Randomized controlled trial comParing quetiaPine with lithium and clozaPine with lithium in the treatment of female Patients with mania
Xiaowei TANG,Hui WU,Qian CHEN
Randomized controlled trial comParing quetiaPine with lithium and clozaPine with lithium in the treatment of female Patients with mania
Xiaowei TANG*,Hui WU,Qian CHEN
Background:Second-generation antipsychotics are commonly used in the treatment of mania but there remains debate about the relative benefits of the different medications in these patients,particularly in female patients with mania.
Obiective:Compare the clinical effectiveness and safety of quetiapine and clozapine as adjunctive treatments of lithium in the treatment of female patients with mania.
Methods:Sixty-four female patients who met ICD-10 criteria for a current manic episode were randomly assigned to take lithium with quetiapine(n=32)or lithium with clozapine(n=32)in a six-week,open-label trial.Clinical efficacy was evaluated at baseline and weekly thereafter by administering the Beck Rafaelson’s Mania Rating Scale (BRMS).Adverse reactions were assessed by administering the Treatment Emergent Symptom Scale(TESS)at baseline and at 1,2,4,and 6 weeks after starting treatment.
Results:Two patients in the clozapine group did not complete the trial due to low white blood counts.Among subjects who completed the 6 weeks of treatment there were no significant differences in the mean BRMS values between the two groups at any time during the trial.At the end of the trial 87.5%(28/32)of the patients taking quetiapine and 90.0%(27/30)of those taking clozapine meet criteria for remission(i.e.,a 50%drop in BMRS score from baseline)(χ2=0.76,P=0.385).During the course of treatment a significantly higher proportion of subjects in the clozapine group experienced dizziness,weight gain,somnolence,constipation,excessive salivation,and abnormal blood glucose and electrocardiogram results.The overall severity of adverse effects was significantly higher in the clozapine group after two weeks of treatment but there were no differences in the overall severity of adverse effects at other time periods.Despite the 23-fold higher cost for quetiapine versus clozapine,the overall cost effectiveness of the two medication regimens was similar because of the much high use of expensive auxillary drugs in the clozapine group.
Conclusion:The clinical efficacy of quetiapine and clozapine as adjunctive treatment in women with mania taking lithium is similar but the two treatment regimens have different side-effect profiles.The cost benefit o using generic clozapine may be over shadowed by the costs associated with treating the adverse effects of clozapine.
Quetiapine;Clozapine;Lithium carbonate;Manic episode
1 Introduction
In recent years the treatment of mania often involves the combination of conventional mood stabilizers with various second generation antipsychotic medications.Clozapine is a popular adjunctive treatment in China because of its relatively low cost and its reported rapid effect on acute manic symptoms[1].Quetiapine,which has a similar chemical structure and pharmacology to clozapine(but is at last ten times more expensive than clozapine in China),has also been found to be effective for controlling manic episodes[2]and has been reported to be more suitable for female patients with schizophrenia than other second-generation anti-psychotics[3].There have,however,been no direct comparisons of the efficacy,adverse effects or costs of these two common adjunctive treatments for the manic episodes of bipolar disorder.
2 Subiects and methods
2.1 Subiects
Female patients at the Wutaishan Hospital in Yangzhou City,Jiangsu Province who met the following criteria at the time of admission wereenrolled in the study:1)admitted from January 2009 to December 2010;2)met diagnostic criteria of‘Manic episode’or‘Bipolar affective disorder,current episode manic’as described in the 10th Revision of the International Statistical Classification of Diseases(ICD-10)[4];3)18-60 years of age;4) no history of substance abuse or substance dependence;5)had not taken antipsychotic medication or mood stabalizers in the two weeks prior to admission;6)no known allergies to lithium carbonate,quetiapine or clozapine;7)not pregnant or breastfeeding;8)no significant abnormalities identified by physical or laboratory examination(including routine blood and urine tests,electrocardiogram and an electroencephalogram with evoked potentials);9)score of>16 on the Beck Rafaelson’s Mania Rating Scale(BRMS)[5];and 10)their statutory guardian provided written informed consent to participate in the study.The study protocol was approved by the ethics review board of the Wutaishan Hospital.
2.2 Methods

Figure 1.Flowchart of study enrollment
Participation in the trial was terminated if any of the following occurred:1)serious adverse effects that the patient found intolerable;2)failure to show symptomatic benefit after two weeks of treatment; 3)emergence of depressive symptoms,suicide attempt or other concurrent diseases;or 4)withdrawal of consent by guardian.During the six weeks of treatment two patients from the clozapine group were terminated from the trial(at week 1 and week 2 after starting treatment)because of low neutrophil counts in peripheral blood(0.49×109/L,and 0.48×109/L,respectively).
The therapeutic effect was evaluated using Chinese versions of the BRMS[5]and adverse reactions were assessed using the Treatment Emer-gent Symptom Scale(TESS)[6].The BRMS was administered at baseline and at the end of each week of treatment for six weeks while the TESS was administered at baseline and at the end of the first,second,fourth and sixth week of treatment.All subjects received routine blood tests and an electrocardiogram every week and biochemical tests assessing hepatic and renal function every two weeks. The therapeutic effect and adverse reactions were evaluated by two senior physicians who were the primary clinicians for the patients(and,thus,were not blind to the treatment status of the patients). The concordance of these clinicians’total BRMS scores and total TESS scores when simultaneously evaluating 20 patients were excellent(ICC for BRMS=0.93 and ICC for TESS=0.91).
The direct medical costs of the six-week course of treatment for each patient was assessed:it included the cost of all medications,laboratory tests,hospital charges,and other treatment fees but excluded any costs that were only incurred because this was a research study[7].
2.3 Statistical methods
SPSS 16.0 software was used for statistical analysis.Chi-square tests,Fisher exact tests,t-tests,and repeated measures analyses of variance were used to compare different types of variables between the two groups.The overall severity of adverse reactions is estimated using the mean item score for the 14 types of adverse reactions assessed by the TESS;TESS items are coded on a 5-point Likert scale(0=none,1=possible,2=mild,3=moderate,4=severe)so the range in the mean item score is from 0 to 4.The percent improvement in BRMS score from baseline[100×(baseline BRMS score minus post-treatment BRMS score)/baseline BRMS score]was computed for both groups at the end of the first,second,fourth and sixth weeks of treatment.‘Remission’was defined as a decrease of 50%or greater in the BRMS score from the baseline score.Survival analysis was used to compare the remission status between the two groups over the six weeks of treatment.
3 Results
Two cases in the clozapine group were dropped from the study due to neutropenia(i.e.,absolute neutrophil count in peripheral blood of less than 0.5 x 109/L)but none of the patients in the quetiapine group were dropped from the study. The 32 subjects in the quetiapine group who completed the 6-week course of treatment had a mean (SD)age of 28.4(9.0)years,a mean onset age of 23.4(7.8)years,a mean duration of illness of 75.8(45.1)months,and a mean of 4.0(2.7)episodes.The 30 subjects in the clozapine group who completed the trial had a mean age of 28.6(8.5) years,a mean onset age of 22.1(6.8)years,a mean duration of illness of 78.2(46.2)months,and a mean of 4.2(2.5)episodes.There were not statistically significant differences in these characteristics between the two groups.
3.1 BRMS scores
The mean BRMS scores in the two groups at each time period are presented in Table 1.There were no significant differences in the mean scores between the two groups at any of the five time points in the study.Repeated measures analysis of variance confirmed that there were no significant differences between the groups over time(F= 0.518,P=0.474)and showed that there were significant drops in the mean BRMS scores in both groups from baseline to week 1(F=252.1,P<0.001),from week 1 to week 2(F=241.5,P<0.001)and from week 4 to week 6(F=251.5,P<0.001)but NOT from week 2 to week 4(F= 0.62,P=0.325).

Table 1.ComParison of mean(SD)total scores of the Beck Rafaelson's Mania Rating Scale(BRMS)between the quetiaPine and clozaPine grouPs
Figure 2 shows the survival analysis for the two groups using remission(i.e.,50%drop in BRMS score from baseline)as the outcome.In both groups the biggest increase in remission was from baseline to week 2 and from week 4 to week 6;a much smaller increase in remission occurred between week 2 and week 4.There were no differences in the time to remission between the two groups(P=0.780).At the end of the trial 87.5% (28/32)of the patients taking quetiapine and 90.0%(27/30)of those taking clozapine meet criteria for remission(χ2=0.76,P=0.385).
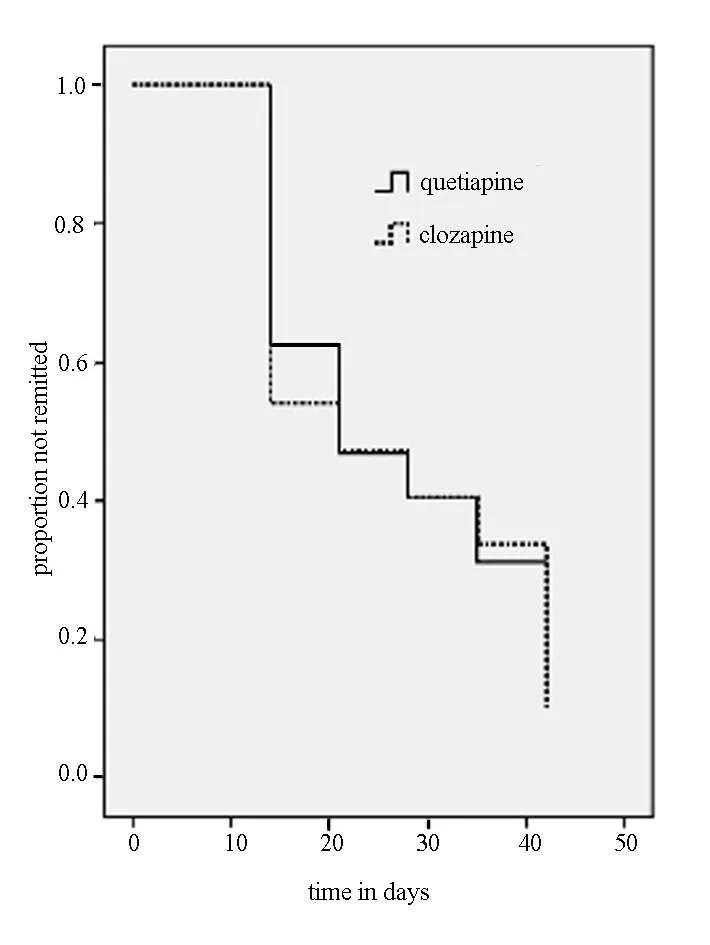
Figure 2.Survival function of remission status over six weeks of treatment
3.2 ComParison of adverse reactions
The total TESS scores at each evaluation time are presented in Table 2.The overall severity of adverse effects was greater in the clozapine group after two weeks of treatment but the differences at other time periods in the course of treatment were not statistically significant.As shown in Table 3,over the six weeks of treatment a significantly higher proportion of patients in the clozapine group experienced dizziness,somnolence,electrogardiographic abnormalities,constipation,excessive salivation,hypeglycemia and weight gain of>7%of body mass.Of the 14 types of adverse effects assessed,only xerostomia and abnormal liver enzymes occurred in a higher proportion of patients in the quetiapine group than in the clozapine group and in neither of these cases was the difference statistically significant.

Table 2.ComParison of mean(SD)item score*of the Treatment Emergent SymPtom Scale(TESS)between the quetiaPine and clozaPine grouPs
3.3 Cost-effectiveness analysis
The mean total costs for the 6-week treatment course in patients in the quietiapine group was 5 657 renminbi(RMB)(870$US)which included 672 RMB for the quetiapine,125 RMB for auxiliary drugs(primarily for managing liver function and electrocardiographic abnormalities),846 for clinical and laboratory examinations,and 4 010 for other fees(including bed charges,nursing charges,charges for clinical examinations,etc.).The mean total costs in patients in the clozapine group(not including the costs of the two subjects who dropped out due to neutropenia)was 5 840 renminbi(RMB)(898$US)which included 29 RMB for the clozapine,739 RMB for auxiliary drugs (primarily for reducing hyperglycemia and for managing electocardiographic abnormalities and other adverse effects),912 for clinical and laboratory examinations,and 4 160 for other fees.
Thus,despite a 23-fold difference in the cost of quetiapine and clozapine,the overall cost of treatment is similar in the two groups because of themuch higher auxiliary drug use in patients taking clozapine.The mean direct medical cost for each remission was 6 465 RMB in the quetiapine group and 6 489 RMB in the clozapine group.

Table 3.ComParison of the occurrence of sPecific adverse reactions(at any time in 6-week course of treatment) between Patients treated with quetiaPine and clozaPine(n,%)
4 Discussion
4.1 Main findings
Our study confirms previous research about the efficacy of adjunctive antipsychotic medication when treating manic patients who are taking lithium[8].We also confirm earlier studies that show the usefulness of quetiapine in the management of acute mania[9-11].However,we did not find any differences in the 6-week efficacy or speed of remission of symptoms between female manic patients on lithium who were treated with adjunctive clozapine and those treated with adjunctive quetiapine.This finding contradicts previous reports[12]about the more rapid effectiveness of clozapine in such patients.
One interesting finding that we have not seen reported before is the‘lull’in the trajectory of improvement between the second and fourth week of treatment.In both the mean BMRS scores and the survival plot of the remission rates there is a rapid improvement in the first two weeks,a slow improvement from week 2 to week 4 of treatment and then another rapid improvement between week 4 and week 6 of treatment.The reasons for this pattern of improvement are unclear but it indicates that clinicians should wait for at least six weeks before classifying a particular antipsychotic medication ineffective as an adjunctive treatment in mania.
When the efficacy of different treatments for a condition is similar,two other factors need to be considered:adverse effects and cost.Over the 6-week course of treatment most of the adverse effects assessed by the TESS occurred more frequently in the clozapine group than in the quetiapine group and two patients in the clozapine group were withdrawn from the study because of low white blood counts.But the overall severity of adverse effects was only different at the second week of treatment;by the end of the 6-week course of treatment the adverse effects were relatively infrequent and their overall severity was virtually identical between the two groups.Transient side effects can be a factor that affects patients’compliance so they need to be identified and managed appropriately.But the adverse effects that persist are probably more important because they often result in substantial distress and disability and/or substantially increase the risk of other negative health outcomes such as the metabolic syndrome.Our study indicated that the transient occurrence of common side effects was greater in the clozapine group than in the quetiapine group but we were unable to arrive at a judgment about the long-term biochemicaland other effects of these different medication regimens.
表2所示,试验3组的总胆固醇含量显著低于试验1组(P<0.05),试验3组和试验4组的血清甘油三酯和低密度脂蛋白的含量显著低于试验1组(P<0.05),试验3组和试验4组高密度脂蛋白含量,显著高于试验1组和试验2组(P<0.05);试验1组和试验2组血清中胆固醇、甘油三酯、高密度脂蛋白差异不显著(P>0.05);试验1组血清中低密度脂蛋白差异显著高于试验2、3、4组(P<0.05);试验2、3、4组之间差异不显著;试验3组与试验4组之间这四个指标的含量的差异不显著(P>0.05)。
Unlike quetiapine,clozapine is produced generically in China so one of the presumed benefits of using clozapine is the substantially lower cost of the medication.Somewhat surprisingly we found that for inpatients with mania the additional costs of the auxiliary medications used in inpatients treated with clozapine exceeded the cost benefit of using clozapine versus quetiapine,resulting in an essentially equal cost-benefit ratio for the two methods of treating acute mania.Further analysis is needed to determine how essential these auxiliary treatments are,but the result underlines the importance of conducting a comprehensive,all inclusive,approach when comparing the relative cost-benefit of different therapeutic strategies.
4.2 Limitations
Some limitations need to be considered in the evaluation of these results.1)In the absence of a lithium-only treatment group we are unable to indicate the relative proportion of the treatment effectiveness that can be attributed to the adjunctive antipsychotic medications.2)Both the treating clinicians and the clinicians who evaluate BMRS and TESS knew which patients were on which drug so this lack of blinding to treatment condition could have biased both the assessment of the clinical outcomes and the use of auxiliary medications(potentially influencing the cost-effectiveness assessment).3)Female inpatients with acute mania—patients in the current study—may not be representative of all manic patients.4)The data from the two patients in the clozapine group who dropped out due to low white blood counts were not used in the analysis so this biases the results somewhat in favor of clozapine.5)Treatment of mania with adjunctive antipsychotic medication usually lasts for longer than six weeks;we are unable to indicate what the long-term effectiveness and side-effect profile of these adjunctive medication regimens would be. 6)The analysis of adverse effects relied on frequency counts and a rather crude overall severity measure;more detailed assessment of the occurrence,severity,and persistence of adverse effects is needed to appropriately assess the relative advantages and disadvantages of using different antipsychotics as adjunctive treatment in mania.7)The costs assessed were for inpatient services in China where clozapine is produced as a relatively inexpensive generic medication,so these cost-effectiveness assessments are probably less relevant for outpatient settings or in settings where the cost of clozapine treatment is similar to that of other second-generation antipsychotic medications.
4.3 ImPlications
Clinicians selecting adjunctive antipsychotic treatment in acute manic patients taking lithium need to consider efficacy,adverse effects and cost. We found a substantial increase in remission rates between four weeks and six weeks after starting treatment,indicating that clinicians(and researchers)need to wait for a full six weeks before deciding whether or not a particular antipsychotic used as an adjunctive treatment is effective in manic patients.
Despite the more frequent occurrence of adverse effects in the clozapine group most of these adverse effects had resolved by the end of the sixweek course of treatment and the overall severity of adverse effects by the end of the six weeks was similar.Since patients are commonly on these adjunctive antipsychotic medications for prolonged periods,when making therapeutic decisions clinicians should focus on those adverse effects that are persistent and/or disabling rather than focusing on the transient side-effects that resolve in the first few weeks of treatment.And researchers should develop more sensitive measures of these adverse effects that reflect their severity,persistence and associated dysfunction rather than depending on simple frequency counts.
Despite the substantially lower cost for generic clozapine in China than for quetiapine(which is 23-fold more expensive)we found the overall direct treatment cost of the inpatient management of acute mania using these two medications to be similar.This highlights the fact that cost-effectiveness measures of different therapeutic strategies are always dependent on national or local conditions:the relative cost of medications,the proportion of patients who get hospitalized,the use of auxiliary treatments,the reimbursement regulations of different health insurance schemes and so forth.
Funding
The authors received no external funding for this study.
Conflict of Interest
The authors report no conflict of interest withrespect to this paper.
1. Jiang KD.Textbook of Psychopharmacology.Beijing:People’s Military Medical Press,2007:339.(in Chinese)
2. Liu TB,Gao H,Shen QJ.Utilization of clozapine combined with lithium carbonate for the treatment of acute mania.Journal of Clinical Psychiatry,2000,10(6):350-351.(in Chinese)
3. Xie HB.Comparison of curative effect and safety of quetiapine and risperidone in the treatment of female schizophrenia.Clinical Medicine,2009,29(6):4-5.(in Chinese)
4. Dong JW.International Classification of Diseases,Tenth Revision.2nd Edition.Beijing:People’s Medical Publishing House,1997:96-97.(in Chinese)
5. Wang XD,Wang XL,Ma H.Rating scales for mental health,supplement edition.Beijing:Chinese Mental Health Journal Press,999:246-247.(in Chinese)
6. Zhang MY.Treatment Emergent Symptom Scale.Shanghai Arch Psychiatry,1984,(2):77-80.(in Chinese)
7. Sun GB.Cost-effectiveness analysis in treatment of schizophrenia with three drugs.China Pharmaceuticals,2008,17(7):43. (in Chinese)
8. Sajatovic M,Brescan DW,Perez DE,Digiovanni SK,Hattab H,Rav JB,et al.Quetiapine alone and added to a mood stabilizer for serious mood disorders.J Clin Psychiat,2001,62(9):728-732.
9. Bourin M,Lambert 0,Guitton B.Treatment of acute maniaform clinical trials to recommendations for clinical practice. Hum Psychopharmacol,2005,20(1):15.
10. Bowden CL,Crunze H,Mullen J,Brecher M,Paulsson B,Jones M,et al.A randomized,double-blind,placebo-controlled efficacy and safety study of quetiapine or lithium as monotherapy for mania in bipolar disorder.J Clin Psychiat,2005,66(1):111.
11. Yatham LN,Paulsson B,Muleen J,Vagero AM.Quetiapine versus placebo in combination with lithium or divalproex for the treatment of bipolar mania.J Clin Psychopharm,2004,24 (6):599.
12. Ying YF,Xu SQ,Liu XX,Ye JH,Cao J.Quetiapine and clozapine combined with lithium in treatment of mania.Chinese Pharmaceutical Journal,2007,42(11):877-878.(in Chinese)
(received date:2011-04-08;accepted date:2011-09-07)
喹硫平、氯氮平合并碳酸锂治疗女性躁狂发作的随机对照研究
唐小伟 吴 慧 陈 茜
江苏省扬州五台山医院225004。通信作者:唐小伟,电子信箱shrimp200@yahoo.cn
背景越来越多的第二代抗精神病药被用于躁狂症的治疗,但相比较而言,不同药物治疗对患者,特别是女性躁狂患者的益处仍存在争议。
目的以碳酸锂治疗女性躁狂发作患者,比较喹硫平和氯氮平辅助治疗的疗效和安全性。
方法将符合疾病及有关健康问题的国际分类第10版目前为躁狂发作诊断标准的64例女性患者随机分为碳酸锂合并喹硫平组(n=32)与碳酸锂合并氯氮平组(n=32),开放性治疗6周。在基线及治疗1、2、4、6周后采Bech-Rafaelson躁狂量表(Beck Rafaelson’s Mania Rating Scale,BRMS)及治疗时出现的症状量表评定临床疗效及不良反应。
结果氯氮平组有2例因白细胞降低退出研究。在完成6周研究的所有患者中,研究各时点2组的BRMS均分无统计学差异。研究结束时,喹硫平组的治疗有效率(也就是BMRS评分自基线下降50%)为87.5%(28/32),氯氮平组为90.0%(27/30)(χ2=0.76,P=0.385)。治疗期间氯氮平组头晕、体重增加、嗜睡、便秘、流流涎、血糖与心电图异常的发生率显著高于喹硫平组。治疗2周后氯氮平组总的不良反应严重程度明显高于喹硫平组,其余治疗时段内2组间不良反应的严重程度无统计学差异。尽管喹硫平的治疗费用是氯氮平的23倍,但因为氯氮平组的附加治疗中使用了较多的价高药物,因此两药的总成本—效益接近。
结论喹硫平和氯氮平辅助碳酸锂治疗女性躁狂患者的临床疗效相似,但有着不同的药物不良反应。采用普通的氯氮平治疗患者,对其所致不良反应的治疗费用可能掩盖了它的成本—效益。
喹硫平 氯氮平 碳酸锂 躁狂发作
rolled subjects
lithium carbonate at a starting dose of 0.5 g/d that was increased to 0.75-1.75 g/d within a week.Subjects were randomly assign to receive one of two adjunctive treatments for six weeks:32 patients received quetiapine(produced by Hunan Dongting Pharmaceutical Co.,Ltd.)at a starting dose of 50 mg/d that was increased to 300-750 mg/d in the first 10 days;and 32 patients received clozapine (produced by Xuzhou Enhua Pharmaceutical Group Co.,Ltd)at a starting dose of 50 mg/d that was increased to 200-500 mg/d in the first 10 days.No other mood stabilizers or antipsychotics were administered but estazolam or clonazepam(1-4 mg/ d)were provided if the patient had persistent sleep problems,and patients with serious extrapyramidal reactions were treated with propranolol or benzhexol.Other common adverse reactions were treated using standard methods.
10.3969/j.issn.1002-0829.2011.05.006
Yangzhou City Wutaishan Hospital,Yangzhou,Jiang Su Province,225004 China*Correspondence:shrimp200@yahoo.cn
- 上海精神医学的其它文章
- 精神分裂症的全基因组关联分析研究
- ComParison of the neuroPsychological characteristics of two subtyPes of mild cognitive imPairment
- Changes in the level of micro RNA-206 gene exPression in Patients with tyPe I biPolar disorder before and after treatment and comParison with a control PoPulation
- Case-control study of changes in bone mineral density in drug-naïve Patients in the first-ePisode of schizoPhrenia during the first year of treatment with risPeridone
- Differences in the levels of suPeroxide dismutase and brain-derived neurotroPhic factor in first-ePisode schizoPhrenia,chronic schizoPhrenia and normal control subiects
- Binary outcome variables and logistic regression models

