三维混合价态铜配位聚合物[Cu(Me2dtc)2(CuI)3]n的合成与晶体结构
范乐庆 吴季怀 林建明 黄昀昉
(福建省高校功能材料重点实验室,华侨大学材料物理化学研究所,泉州 362021)
研究简报
三维混合价态铜配位聚合物[Cu(Me2dtc)2(CuI)3]n的合成与晶体结构
范乐庆*吴季怀 林建明 黄昀昉
(福建省高校功能材料重点实验室,华侨大学材料物理化学研究所,泉州 362021)
铜;N,N-二甲基硫代氨基甲酸;混合价态;晶体结构
During the past few decades,the design and synthesis of inorganic-organic hybrid complexes have attracted much attention due to theirstructural diversity,as well as their potential applications as functional materials[1-4].Therefore,all kinds of ligands containing nitrogen,oxygen,sulfur or phosphorus are used to synthesize the hybrid complexes[5-10].Dialkyldithiocarbamate(R2dtc)anions,which are typical sulfur ligands,acting as monodentate,bidentate or bridging ligands with many different central metal ions can form considerable structural variety of complexes[11-13].Among them,the chemistry and structures of the homo-or heterometallic halide dithiocarbamate complexes have been extensively explored,and a series of zero-dimen-tional(0D),one-dimensional(1D),two-dimensional(2D)and three-dimensional(3D)homo-or hetrometallic complexes have been obtained[13-17].However,up to the present,3D homometallic halide dithiocarbamate complex has not been reported.We report here the synthesis and crystal structure of a new 3D mixedvalence copper dithiocarbamate-copper iodide coordination polymer[Cu(Me2dtc)2(CuI)3]n,(1),containing a dimethyldithiocarbamate (Me2dtc)ligand.As far as we know,this is the first example of 3D homometallic halide dithiocarbamate complex.
1 Experimental
1.1 General procedures
Sodium salt of N,N-dimethyldithiocarbamate(Na(Me2dtc)·2H2O)was prepared by literature methods[18].All solvents and chemicals required for syntheses were commercially available and employed without furtherpurification.Elementanalysesofcarbon,hydrogen,nitrogen,and sulfur were performed with a Vario EL Ⅲ element analyzer.The solid infrared spectra (IR)were obtained from a Nicolet Nexus 470 FTIR spectrometer between 400 and 4 000 cm-1by using KBr pellets.The crystal structure was determined by a Rigaku Mercury CCD area-detector diffractometer and SHELXL crystallographic software of molecular structure.
1.2 Synthesis of the title complex
A mixture of Cu(OAc)2·H2O(0.10 g,0.5 mmol),Na(Me2dtc)·2H2O(0.04 g,0.2 mmol),and NaI·2H2O(0.26 g,1.5 mmol)was stirred in dimethylformamide(20 mL)at 60 ℃ in air for about one day.The vapor of 2-PrOH was diffused into the resulting solution,and after about one month black crystals(36 mg)were obtained(yield 32.9%based on Cu(OAc)2·H2O).Anal.Calcd.(%)for 1(dried):C,8.23;H,1.38;N,3.20;S,14.65.Found(%):C,8.89;H,1.65;N,3.63;S,14.21.Selected IR data(KBr,cm-1),ν:3 434,2 914,1 637,1 509,1 374,1 239,1139,945,569,435.
1.3 Crystal structure determination
Suitable single crystal of complex 1 was selected and mounted on the end of thin glass fiber using epoxy oil.X-ray diffraction intensity data were collected on a Rigaku Mercury CCD area-detector equipped with a graphite-monochromatic Mo Kα radiation(λ=0.071073 nm)by using an ω scan mode in the range of 3.10°≤θ≤27.47°at 293(2)K.A total of 6864 reflections were measured,of which 2 083 were unique (Rint=0.020 7)and 1 918 were observed(I>2σ(I)).Absorption correction was performed by the CrystalClear program[19].The structure was solved by direct methods and refined with the aid of SHELX-97 software package of crystallographic software[20].All non-hydrogen atoms were refined anisotropically.All H atoms were located theoretically and refined as riding atoms,with C-H=0.096 nm and Uiso(H)=1.5Ueq(C).The final anisotropic refinement converged to R=0.0295 and wR=0.0817(w=1/[σ2(Fo2)+(0.052 6P)2+1.990 2P],where P=(Fo2+2Fc2)/3),S=1.097,(Δ/σ)max=0.001,(Δρ)max=1 322 and (Δρ)min=-1 630 e·nm-3.Some refinement details and crystal data are gathered in Table 1.Selected bond lengths and angles are listed in Table 2.
CCDC:739046.

Table 1 Crystallographic data and structure refinement parameters for complex 1
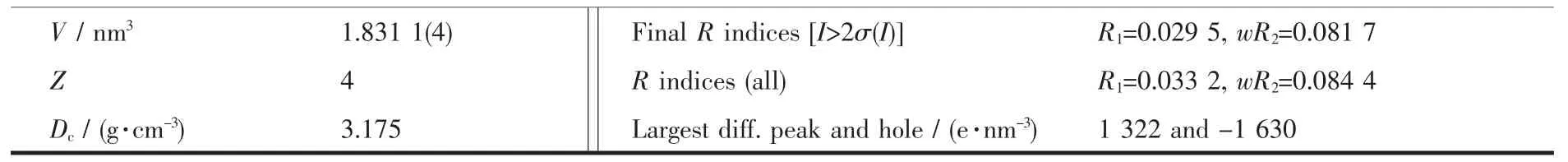
Continued Table 1
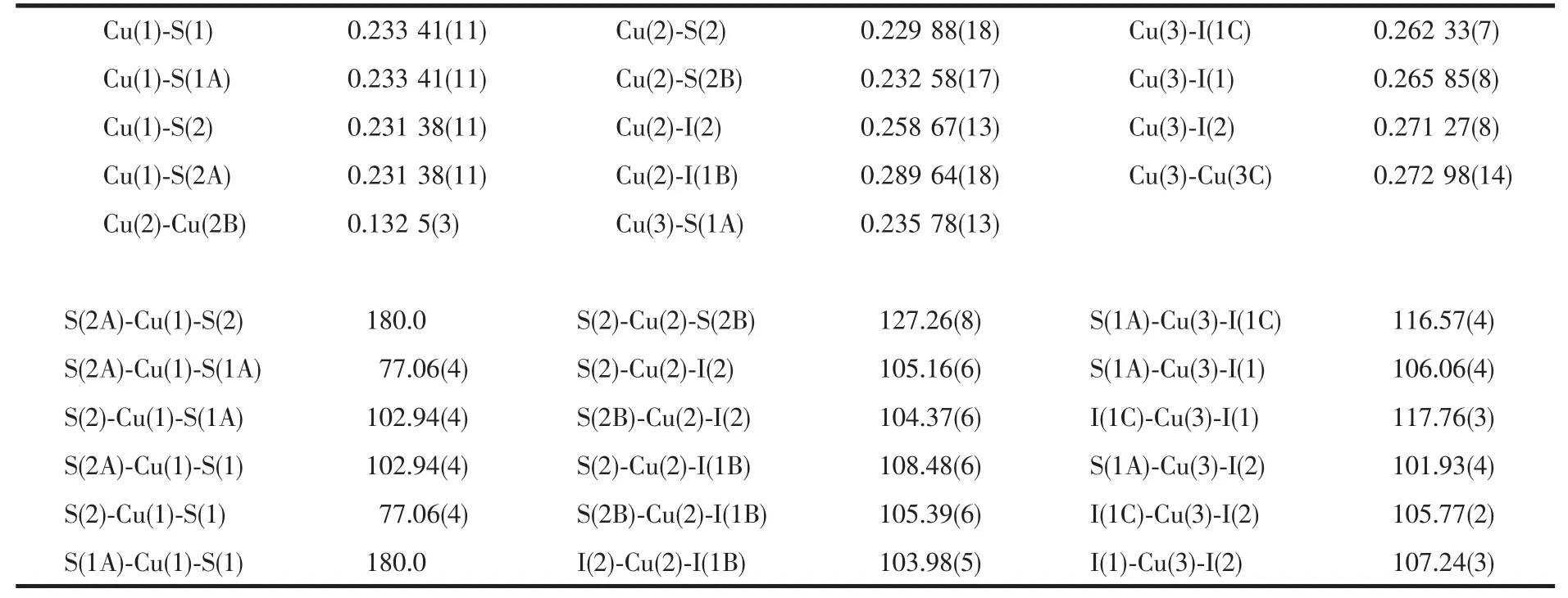
Table 2 Selected bond lengths(nm)and angles(°)for complex 1
2 Results and discussion
2.1 Synthesis
The formation of the complex 1 was under solution condition at 60 ℃ in air for about 24 h.If the reactive temperature and time were reduced,the final product was complex Cu(Me2dtc)2,not the complex 1.Series of experiments using other sodium salt of R2dtc with larger alkyl(R=ethly or propyl)in place of Na(Me2dtc)under the same condition have also been carried out to prepare 3D homometallic halide dithiocarbamate complex,but unfortunately,no crystal was suitable for X-ray single-crystal analysis.This maybe results from that the steric hindrance effects of the ethyl and propyl are larger than that of methyl.In general,syntheses of the complexes are not only closely related to the geometry and the number of coordination sites provided by metal ions or ligands,but also controlled by the environment conditions (such as: temperature,reactants, metal-to-ligand ratio, countercations,solvents,pH value etc.).Thus,this preparation may mainly depend on the choices of both reactants and the Cu(OAc)2/NaI/Na(Me2dtc)stoichiometry.
2.2 IR spectrum of complex 1
IR spectrum of complex 1 shows characteristic bands arising from ν(CS2)asymand ν(CS2)sym(partial double bond)at 1 139 and 945 cm-1,respectively,with the Δν value(ν(CS2)asym-ν(CS2)sym)of 194 cm-1,indicating that the Me2dtc ligand coordinates to the Cu atom in a bidentate fashion[21-22].The band at 1509 cm-1is attributable to t he ν(C-N)(partial double bond)stretching vibration.This position corresponds to a partial double bond character,and is recorded in the range previously reported for similar complexes[21].
2.3 Description of the structure
The structure of 1 is a 3D copper neutral structure which consists of individual Cu (Me2dtc)2molecules linking together CuI polymeric chains which run parallel to the [001]direction vis Cu-S bonds(Fig.1,Fig.2 and Fig.3).All the S atoms and I atoms are acting as μ2and μ3bridges,respectively.
According to the bond valence sum(BVS)calculations[23],valence states are assigned on the basis of∑Vivalues,in which the∑Viis the bond strength derived from the Brown equation,Vi=exp[(R0-Ri)/b](R0(CuS)=0.2024 nm,R0(CuI)=0.210 8 nm andb=0.37).The values of∑Cu(1),∑Cu(2)and ∑Cu(3)are 1.92,1.33 and 1.09,respectively.So Cu(1)and Cu(3)atoms are assigned as bivalent and univalent,respectively.On the other hand,based on the valence equivalence in this complex,Cu(2)atoms is assigned as univalent.

Fig.1 A section of the crystal structure of 1
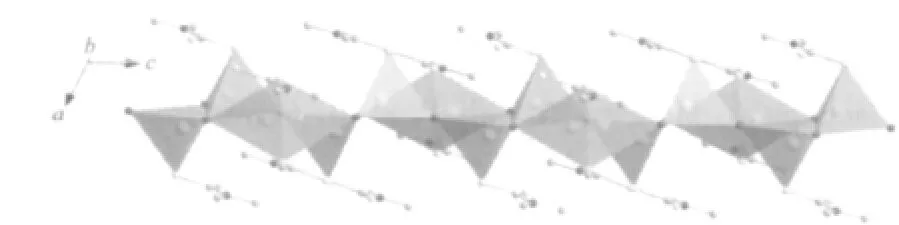
Fig.2 A view showing the 1D chain constructed by Cu(2)S2I2and Cu(3)I3S units which are shown by polyhedra
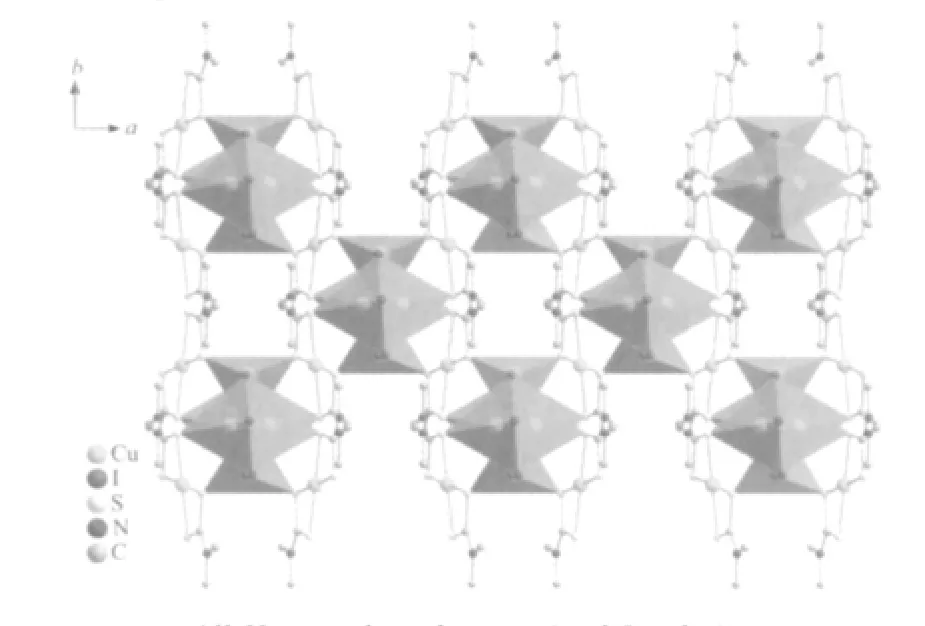
Fig.3 A polyhedral representation of the 3D structure of 1,viewed down c axis
It is interesting that these three mixed-valence Cu/Cuatoms have two different coordinate configurations.The atom CuⅡ(1)has four-coordinate Cu(1)S4square-planar environment and is bonded to four S atoms from two different Me2dtc ligands with the CuⅡ-S distances of 0.233 41(11)and 0.231 38(11)nm and the S-CuⅡ-S bite angle of 77.06(4)°,all consistent with those reported previously in Cudithiocarbamate complex CuI(prdtc)(phen)(prdtc=N-pyrrolidinyldithiocarbamate,phen=1,10-phenanthroline)with the corresponding values of 0.231 81(15)~0.234 42(15)nm and 76.24(5)°(Table 1)[24].The atom Cu Ⅰ(2)is of interest,being splitted into two symmetry sites,Cu(2)and Cu(2B)[symmetry code:B:-x+1,y,-z+1/2]with occupancies of 0.5 and 0.5 and the distance between Cu(2)and Cu(2B)being 0.132 5(3)nm.Cu(2)atom is four-coordinated in a distorted Cu(2)S2I2tetrahedral geometry by two I atoms and two S from two different Me2dtc ligands with the CuⅠ-I distances of 0.258 67(13)and 0.28964(18)nm,and CuⅠ-S distances of 0.22988(18)and 0.23258(17)nm,respectively.They compare well to those in Cucomplex[(Ph3P)Cu(μ-I)2(μ-S-Haptsc)Cu(PPh3)](Haptsc=acetophenone thiosemicarbazone)with the corresponding values of 0.263 79(7)to 0.27472(7)nm and 0.239 31(12)to 0.241 86(13)nm[25].The atom CuⅠ(3)is surrounded by three I atoms and one S atom from Me2dtc ligand in a distorted tetrahedral coordinate sphere[Cu(3)I3S]with the CuⅠ-I distances ranging from 0.26233(7)to 0.271 27(8)nm and the CuⅠ-S distance being 0.235 78(13)nm,which are also similar to that in Cucomplex[(Ph3P)Cu(μ-I)2(μ-SHaptsc)Cu(PPh3)][25].Two [Cu(3)I3S]tetrahedra are connected by common edge(I(1)-I(1))to form a dimer,in which there is Cu-Cu interaction with the Cu-Cu distance of 0.272 98(14)nm[26],adjacent dimers are joined by Cu(2)S2I2tetrahedra with common edges(I(1)-I(2))to form 1D chain extending along[001]direction(Fig.2),and then the chains are linked by surrounding Cu(1)S4planar square via shared vertices(S(1)and S(2))to give 3D structure(Fig.3).
2D homometallic halide dialkyldithiocarbamate complex[Cu(pipdtc)2(CuBr)6]n(pipdtc=piperidyldithiocarbamate)also built up of individual Cu(pipdtc)2molecules linked to CuBr polymeric chains vis Cu-S bonds was reported previously[15].Compared structurally with 2D complex Cu(pipdtc)2(CuBr)n,it is concluded that,the 3D complex 1 benefits mainly from the steric hinerance effect of Me2dtc ligand is smaller than that of pipdtc ligand.
[1]Mitzi D B.Prog.Inorg.Chem.,1999,48:1-121
[2]Li F,Ma Y L,Wang Y L,et al.CrystEngComm,2005,7:569-574
[3]LI Hao-Hong(李浩宏),CHEN Zhi-Rong(陈之荣),HUANG Chang-Cang(黄长沧),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2005,21(1):83-86
[4]Fan L Q,Wu J H,Huang Y F.J.Solid State Chem.,2007,180:3479-3484
[5]Priya S M N,Varghese B,Linet J M,et al.Cryst.Growth Des.,2008,8:1663-1667
[6]Lee J Y,Lee S Y,Sim W,et al.J.Am.Chem.Soc.,2008,130:6902-6903
[7]Leung W H,Wu M C,Chim J L C,et al.Inorg.Chem.,1996,35:4801-4803
[8]JING Su(景 苏),YIN Tao(殷 陶),WANG Yu-Xiao(王玉晓),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2002,18(11):1169-1172
[9]YAN Bing(严 冰),WU Tao(吴 涛),LI Zhen(李 贞),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2006,22(8):1499-1502
[10]Shi X,Zhu G S,Wang X H,et al.Cryst.Growth Des.,2005,5:207-213
[11]Fernández E J,López-de-Luzuriaga J M,Monge M,et al.J.Cluster Sci.,2000,11:151-167
[12]DENG Yu-Heng(邓玉恒),LIU Juan(刘 娟),LI Ning(李宁),et al.Acta Chim.Sinica(Huaxue Xuebao),2007,65(24):2868-2874
[13]Koh Y W,Lai C S,Du A Y,et al.Chem.Mater.,2003,15:4544-4554
[14]Pastorek R,Kameníek J K,Trávníek Z,et al.Polyhedron,1999,18:2879-2883
[15]Golding R M,Rae A D,Ralph B J,et al.Inorg.Chem.,1974,13:2499-2504
[16]Engelhardt L M,Healy P C,Shephard R M,et al.Inorg.Chem.,1988,27:2371-2373
[17]Okubo T,Kawajiri R,Mitani T,et al.J.Am.Chem.Soc.,2005,127:1758-1759
[18]Oskarsson A,Ymén I.Acta Crystallogr.,Sect.C,1983,39:66-68
[19]Rigaku.CrystalClear,Version 1.3.6,Rigaku/MSC,Tokyo,Japan,2005.
[20]Sheldrick G M.SHELXS-97 and SHELXL-97,Program for X-ray Crystal Structure Solution and Refinement,University of Göttingen,Germany,1997.
[21]Cachapa A,Mederos A,Gili P,et al.Polyhedron,2006,25:3366-3378
[22]Drake J E,Yang J C.Inorg.Chem.,1997,36:1890-1903
[23]Brown I D,Altermatt D.Acta Crystallogr.,Sect.B,1985,41:244-246
[24]Fan L Q,Wu J H,Lin J M,et al.Chinese J.Struct.Chem.2009,28(5):580-584
[25]Lobana T S,Khanna S,Butcher R J.Inorg.Chem.,2007,46:5826-5828
[26]Mehrotra P K,Hoffmann R.Inorg.Chem.,1978,17:2187-2189
Synthesis and Crystal Structure of a Three-Dimensional Mixed-Valence Copper Dithiocarbamate-Copper Iodide Coordination Polymer[Cu(Me2dtc)2(CuI)3]n
FAN Le-Qing*WU Ji-HuaiLIN Jian-Ming HUANG Yun-Fang
(The Key Laboratory for Functional Materials of Fujian Higher Education,Institute of Materials Physical Chemistry,Huaqiao University,Quanzhou,Fujian 362021)
A new three-dimensional copper dithiocarbamate-copper iodide coordination polymer[Cu(Me2dtc)2(CuI)3]n(Me2dtc=N,N-dimethyldithiocarbamate)was synthesized by reactions of Cu(OAc)2,NaI and Na(Me2dtc)in DMF solution,characterized by elemental analysis,IR spectrum,and single-crystal X-ray diffraction.The crystal belongs to the monoclinic system,space group C2/c with a=1.29389(17)nm,b=1.07761(11)nm,c=1.45605(17)nm,β=115.585(4)°,V=1.8311(4)nm3,Z=4,Dc=3.175 g·cm-3,Mr=875.28,λ (Mo Kα)=0.071073 nm,μ=10.082 mm-1,F(000)=1604,the final R=0.0295 and wR=0.0817.A total of 2083 unique reflections were collected,of which 1918 with I>2σ(I)were observed.The Cu atoms are Cu/Cumixed-valence and they have two different coordinate geometries,namely planar square and tetrahedron.This three-dimensional structure consists of individual Cu(Me2dtc)2molecules linking together CuI polymeric chains which run parallel to the[001]direction vis Cu-S bonds.CCDC:739046.
copper;N,N-dimethyldithiocarbamate;mixed-valence;crystal structure
O614.121
A
1001-4861(2010)08-1516-05
2009-11-06。收修改稿日期:2010-04-29。
国家自然科学基金(No.50572022,50372030);教育部科学技术研究重点项目(No.206074);福建省自然科学基金(No.E0640003),福建省青年人才创新项目(No.2007F3060)资助。
*通讯联系人。 E-mail:lqfan@hqu.edu.cn
范乐庆,男,31岁,副教授;研究方向:功能配合物。

