共溶剂对超临界CO2注入技术制备聚丙烯/SiO2纳米复合材料的影响
张爱琴 颜 蔚 施立群 施利毅, 方建慧 金鹿江
(1上海大学理学院,上海 200444;2上海大学纳米科技与技术研究中心,上海 200444; 3复旦大学现代物理研究所,上海 200433)
In recent years,the synthesis of polymer/inorganic hybrid materials has become the subject of extensive studies[1-4].These materials,combining the properties of each component,are supposed to achieve the desired material performance[4-6].Sol-gel reaction is now employed as the general method to obtain the polymer/inorganic hybrid materials.During the sol-gel process, it is very important to find solvents to dissolve both inorganic precursors and organic polymers[5-8].However,some polymers, such as polypropylene(PP)and polyethylene(PE),have very bad solubilityin common solvents[7].Anotherproblemassociated with the sol-gel process is that the remaining organic solvents can not be totally removed.New methods to synthesize these polymerbased hybrid materials must be developed.
The supercritical state was first reported in 1822 by Tour et al.[9].There are a large number of substances that can be used as fluidsinsupercriticaltechniques.Amongthem,CO2ismostwidely used for its non-toxicity,non-flammability[10].Moreover,the critical temperature and pressure of CO2are relatively low, which make it quite cost efficient in terms of energy use during operation.Though supercritical CO2(scCO2)is a weak solvent for most polymers,it can effectively swell and plasticize the amorphous region of polymers[7,11].Many low-molecular-weight additives are dissolvable in scCO2,and there have been many reports on impregnation of these additives into polymers with the aid of scCO2[12-16].Upon release of pressure,CO2leaves the polymers rapidly,while the low-molecular-weight additives are left in the polymers for their low diffusivities.Watkins and McCarthy[13]used scCO2to infuse a Pt precursor into poly(4-methyl-1-penetne) (PMP)and polytetrafluoroethylene(PTFE).Taylor et al.[14]achieved impregnation of a silver precursor[Ag(COD)(HFA)](1,5-cyclooctadiene-1,1,5,5,5-hexa-fluoroacetylacetonato silver)into polyimide and poly(etherether ketone)(PEEK)films through scCO2.Recently,PP based hybrid materials have been successfully fabricated by infusing tetraethyl orthosilicate(TEOS)[7]and sodi-um benzoate(NaBZ)into PP films through scCO2[12].
As CO2is a non-polar molecule,many polar compounds have very low solubilities in scCO2.The addition of small amount of polar co-solvent may increase the solubilities of polar compounds in scCO2significantly[15-16].Much attention has been paid to investigating the effects of co-solvents on the solubility of polar substances in scCO2[17].As the impregnation process may be described as a partitioning of the compound between the CO2-and polymer-rich phases,the relative solubility of the compound in both scCO2and polymer is the major factor governing the amount of the compound adsorbed in the polymer[18].The increased solubility of the compound in scCO2is supposed to result in the decreased dispersion of the substance in polymer, which might be the reason why co-solvents were seldom used when researchers tried to prepare polymer/inorganic hybrid materials through scCO2.However,when Hu et al.[12]tried to impregnate NaBz into PP films,they found that the mass uptake of NaBz into PP films increased when co-solvents were added.
To learn the information inside the hybrid polymers,cross sections were performed,and transmission electron microscopy (TEM)and scanning electron microscopy(SEM)images were taken[7,13-14].These images,however,could just give us the information on the size and morphology of the inorganic particles in the hybrid polymers.X-ray photoelectron spectra(XPS)were used,but only obtain the atomic concentrations of elements on the surface of polymers[19-20].Therefore,it is a big challenge to quantitatively analyze the depth profile of the compound infused into the polymer.Rutherford backscattering(RBS)is an analytical technique which can quantitate composition of thin layers or near surface regions of solids by measuring the backscattering spectrometry of a beam of high energy ions impinging on a sample[21].The number of scattered particles and their energies are measured to provide information on elemental concentrations versus depth in a range of several nanometers below the surface. Thus,RBS is a good method to determine the concentrations of the infused elements versus depth in the hybrid polymer[22].In this research,TEOS was infused into PP strips by scCO2,using ethanol as the cosolvent,to investigate the effects of cosolvents on the synthesis of polymer/inorganic hybrid materials through scCO2impregnation.
1 Materials and methods
1.1 Chemicals
All chemicals were of analytical grade.The PP sheet with the thickness of 1 mm was supplied by Wenzhou Guofeng Plastic Co.Ltd.,China,and was cut into PP strips(35 mm×30 mm)before use.TEOSand ethanolwere bought from Sinopharm Chemical Reagent Co.,China,CO2with a purity of 99.99%was supplied by Shanghai Youjiali Liquid Helium Co.,China,and used as received.
1.2 Impregnation of PP strips with TEOS in scCO2
The impregnation of PP strips was carried out in a high-pressure stainless steel vessel(inner volume:110 mL).In a typical experiment,10 mL TEOS was introduce into a small glass beaker.After addition of certain amount of ethanol,the beaker was set at the bottom of the high-pressure stainless steel vessel. The volume ratio of ethanol to TEOS in the glass beaker was denoted as VEtOH/VTEOS.PP strips were exposed to the mixed solution of TEOS and ethanol.The vessel was then closed and suitable amount of CO2was charged into the vessel through a high-pressure manual pump.The impregnation was performed at a given pressure and temperature.After a desired soaking period,the high-pressure vessel was vented rapidly,and the PP strips were taken out and kept in an ambient atmosphere for the releasing of CO2.
1.3 Post-treatment
A Teflon lined steel hydrothermal autoclave was used for the post-treatment of PP strips.1 mL ethanol was mixed with 19 mL hydrochloric acid(0.1 mol·L-1).The mixed solution was set at the bottom of the autoclave.The impregnated PP strips were immersed in the solution.The autoclave was heated to 110℃in an oven and maintained at that temperature for 20 h.Finally,the PP strips were washed with water and ethanol and then dried at ro om temperature.
1.4 RBS detection
The incident proton beams used in the RBS measurement with the energy of 2.0 MeV,were provided by the NEC 9SDH-2 2×3 MV tandem accelerator at Fudan University.The Au/Si sur-face barrier detector was placed at the laboratory backward angle of 165°and subtended a solid angle of 1.87×10-3sr,set by a defining slit of 3 mm×4 mm.The angular resolution of the detector was thus 1°.The He+(2 MeV)beam was confined to a diameter of 0.6 mm before bombarding the target and the beam currents were limited to less than 40 nA.The beam current should be kept sufficiently low to ensure the dead time of analyzer was negligible and pulse pileup was minimized.The experiment spectra were analyzed with SIMNRA software.
1.5 Characterization
Thin cross sections for TEM were prepared using a LKB-2088 ultramicrotome,and TEM images were taken on a Hitachi H-800 transmission electron microscope.
2 Results and discussion
2.1 Mass change of the infused PP strip
When the PP strips were taken out after infusion and kept in an ambient atmosphere for the releasing of CO2.It can be seen from Fig.1 that the mass of infused PP strips decreases over time, but it does not change any more after 15 h,suggesting that the CO2adsorbed in the PP strip had been totally diffused into the air.Then the PP strips were weighted.The mass gain of the PP strips against its initial mass could be regarded as the mass uptake of PP strips(Eq.(1))

where m0stands for the initial mass of PP strips,and mgstands for the mass gain of PP strips after impregnation.
2.2 Effect of ethanol on mass uptake of TEOS into PP strips
The effect of ethanol on the mass uptake of TEOS into PP strips is shown in Fig.2.The impregnation experiments were performed at 40℃and 16 MPa for 10 h soaking in scCO2.It can be seen that when no co-solvent is added (VEtOH/VTEOS=0.0),the mass uptake of TEOS into PP strips is 0.4%.The mass uptake increases with the increase of the value of VEtOH/VTEOS,but it decreased when the VEtOH/VTEOSis larger than 0.3.

Fig.1 Mass change of the infused PP strip over time

Fig.2 Effect of VEtOH/VTEOSon mass uptake of TEOS into PP strips
It is known that the solubility of polar compounds in non-polar scCO2is very low.Researchers found that addition of a small amount of polar co-solvent could increase the solubility of polar compounds in scCO2significantly[15,23-24].The solubility enhancement can be attributed to two reasons.One is that the density of scCO2will increase if the cosolvent is added,which can improve the solvent strength of scCO2[23].The other is that there are strong interactions between the compound and the cosolvent[15].Since TEOS and ethanol are both polar molecules,the dipole-dipole interaction between TEOS and ethanol is one of the main reasons accounting for the increasing solubility of TEOS in scCO2. As the process of infusion of a low-molecular-weight compound into polymers by scCO2can be described as a partitioning of the additive between the CO2-and polymer-rich phases,the increasing solubility of TEOS in scCO2is supposed to result in the decreasing dispersion of TEOS into the PP strips.
In a“co-solvent free”system,the mass uptake of a compound into polymer phase depends on the relative solubility of the compound in both scCO2and polymer.The increased solubility of the compound in scCO2would lead to a decreased dispersion of the compound in the polymer substrate.However,the added co-solvent is soluble in both scCO2and polymer phase[24].The co-solvent in the polymer phase can form clusters with the polar compound through dipole-dipole interaction,which will increase the dispersion of the polar compound in the polymer phase. Brantley et al.[18]found that at high pressure,the partition coefficients(K)of polar compounds between scCO2and polymer phase after addition of co-solvents were almost the same as that without addition of co-solvents.K can be defined as follows:

where Cpand Cfstand for the concentration of the polar compound in polymer and scCO2fluid phase,respectively.The solubility of compounds was reported to increase significantly with the concentration of co-solvent,and Cpwill increase with the increase of Cfaccording to Eq.(2)because the value of K nearly keeps constant at high pressure no matter whether co-solvent was added or not.Thus,the increasing solubility of TEOS in scCO2,which resulted from the addition of ethanol,would lead to the increasing mass uptake of TEOS into PP strips.
In addition to having interactions with TEOS,ethanol can self-associate through hydrogen bonding[15],which will reduce its effect on enhancing the solubility of the polar compound in CO2phase.When the concentration of ethanol is too high,the interactions between ethanol molecules are so strong that the actual number of ethanol molecules to form clusters with TEOS de-creases,leading to the decrease in the Cfof TEOS.Since K is a constant under the condition of high pressure,the concentration of TEOS in PP strips will decrease with the solubility reduction of TEOS in scCO2phase.That is the reason why when VEtOH/VTEOSwas larger than 0.3,the mass uptake of TEOS into PP decreased with the increase in the volume ratio of ethanol to TEOS.

Fig.3 Effect of soaking pressure on mass uptake of TEOS into PP strips
2.3 Effect of soaking pressure on mass uptake of TEOS into PP strips
Fig.3 displays the effect of soaking pressure on the mass uptake of TEOS into PP strips when VEtOH/VTEOSis 0.3 at 40℃for 10 h soaking in scCO2.It can be seen from Fig.3 that the mass uptake of TEOS into PP strips increases to a maximum and then decreases with increasing soaking pressure,and the maximum mass uptake could be observed at 16 MPa.The value is very close to the pressure at which the maximum mass uptake of TEOS into PP films could be observed when no co-solvent was added[7].
It is known that scCO2can effectively swell and plasticize the amorphous region of polymers,and the inter-chain distance of polymers increases upon plasticization.The degree of plasticization of polymers will increase with the increasing scCO2pressure,which allowing more TEOS and ethanol molecules to permeate into the PP strips and leading to the increasing in mass up-take of TEOS into PP strips.However,the increase of free volume will be ultimately limited by the restriction of the rigid crystalline phase,indicating that the dispersion amount of the compound and co-solvent into polymer phase will be limited. On the other hand,the solubility of TEOS in the scCO2increases rapidly with increasing scCO2pressure.Li et al.[25]also tried to disperse 2,2-methylene-bis(4,6-di-tert-butylphenyl)phosphate (NA40)into PP pellets,using acetone as the co-solvent.The maximum mass uptake of NA40 into PP pellets was observed at ca 10 MPa.They found that the partition coefficient of NA40 de creased as the pressure increased,while the solubility of NA40 increased almost linearly with the CO2pressure,implying that the solubility of NA40 in PP pellets increased less than in scCO2as the scCO2pressure increased.That is the reason why the maximum mass uptake could be observed.
2.4 Effect of soaking temperature and time on mass uptake of TEOS into PP strips
Fig.4A displays the effect of soaking temperature on the mass uptake of TEOS into PP strips at 16 MPa for 10 h when VEtOH/VTEOSis 0.3.It can be seen from Fig.4A that the mass uptake of TEOS into PP strips increased with the increasing temperature.The degree of plasticization of the polymer substrates increased when the soaking temperature raised,and the larger free volume allowed more TEOS molecules to partition into PP strips.
Fig.4B shows the effect of soaking time on the mass uptake of TEOS into PP strips at 16 MPa and 80℃when VEtOH/VTEOSis 0.3. It can be seen that the mass uptake of TEOS into PP strips increases almost linearly with time,and even after 25 h,the mass uptake still increases.
2.5 Characterizations
Fig.5A shows the RBS spectra of a PP strip impregnated with TEOS at 16 MPa and 80℃for 10 h when VEtOH/VTEOSis 0.3,then followed by hydrolysis.The peaks have been observed at 0.7 and 1.4 MeV,corresponding to O and Si,respectively.A broad wave due to C element appears below 0.5 MeV.The gentle slopes to the lower energy side of RBS peaks for O and Si indicate the diffusion of the elements to PP strips.

Fig.4 Effects of soaking temperature(A)and time(B)on the mass uptake of TEOS into PP strips
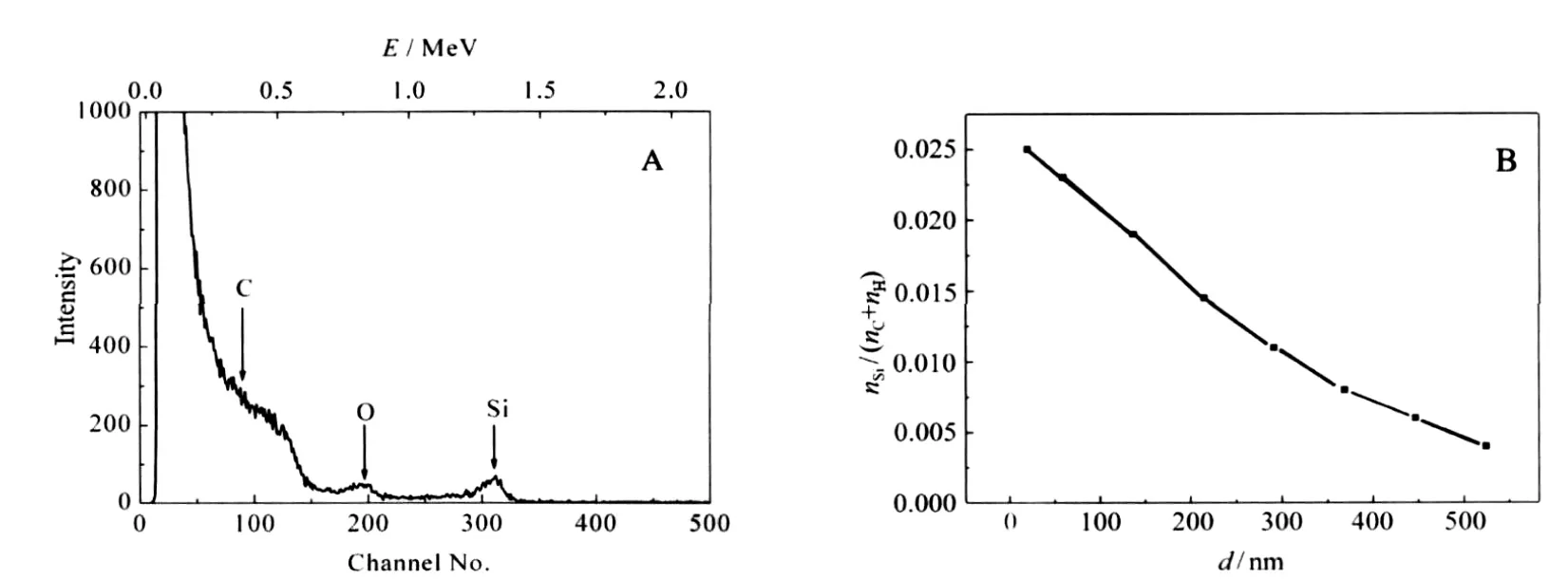
Fig.5 RBS spectrum a PP strip impregnated with TEOS followed by hydrolysis(A),depth(d)profile of Si element in the PP strip(B)
Taylor et al.[14]have impregnated a silver precursor into polyimide and PEEK films with the aid of scCO2;from the cross-section TEM images of the films,it can be seen that high-density silver particles with the diameter of tens of nanometers presented on the surface of the films,and low-density silver nano-sized particles extended less than 300 nm into the films.The TEM image shows the distribution of the silver particles in the polymer. It can be concluded from the image that the concentration of silver element near the surface of the polymer is high,and the concentration of silver element inside the polymer is low.RBS is an effective technique to quantitate composition analysis of thin layers or near surface regions of solids by measuring the back-scattering of a beam of high energy ions impinging on a sample.After analysis of the RBS spectra,depth distributions of the infused elements can be obtained.Fig.5B shows the Si element profile in a PP strip,which was obtained by analysis of the RBS spectra with SIMNRA software.It can be seen that the Si ele-ment extends several hundred nanometers into the PP strip, and decreases with the infusion depth.
Li and Vogt[26]have tried to swell polymer films with CO2containing dilute concentrations of water and TEOS to form a rigid silica network within the hydrophilic domains.After calcinations,mesoporous silica films formed,and the CO2concentration profile in polymer films could be visualized from the porosity and pore size of the silica films.They found that the film synthesized at 7 MPa exhibited a very uniform pore size throughout the film.The film synthesized at 8.7 MPa had a gradient in the pore size that extended approximately 150 nm into the film from the free surface and a short gradient(<10 nm)at the buried interface,and the film synthesized at 10.4 MPa also had larger pores close to the free surface and buried interface,but to a less degree.The pore size is an indirect but accurate measure of the CO2induced swelling of the hydrophobic domains within the polymer film.During the impregnation process,as the compounds are brought into polymers by scCO2through swelling of the polymers,it can be deduced that the concentration of TEOS is related to the concentration of CO2in the polymer.Sun et al.[7]also found that the silica particles were uniformly distributed in the PP matrix.However,it can be seen from Fig.5B that the concentration of Si element in the polymer was not uniform.Instead,it decreased with the infusion depth.The deviation might result from the difference in the thickness of PP substrate.Either Sun et al.[7]or Li and Vogt[26]used PP thin films,and the thickness of the PP films was 500 nm or 100 μm,respectively.While during our experiments,the thickness of PP strips was 1 mm, which might be the reason why the concentration of Si element in the PP strip decreased with the infusion depth.

Fig.6 TEM image of SiO2nanoparticles within PP strips
Fig.6 displays the cross-section TEM image of the PP/SiO2hybrid material.The SiO2particle size in the image ranged from 40 to 100 nm.
3 Conclusions
When TEOS was infused into PP strips by scCO2using ethanol as the co-solvent,the mass uptake of TEOS into PP strips increased with the addition of ethanol because the solubility of TEOS in scCO2increased when ethanol was added.But when the concentration of ethanol was too high,the concentration of TEOS in scCO2would decrease for the self-association of ethanol molecules,leading to the reduction of the mass up-take of TEOS into PP strips.The effects of impregnation experimental conditions(suchassoakingtemperature,pressure,andtime)on the mass uptake of TEOS into PP strips under the existence of cosolvent were also discussed.The depth profile of the infused element inside the hybrid polymer material was obtained by RBS technology.It can be seen the Si element extends several hundred nanometers into the PP strip.The concentration of Si element decreased with the infusion depth.
1 Spange,S.;Graser,A.;Muller,H.;Zimmermann,Y.;Rehak,P.; Jager,C.;Fuess,H.;Baehtz,C.Chem.Mater.,2001,13:3698
2 Sun,D.;Sue,H.J.;Miyatake,N.J.Phys.Chem.C,2008,112: 16002
3 Liang,X.;George,S.M.;Weimer,A.W.;Li,N.H.;Blackson,J. H.;Harris,J.D.;Li,P.Chem.Mater.,2007,19:5388
4 Shah,M.S.A.S.;Nag,M.;Kalagara,T.;Singh,S.;Manorama,S. V.Chem.Mater.,2008,20:2455
5 Mazzocchetti,L.;Cortecchia,E.;Scandola,M.ACS Appl.Mater. Interface,2009,1:726
6 Beek,W.J.E.;Wienk,M.M.;Kemerink,M.;Yang,X.;Janssen,R. A.J.J.Phys.Chem.B,2005,109:9505
7 Sun,D.;Zhang,R.;Liu,Z.;Huang,Y.;Wang,Y.;He,J.;Han,B.; Yang,G.Macromolecules,2005,38:5617
8 Kim,K.M.;Adachi,K.;Chujo,Y.Polymer,2002,4:1171
9 Cagniard de la Tour,C.Ann.Chim.Phys.,1822,21:127
10 Green,J.W.;Rubal,M.J.;Osman,B.M.;Welsch,R.L.;Cassidy, P.E.;Fitch,J.W.;Blanda,M.T.Polym.Adv.Technol.,2000,11: 820
11 Watkins,J.J.;McCarthy,T.J.Macromolecules,1995,28:4067
12 Li,B.;Hu,G.H.;Cao,G.P.;Liu,T.;Zhao,L.;Yuan,W.K. J.Appl.Polym.Sci.,2006,102:3212
13 Watkins,J.J.;McCarthy,T.J.Chem.Mater.,1995,7:1991
14 Boggess,R.K.;Taylor,L.T.;Stoakley,D.M.;StClair,A.K. J.Appl.Polym.Sci.,1997,64:1309
15 Zhong,M.;Han,B.;Yan,H.;Peng,D.Y.Fluid Phase Equilibria, 1997,134:175
16 Ke,J.;Mao,C.;Zhong,M.;Han,B.;Yan,H.J.Supercrit.Fluid, 1996,9:82
17 Nilsson,W.B.;Seaborn,G.T.;Hudson,J.K.Journal of the American Oil Chemists′Society,1992,69:305
18 Brantley,N.H.;Bush,D.;Kaarian,S.G.;Eckert,C.A.J.Phys. Chem.B,1999,103:10007
19 Rosolovsky,J.J.Mater.Res.,1997,12:3127
20 Nazem,N.;Taylor,L.T.;Rubira,A.F.J.Supercrit.Fluid,2002, 23:43
21 Jiang,L.;Liu,B.;Zhou,Z.Y.;He,M.H.;Zhao,G.Q.;Zong,X.F. Chin.Phys.Lett.,1999,10:770
22 Bubert,H.;Jenett,H.Surface and thin film analysis:principles, instrumentation,applications.Weinheim:Wiley-VCH,2002
23 Sahle-Demessie,E.;Pillai,U.R.;Junsophonsri,S.;Levien,K.L. J.Chem.Eng.Data,2003,48:541
24 Kazarian,S.G.;Vincent,M.F.;West,B.L.;Eckert,C.A. J.Supercrit.Fluid,1998,13:107
25 Li,B.;Hu,G.H.;Cao,G.P.;Liu,T.;Zhao,L.;Yuan,W.K. J.Supercrit.Fluid,2008,44:446
26 Li,X.;Vogt,B.D.Macromolecules,2008,41:9306
———理学院

