Changes of PKAC-β,c-Fos and BDNF in cerebral cortex after intracerebral hemorrhage in rats treated with WIN55-212-2
ZHU Li, DONG Zhi, ZHANG Guo- dong
(Department of Biochemistry and Molecular Pharmacology Laboratory,Chongqing Medical University,Chongqing 400016,China.E -mail:zhidong073@hotmail.com)
Intracerebral hemorrhage(ICH)is the bleeding caused by the rupture of blood vessels in brain.Blood irritates the brain tissues,causing cerebral swelling and collecting it into a mass called hematoma.Either swelling or a hematoma will increase pressures on nearby brain tissues and then quickly destroy them.So the clinical symptoms usually develop suddenly without warning,often during activity.Intracerebral hemorrhagic stroke accounts for 10% -25%of all strokes.
In central nervous system(CNS),cannabinoids(CBs)is broadly defined as compounds with actions on cannabinoid receptor I(CB1R)to protect the neurons.The signaling pathways involved in the mechanisms of cannabinoids[1]are related to the expression of BDNF through regulation of cyclic adenosine 3',5'- monophosphate(cAMP)response element binding protein(CREB)[2].Verification of the relationship between CB1R and BDNF,and the evidence of increase in the levels of BDNF by PKA and c-Fos are important to understand the mechanisms of cannabinoids[3-5].The main purpose of the present study is to explore the effect of WIN55-212-2,a CB1R agonist,on the expression of PKAC-β,c-Fos and BDNF in ipsilateral cortex,so as to provide the experimental evidence for using CBs in treating ICH.
MATERIALS AND METHODS
1 Animals and surgery
The study was performed according to the guidelines of the Institutional Animal Care Committee.Thirty-six(36)male SD rats,weighing 180 -220 g,were provided by Animal Center of Chongqing Medical University.The surgical protocol of collagenaseⅦ (Sigma)injection was performed according to the procedure described by Rosenberg GA et al[6].Briefly,30 rats were deeply anesthetized with chloral hydrate(30%in saline solution)intraperitoneally,and the rats were placed in a stereotaxic apparatus.The rats were injected with collagenase Ⅶ (0.5 U in 2 μL saline)unilaterally into the right globus pallidus,using the stereotactic coordinates 1.0 mm posterior and 3.2 mm lateral of bregma,and 5.6 mm in depth.CollagenaseⅦ was delivered over 3 min and the needle stayed in place for an additional 5 min to prevent any reflux.Six rats were deeply anesthetized and sham - operated as controls.After injured,30 animals were housed separately with free access to food and water ad libitum for 5 different treatments.Dissolved in dimethylsulfoxide(DMSO),the CB1R agonists WIN55 -212 -2 at dose of 1 μg/g,3 μg/g or 5 μg/g or an equal volume of the vehicle(DMSO)was administered 30 min after ICH.Nimodipine was also injected at dose of 1 μg/g by the intraperitoneal route(ip),30 min after ICH.After postanaesthetic recovery,the rats were scored for neurological deficits using a longa's neurological scoring system.The rats graded 3 scores were chosen for the further experiments,by an observer blinded to the experimental treatment.
2 Immunohistochemistry
The rats were sacrificed 24 h after the operation.The rats were deeply anesthetized with chloral hydrate(300 mg/kg,ip)and transcardially perfused with phosphate- buffered saline(PBS,pH 7.4)followed by 4%paraformaldehyde in PBS.The brains were removed,post-fixed in the same fixative for 24 h.The serial tissue sectioning was performed in the vertical meridian.The thickness of the sections was 5 μm,which were taken for HE staining and immunohistochemical staining.HE staining was used for re-confirming the pathological results.The protein expression of PKAC -β,c-Fos and BDNF was determined by immunohistochemical staining according to the instruction of sABC kit(Boster,China).The sections were incubated with mouse anti-rat polyclonal antibody for PKAC-β (1∶120,Bioworld),c-Fos(1∶120,Bioworld)or BDNF(1∶50,Santa Cruz)followed by the incubation with biotinilated anti-rabbit IgG and the streptavidiperoxidase complex.The positive cells were observed and the images were obtained under light microscope(Leica).All sections were analyzed under the same magnification times and the same light intensity.
3 Semi-quantitative RT-PCR analysis
The rats were sacrificed at 24 h after reperfusion with 0.9%saline solution.The ipsilateral cortex of cerebrum was rapidly harvested,frozen and pooled under-80℃ for future use.The total RNA was isolated from cortex according to the manufacturer's(Bio-flux,China)instructions.The total RNA(1 μg)was transcribed into cDNA using AMV reverse transcriptase(Bio-flux)with 0.5 μL oligo-(dT)18(Bio-flux)as primers.The mRNA expression of β - actin was used as an internal standard to normalize the mRNA levels of PKAC-β,c-Fos and BDNF.The nucleotide sequences of the sense and antisense primers and the expected product size were listed in Table 1.The PCR specific optimum cycle numbers of amplification were operated as follows:30 cycles for PKAC -β or c-Fos,35 cycles for BDNF,and 28 cycles for β - actin.Amplified cDNA was then separated by electrophoresis in 2%agarose(Biowest,Bio Sun,China)gels stained with ethidium bromide(Promega).The band size was recognized using DNA ladder.The optical density of the bands was measured using Bio-Rad video imaging system.The quantification was performed by expressing the absorbance(A)of the respective bands as percentage of β - actin.
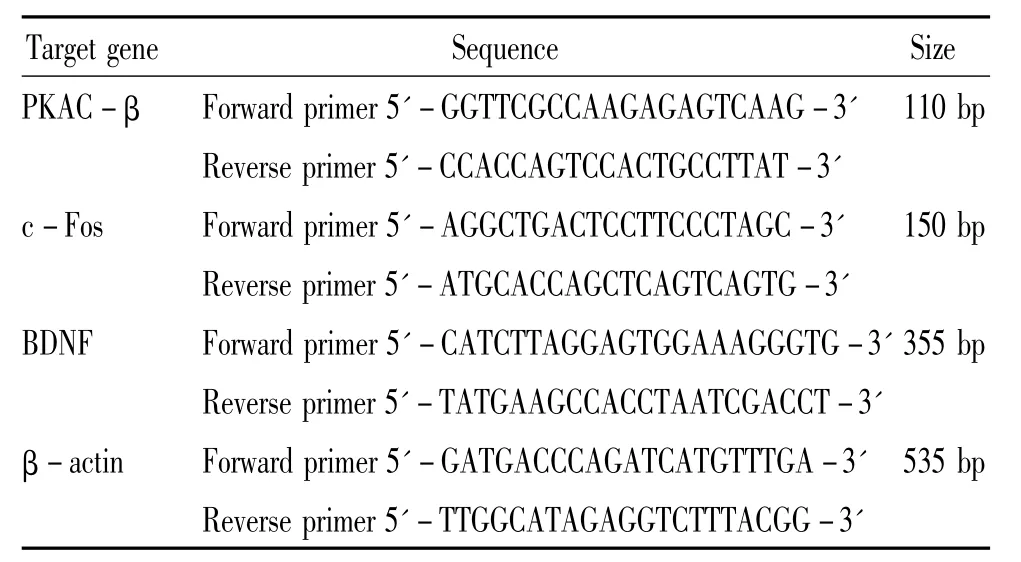
Table 1.The primer sequences of PKAC - β,c-Fos,BDNF and β-actin
4 Western blotting
The cerebral cortex was homogenized in lysis buffer.The cell lysates were centrifuged at 12000 ×g for 15 min,and protein content was measured using the BCA assay.The samples were diluted 1∶1 in PBS and denatured at 95 ℃ for 5 min.The proteins(50 μg/lane)were separated by SDS-PAGE(10%resolving gel)and transferred onto PVDF membranes by semidry transfer.The blots were blocked for 1 h at room temperature with blocking buffer,and incubated with the antibodies of rabbit polyclonal anti-PKAC - β (1∶120;Bioworld),rabbit polyclonal anti- BDNF(1∶100;Santa Cruz)or rabbit polyclonal anti-β-actin(1∶500;Abzoom)in blocking buffer overnight at 4 ℃.After incubation with a horseradish peroxidase-coupled secondary antibody(1∶3000,Beyotime,China)for 2 h at room temperature,the bands were visualized using the ECL detection system.The membrane was subsequently exposed to a photographic film.The A value of the bands were calculated using Bio-Rad video imaging system.The quantification was also performed by expressing the optical density of the respective bands as percentage of β - actin.
5 Statistical analysis
The data were expressed as mean±standard deviation(),and data processing was performed with SPSS 13.0 statistical package.
RESULTS
1 The effect of WIN55-212-2 on hematoma volume in HE staining
The pathomorphology of cerebral tissues was observed with HE staining under microscope.In sham group(Figure 1A),the cerebral tissue was normal,no bleeding and no degenerating neurons was observed.However,in model group(Figure 1B),there was a significant bleeding area,which was full of red blood cells without normal structure.Compared to model group,nimodipine(Figure 1C)reduced the hematoma volume in thelesioned (ipsilateral)hemisphere.WIN55-212-2(Figure 1D,E,F)treated rats had smaller hematoma volumes than that in nimodipine treated animals in a dose -dependent manner.
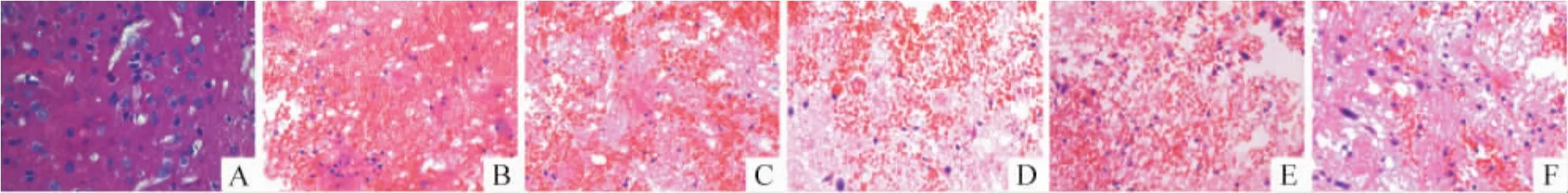
Figure 1.Intracerebral hemorrhage in cerebral cortex after collagenaseⅦ injection with or without WIN55-212-2 treatments(HE staining,×400).A:sham group;B:model group;C:nimodipine group;D.WIN55-212-2,1 μg/g group;E:WIN55 -212 -2,3 μg/g group;F:WIN55 -212 -2,5 μg/g group.
2 The position of PKAC -β,c-Fos and BDNF expression in the cells detected by immunohistochemistry
The proteins of PKAC-β,c-Fos,and BDNF were expressed on the membrane of neurons,in the nucleus of neurons or the cytoplasm of glial cells respectively(Figure 2).
3 The effect of CB1R agonist WIN55-212-2 on the mRNA expression of PKAC -β,c-Fos and BDNF in ipsilateral cortex of cerebrum
Compared to sham group,the mRNA expression of PKAC-β,c-Fos and BDNF were all increased significantly in model group(P<0.05).The mRNA expression of c-Fos and BDNF in nimodipine group was higher than that in model group(P<0.05),but the application of nimodipine did not show any statistically significant change of PKA mRNA levels as compared to model group(P>0.05).Compared to nimodipine group,the mRNA expression of c-Fos and BDNF increased significantly,while the mRNA expression of PKAC-β reduced significantly after treatment with WIN55 -212 -2 at doses of 1 μg/g,3 μg/g or 5 μg/g for 24 h,respectively(P<0.05).The results showed that the activation of c-Fos and BDNF or the inhibition of PKA after WIN55-212-2 treatment were both in a dose-dependent manner(Figure 3).
4 The effect of CB1R agonist WIN55-212-2 on the proteins expression of PKAC-β and BDNF in ipsilateral cortex of cerebrum
Compared to sham group,the proteins levels of PKAC-β and BDNF were increased significantly in model group(P<0.05).The protein expression of BDNF in nimodipine group was higher than that in model group(P<0.05),but the application of nimodipine did not show any statistically significant change of PKA protein level as compared to model group(P >0.05,Figure 3).Compared to nimodipine group,the protein expression of BDNF increased obviously after treated with WIN55 - 212 - 2 at doses of 1 μg/g,3 μg/g or 5 μg/g for 24 h,respectively,but the protein expression of PKAC-β reduced obviously(P<0.05).The results showed that the activation of BDNF or the inhibition of PKA after WIN55-212-2 treatment was both in a dose-dependent manner(Figure 4).
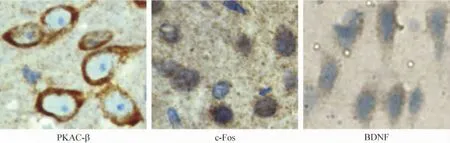
Figure 2.Effect of WIN55-212-2 treatment on the protein expression of PKAC-β,c-Fos and BDNF in ipsilateral cortex 24 h after ICH.All drugs were injected ip 30 min after operation.The protein expression of BDNF,PKAC -β and c-Fos in cortex was determined by immunohistochemistry(sABC,×400).
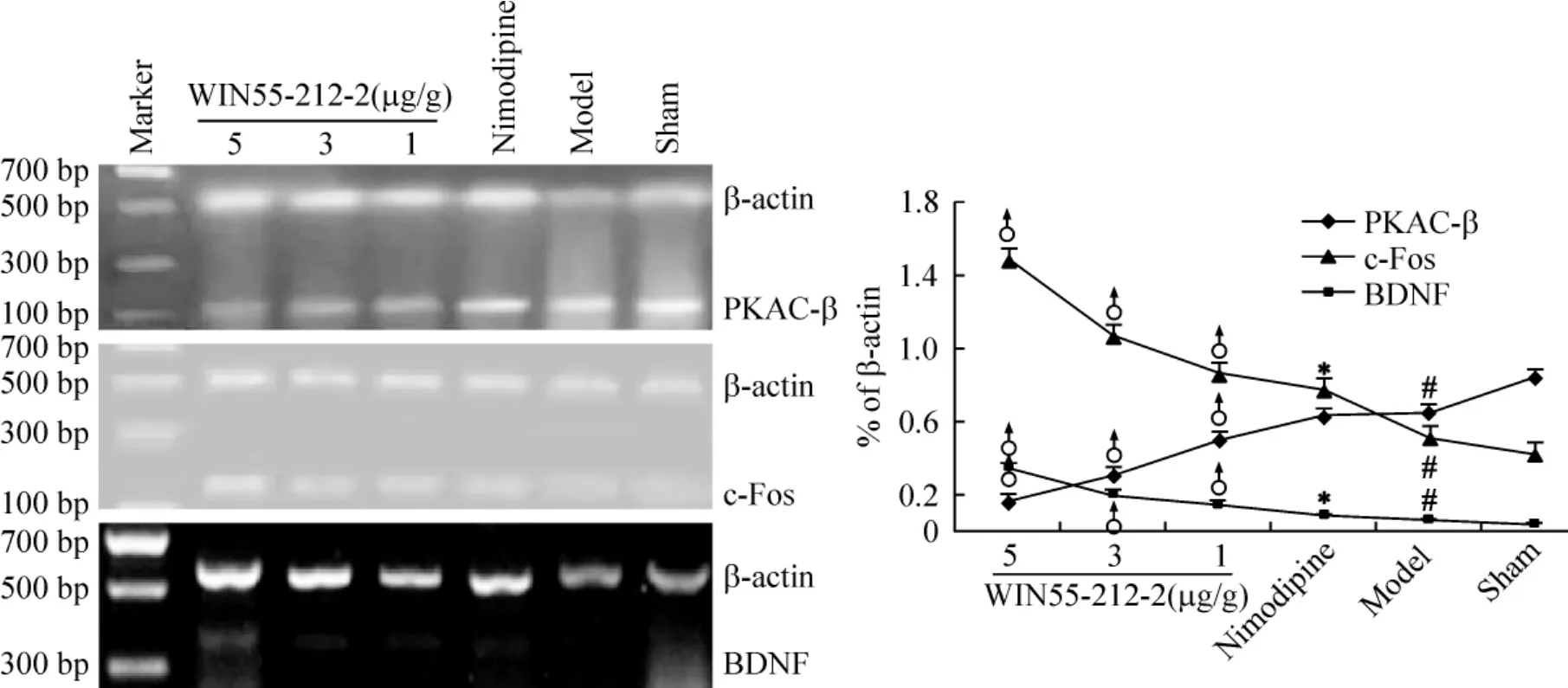
Figure 3.Effect of WIN55-212-2 treatment on the mRNA expression of PKAC-β,c-Fos and BDNF in ipsilateral cortex 24 h after ICH.All drugs were injected ip 30 min after operation..n=3.*P <0.05 vs model;♂P <0.05 vs nimodipine;#P<0.05 vs sham.
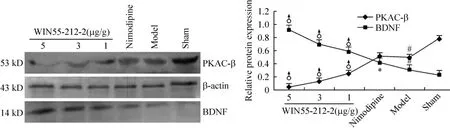
Figure 4.Effect of WIN55-212-2 treatment on the protein expression of PKAC-β and BDNF in ipsilateral cortex 24 h after ICH by Western blotting.All drugs were injected ip 30 min after operation..n=3.*P<0.05 vs model;♂P<0.05 vs nimodipine;#P<0.05 vs sham.
DISCUSSION
In recent years,the scientists are interested in the relationship between CB1R and BDNF.Khaspekov et al[7]and Marsicano et al[8]reported that genetic suppression or pharmacological antagonism of CB1R blocks the production of BDNF following the toxic administration of kainic acid,suggesting that BDNF may be an another important mediator of the neuroprotective effects of CBs.Derkinderen et al[5]and Marsicano et al[8]also found that CBs induced the expression of BDNF mRNA through activation of“microtubule associated protein kinase”and“extracellular signal- regulated kinases”(MAPK/ERK)pathway.In 2008,De March et al[9]further proved that BDNF upregulated coincides with a higher binding activity and an increased protein expression of CB1R in the rat model of striatal excitotoxicity.In short,WIN55-212-2 can induce the expression of BDNF.In contrast,some scholars found that WIN 55 -212-2 significantly reduced the levels of BDNF in hippocampus,cortex or cerebellum.As for this phenomenon,the differences in the model systems used should be taken into account when discussing such discrepancies[10,11].Our results showed that the expression of BDNF at mRNA and protein levels increased obviously in WIN55-212-2 treated rats in ipsilateral cortex.
Various signaling pathways,through CREB -mediated,can regulate a number of genes known as late response genes,which control the expression of neurotrophic factors,such as BDNF.This mechanism may be particularly important for long-term changes in gene expression and necessary for neuron protection.cAMP/PKA is one of the best characterized cannabinoid signaling pathways.Childers et al[12]first reported that cannabinoids bound to the receptors that coupled to Gi/oproteins and inhibited adenylyl cyclase in 1996.After nearly 10 years,Kim et al[13,14]also demonstrated that the neuroprotective effectsofcannabinoids,acting through CB1R and Gi/o proteins,depend on the suppression of cAMP/PKA signaling.Our results are consistent with this conclusion.WIN55-212-2 reduced the PKAC-β at mRNA and protein levels in ipsilateral cortex.PKA contains catalytic subunits of α,β and γ,and the β subunit level is the highest in neurons,which is necessary for synaptic plasticity.So we choose β subunit in our experiment.Although PKA was reduced by WIN55-212-2,it may induce the expression of BDNF[3-5].This mechanism needs for further demonstration.
c-Fos undergoes rapid induction after extracellular stimulus,which is not only implicated in neuronal apoptosis but also important for neuronal recovery.c-Fos contains both serum response element(SRE)and cAMP response element(CRE),which is important for regulating the expression of BDNF[5].Therefore,we focus our attention on c-Fos,in addition to PKA.
As early as 10 years ago,Mailleux et al[15]discovered that intraperitoneal injection of cannabinoids significantly increased the mRNA concentrations of c-Fos,c-Jun and ZIF-268 in the cingulate cortex.The result of our experiment also supported this conclusion.At the time point of 24 h after intraperitoneal injection of WIN55-212-2,BDNF was increased accompany with c-Fos,suggesting that c-Fos has a positive regulatory effect on the expression of BDNF in WIN55-212-2 treated rats.
Collectively,our results show that WIN55-212-2 evokes a concentration-dependent increase in BDNF.The functional crosstalk between CB1R and BDNF signaling is so complicated that is still not clear.However,evidence is emerged to suggest that cannabinoids increase the levels of BDNF.This conclusion is helpful in guiding our future studies on the neuroprotective mechanism of CBs and might represent a promising target for the treatment of cerebrovascular diseases.
[1]Demuth DG,Molleman A.Cannabinoid signaling[J].Life Sci,2006,78(6):549 -563.
[2]YU Qi,JIN GL.The effects of three complex prescriptions on BDNF and CREB expression of liver depression model by chronic unpredicatable mild stress in rats[J].Chinese Journal of Pathophysiology,2009,25(3):591-594.
[3]Melck D,Rueda D,Galve-Roperh I,et al.Involvement of the cAMP/protein kinase A pathway and of mitogenactivated protein kinase in the anti-proliferative effects of anandamide in human breast cancer cells[J].FEBS Lett,1999,463(3):235-240.
[4]Davis MI,Ronesi J,Lovinger DM.A predominant role for inhibition of the adenylate cyclase/protein kinase A pathway in ERK activation by cannabinoid receptor 1 in N1E-115 neuroblastoma cells[J].J Biol Chem,2003,278(49):48973-48980.
[5]Derkinderen P,Valjent E,Toutant M,et al.Regulation of extracellular signal-regulated kinase by cannabinoids in hippocampus[J].J Neurosci,2003,23(6):2371 -2382.
[6]Rosenberg GA,Mun-Bryce S,Wesley M.Collagenaseinduced intracerebral hemorrhage in rats[J].Stroke,1990,21(5):801-807.
[7]Khaspekov LG,Brenz Verca MS,Frumkina LE,et al.Involvement of brain-derived neurotrophic factor in cannabinoid receptor-dependent protection against excitotoxicity[J].Eur J Neurosci,2004,19(7):1691 -1698.
[8]Marsicano G,Goodenough S,Monory K,et al.CB1 cannabinoid receptors and on-demand defense against excitotoxicity[J].Science,2003,302(5642):84 -88.
[9]De March Z,Zuccato C,Giampà C,et al.Cortical expression of brain derived neurotrophic factor and type-1 cannabinoid receptor after striatal excitotoxic lesions[J].Neuroscience,2008,152(3):734-740.
[10]Bayatti N,Hermann H,Lutz B,et al.Corticotropin-releasing hormone-mediated induction of intracellular signaling pathways and brain-derived neurotrophic factor expression is inhibited by the activation of the endocannabinoid system[J].Endocrinology,2005,146(3):1205-1213.
[11]Maj PF,Collu M,Fadda P,et al.Long-term reduction of brain-derived neurotrophic factor levels and signaling impairment following prenatal treatment with the cannabinoid receptor 1 receptor agonist WIN 55 -212 -2[J].Eur J Neurosci,2007,25(11):3305 -3311.
[12]Childers SR,Deadwyler SA.Role of cyclic AMP in the actions of cannabinoid receptors[J].Biochem Pharmacol,1996,52(6):819-827.
[13]Kim SH,Won SJ,Mao XO,et al.Molecular mechanisms of cannabinoid protection from neuronal excitotoxicity[J].Mol Pharmacol,2006,69(3):691 -696.
[14]Kim SH,Won SJ,Mao XO,et al.Involvement of protein kinase a in cannabinoid receptor mediated protection from oxidative neuronal injury[J].J Pharmacol Exp Ther,2005,313(1):88-94.
[15]Mailleux P,Verslype M,Preud'homme X,et al.Activation of multiple transcription factor genes by tetrahydrocannabinol in rat forebrain[J].Neuroreport,1994,5(10):1265-1268.

