pre-exponential factor andis ideal gas constant.
This assumption of three pseudocomponents is consistent with the fact that most of biomass consists of hemicellulose, cellulose and lignin. In fact, three pseudocomponents represent a pool of fractions of the main biomass components. Depending on the reaction order, the following kinetic equations are used for each pseudocomponent:


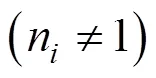
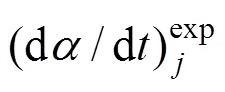
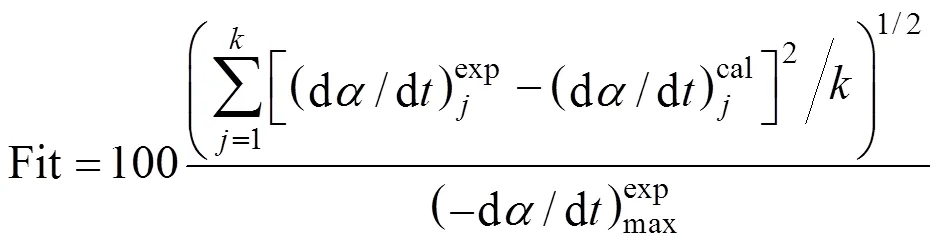
Hu. [6] pointed out that the three- pseudocomponent model with a reaction order of one could have sufficiently high accuracy to represent biomass pyrolysis for practical utilization. Therefore, the TPM model is used to evaluate the thermal decomposition of rice straw, as shown in Fig. 3. In the view of the previous discussion [6], the decomposition of the raw material is considered as the result of the degradation of its main constituents. Therefore, the three reactions utilized for the simulation of rice straw correspond to the thermal degradation of cellulose, hemicellulose and lignin. The kinetic parameters for rice straw are summarized in Table 2.

Figure 3 indicates that the pyrolysis of rice straw is quite well described by the three-pseudocomponent model with a reaction order of one. According to the kinetic estimations, the first reaction, which corresponds to hemicellulose, occurs between 250 and 360°C, and the second corresponding to cellulose happens between 200 and 300°C. The third reaction corresponding to the decomposition of lignin appears over a wide temperature range (between 250 and 550°C). Fundamental studies of the decomposition of lignocellulosic materials show the same ranges for the decomposition of hemicellulose, cellulose and lignin, confirming the validity of the results [9-11]. The activation energy of hemicellulose range between the values of 91-114 kJ·mol-1. The corresponding values for the pyrolysis of cellulose are higher and range between 143 and 200 kJ·mol-1. The lowest activation energy values are exhibited by lignin and scattered between 20 and 27 kJ·mol-1. Similar values for activation energies of hemicellulose, cellulose and lignin are also found in the Ref. [12].
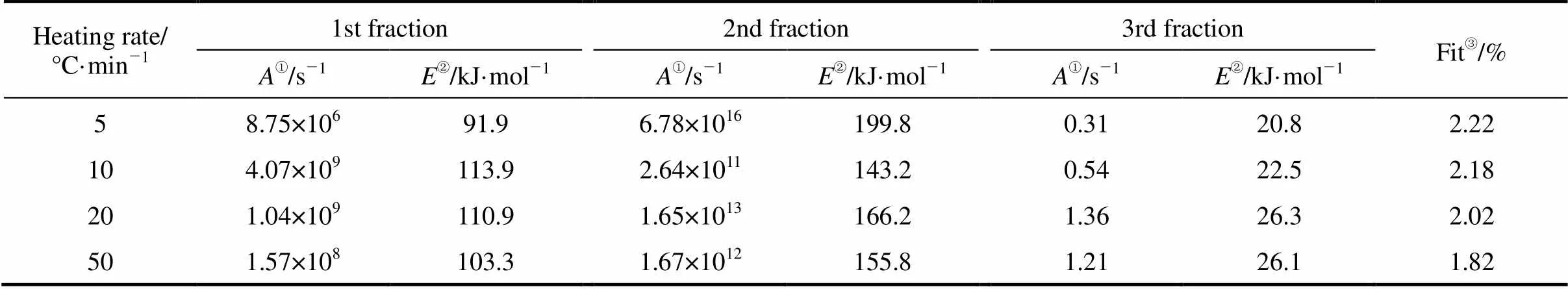
Table 2 Kinetic parameters of rice straw pyrolysis
① Pre-exponential factor.
② Activation energy.
③ Fit represents the calculation quality of the three-pseudocomponent model.
3.3 FTIR analysis of gas products
Figure 4 shows the typical IR spectra of gas products in the three different pyrolysis stages for rice straw. Several representative temperatures in each pyrolysis stage are chosen to study the formation mechanism of main gas products. In the first stage, the mass loss is mainly caused by the release of water [Fig. 4 (a)], with the characteristic bands of H2O at 3500-4000 and 1275-1875cm-1. The water attached onthe surface of the sample is released out by evaporation.


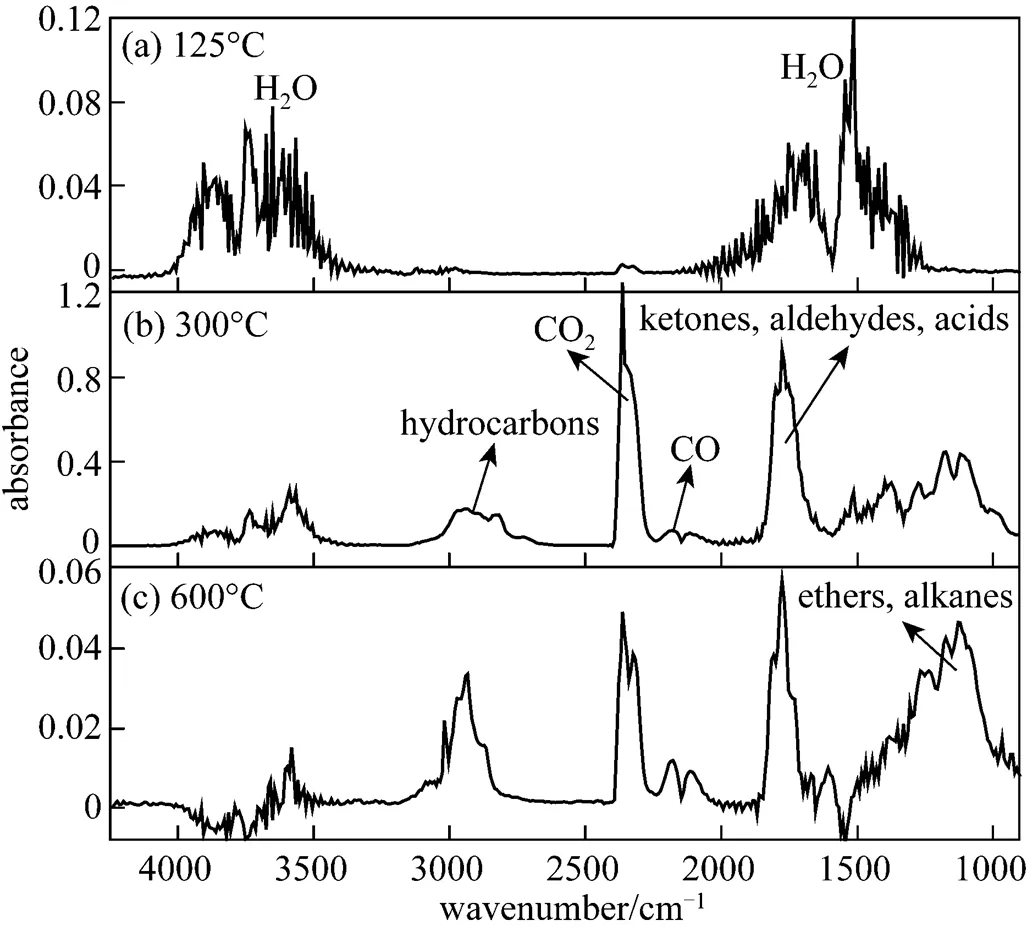
Figure 4 FTIR spectra of gas products at (a) 125°C, (b) 300°C and (c) 600°C during the pyrolysis of rice straw
The main constituents of biomass are lignin, hemicellulose and cellulose [4, 17]. Lignin is a very complex aromatic structure and hemicellulose is a polymer of 5- and 6-carbon sugars, while cellulose is a polymer of glucose [4]. Those structural differences have an influence on the thermal decomposition behaviors of the biomass. Yang. [1] pointed out that the releasing of CO2was mostly contributed by hemicellulose at low temperature (<500°C) and by lignin at high temperature (>500°C), CO releasing was mostly caused by the pyrolysis of hemicellulose in the whole temperature range and that of lignin at high temperatures (>600°C), and hemicellulose, cellulose and lignin all contributed to the releasing of CH4at low, middle and high temperatures.
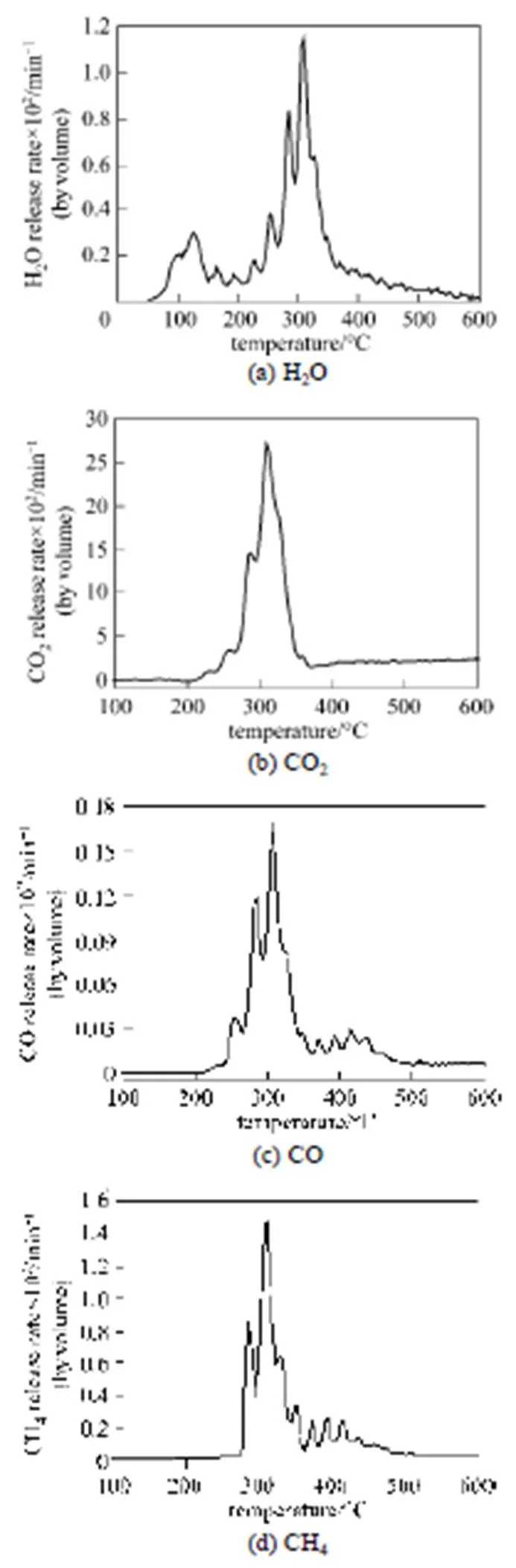
Figure 5 The profiles of (a) H2O, (b) CO2, (c) CO and (d) CH4evolving from rice straw pyrolysis
3.5 FTIR analysis of rice straw

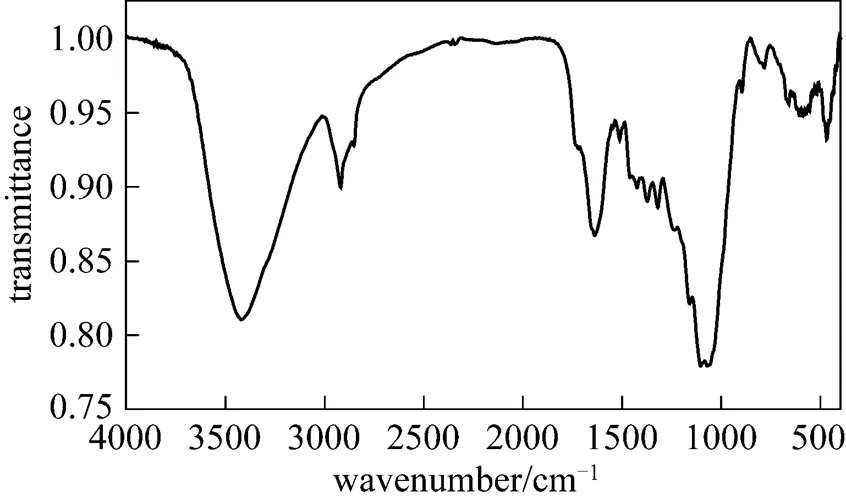
Figure 6 FTIR spectra of rice straw


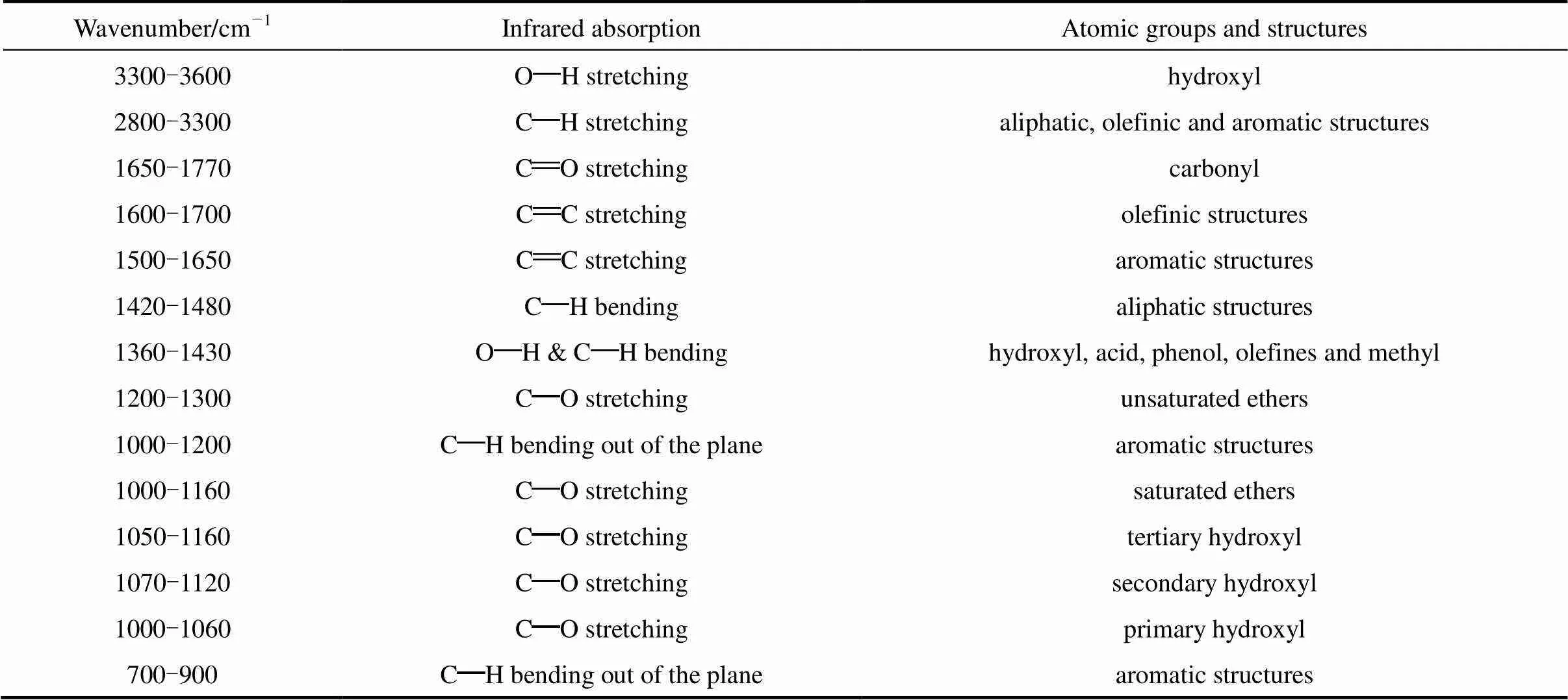
Table 3 The main atomic groups and structures of rice straw
3.6 Changes in the surface chemistry



Figure 7 The changes in the FTIR spectra through the pyrolysis of rice straw
4 CONCLUSIONS
From the above results, the following conclusions can be drawn:
(1) The maximum pyrolysis rate increases with the heating rate increasing and the corresponding temperature also increases. The three-pseudocomponent model could describe the pyrolysis behavior of rice straw accurately.
(2) The main gas products of rice straw pyrolysis are H2O, CO2, CO, CH4, formaldehyde, formic acid, methanol, phenols,. The releasing of H2O, CO2, CO and CH4mainly focuses at 220-400°C. The H2O formation process is separated into two stages corresponding to the evaporation of free water and the formation of primary volatiles. The release of CO2first increases with increasing temperature and gets the maximum at 309°C. The releasing behavior of CO is similar to H2O and CO2between 200 and 400°C. The production of CH4happens at higher temperatures of 275-400°C with the maximum at 309°C.

1 Yang, H., Yan, R., Chen, H., Lee, D.H., Zheng, C., “Characteristics of hemicellulose, cellulose and lignin pyrolysis”,, 86, 1781-1788 (2007).
2 Ren, Q., Zhao, C., Wu, X., Liang, C., Chen, X., Shen, J., Tang, G., Wang, Z., “Effect of mineral matter on the formation of NOprecursors during biomass pyrolysis”,..., doi: 10.1016/j.jaap.2008.08.006 (2008).
3 Manya, J.J., Velo, E., Puigjaner, L., “Kinetics of biomass pyrolysis: A reformulated three-parallel-reactions model”,...., 42, 434-441 (2003).
4 Becidan, M., Skreiberg, Ø., Hustad, J., “Products distribution and gas release in pyrolysis of thermally thick biomass residues samples”,..., 78, 207-213 (2007).
5 Yan, R., Yang, H., Chin, T., Liang, D.T., Chen, H., Zheng, C., “Influence of temperature on the distribution of gaseous products from pyrolyzing palm oil wastes”,, 142, 24-32 (2005).
6 Hu, S., Jess, A., Xu, M., “Kinetic study of Chinese biomass slow pyrolysis: Comparison of different kinetic models”,, 86, 2778-2788 (2007).
7 Babu, B.V., Chaurasia, A.S., “Pyrolysis of biomass: Improved models for simultaneous kinetics and transport of heat, mass and momentum”,, 45, 1297-1327 (2004).
8 Park, Y.H., Kim, J., Kim, S.S., Park, Y.K., “Pyrolysis characteristics and kinetics of oak trees using thermogravimetric analyzer and micro-tubing reactor”,, 100, 400-405 (2009).
9 Muller-Hagedorn, M., Bockhorn, H., Krebs, L., Muller, U., “A comparative kinetic study on the pyrolysis of three different wood species’’,..., 68/69, 231-249 (2003).
10 Fisher, T., Hajaligol, M., Waymack, B., Kellogg, D., “Pyrolysis behavior and kinetics of biomass derived materials”,..., 62, 331-349 (2002).
11 Reed, T. B., Gaur, S., “Atlas of thermal data of biomass and other fuels-A report on the forthcoming book”,, 7, 143-145 (1994).
12 Grammelis, P., Basinas, P., Malliopoulou, A., Sakellaropoulos, G., “Pyrolysis kinetics and combustion characteristics of waste recovered fuels”,, 88, 195-205 (2009).
13 Zhu, H.M., Yan, J.H., Jiang, X.G., Lai, Y.E., Cen, K.F., “Study on pyrolysis of typical medical waste materials by using TG-FTIR analysis”,, 153, 670-676 (2008).
14 Liu, Q., Wang, S., Zheng, Y., Luo, Z., Cen, K., “Mechanism of wood lignin pyrolysis by using TG-FTIR analysis”,..., 82, 170-177 (2008).
15 Biagini, E., Barontini, F., Tognotti, L., “Devolatilization of biomass fuels and biomass components studied by TG/FTIR technique”,..., 45, 4486-4493 (2006).
16 Fu, P., Hu, S., Sun, L., Xiang, J., Chen, Q., Yang, T., Zhang, J., “Release characteristics and formation mechanism of gas products during rice straw and maize stalk pyrolysis”,, 29, 113-118 (2009).
17 Worasuwannarak, N., Sonobe, T., Tanthapanichakoon, W., “Pyrolysis behaviors of rice straw, rice husk, and corncob by TG-MS technique”,..., 78, 265-271 (2007).
18 Painter, P.C., Sobkowiak, M., Youtcheff, J., “FT-IR study of hydrogen bonding in coal”,, 66, 973-978 (1987).
19 Gómez-Serrano, V., Piriz-Almeida, F., Durán-Valle, C.J., Pastor-Villegas, J., “Formation of oxygen structures by air activation. A study by FT-IR spectroscopy”,, 37, 1517-1528 (1999).
20 Gómez-Serrano, V., Pastor-Villegas, J., Perez-Florindo, A., Duran-Valle, C., Valenzuela-Calahorro, C., “FT-IR study of rockrose and of char and activated carbon”,..., 36, 71-80 (1996).
21 Pastor-Villegas, J., Meneses Rodríguez, J.M., Pastor-Valle, J.F., García, M.G., “Changes in commercial wood charcoals by thermal treatments”,..., 80, 507-514 (2007).
22 Pastor-Villegas, J., Duran-Valle, C.J., Valenzuela-Calahorro, C., Gómez-Serrano, V., “Organic chemical structure and structural shrinkage of chars prepared from rockrose”,, 36, 1251-1256 (1998).
23 Tang, M.M., Bacon, R., “Carbonization of cellulose fibers (1) Low temperature pyrolysis”,, 2, 211-214 (1964).
24 Shafizadeh, F., Sekiguchi, Y., “Development of aromaticity in cellulosic chars”,, 21, 511-516 (1983).
2008-12-03,
2009-04-07.
the Special Funds for Major State Basic Research Projects of China (2004CB217704), and the National Natural Science Foundation of China (50721005).
** To whom correspondence should be addressed. E-mail: hssh30@163.com
 Chinese Journal of Chemical Engineering2009年3期
Chinese Journal of Chemical Engineering2009年3期
- Chinese Journal of Chemical Engineering的其它文章
- On-line Monitoring for Phosphorus Removal Process and Bacterial Community in Sequencing Batch Reactor*
- Simultaneously Designing and Targeting for Networks with Multiple Resources of Different Qualities*
- Modeling and Control of Nonlinear Discrete-time Systems Based on Compound Neural Networks*
- Effect of Doping Cerium in the Support of Catalyst Pd-Co/Cu-Co-Mn Mixed Oxides on the Oxidative Carbonylation of Phenol
- Biodegradation of Aniline by a Newly Isolated Delftia sp. XYJ6*
- Combination of Supercritical Fluid Extraction with Ultrasonic Extraction for Obtaining Sex Hormones and IGF-1 from Antler Velvet*
