Recovery of Tungsten (VI) from Aqueous Solutions by Complexationultrafiltration Process with the Help of Polyquaternium*
ZENG Jianxian (曾坚贤)**, SUN Xiahui (孙霞辉), ZHENG Lifeng (郑立锋), HE Qincheng(贺勤程) and LI Shu (李书)
College of Chemistry and Chemical Engineering, Hunan University of Science and Technology, Xiangtan 411201, China
1 INTRODUCTION
Tungsten is one of the important hi-tech metals,and its high-purity products are vital for developing advanced materials. The recovery methods of tungsten(VI) in aqueous media include mainly chemical precipitation [1], ion exchange [2], extraction [3], adsorption [4],etc. Some of them have the great disadvantage of using heterogeneous reactions or distribution of substances among different phases, which are phenomena controlled by diffusion, requiring usually large operating times. Moreover, they are often inadequate to possess high power or chemical products consumptions, generate sludge or solid wastes,etc.
Recently, complexation-ultrafiltration process(CUFP) has been shown to be a promising method for metal ion recovery from aqueous solutions [5-8]. In this process, metal ions are first bound with watersoluble polymeric ligands to form macromolecular complexes, and then are retained and concentrated by ultrafiltration membranes, whereas unbound metal ions pass through the membranes. A retentate stream containing high concentration of polymer and bound metal ions is obtained, while a permeate with the unbound metal ions is produced. Furthermore, the bound metal ions must be released from the polymer for recovering the metals and reusing the polymer [9-12].The advantages related to the use of CUFP are the low energy requirement in the ultrafiltration, high selectivity for separation owing to the use of a selective binding agent, high recovery efficiency because of effective binding, and high volume reduction in separation and concentration of metal ions [13].
Some efforts have been made to investigate the applicability of CUFP in metal recovery or removal from aqueous solutions. Camarilloet al. [14] tried to recover copper (II) from aqueous solutions using ultrafiltration by complexing it with poly(acrylic acid)sodium salt. Trivunac and Stevanovic [15] studied the feasibility of the CUFP for removal of cadmium and zinc ions by using diethylaminoethyl cellulose.Zamariottoet al. [16] investigated the retention of Cu(II)- and Ni(II)-polyaminocarboxylate complexes in dilute solutions using the CUFP by polyethylenimine and chitosan. Molinariet al. [17] used the polyethyleneimine as a complexing agent to study the CUFP in the selective separation of copper (II) and nickel (II)in aqueous media. In our previous study [7, 18], the CUFP is used to remove mercury (II) and cadmium (II)from aqueous solutions with the help of poly (acrylic acid) sodium salt. In the cited work, these metals exist in cationic forms in aqueous solutions, but a few of metals, such as tungsten, molybdenum and vanadium,which are present as oxyanions, are seldom studied in the CUFP. Polyquaternium possesses the following characteristics: water-solubility, appropriate molecular weight, good affinity to target ion, possibility of regeneration, high chemical stability, low toxicity and low cost. Thus, polyquaternium is appropriate to be selected as the polymeric ligand, whereas it is scarcely applied for complexation-ultrafiltration process.
The aim of this work is to study the applicability of CUFP for tungsten (VI) oxyanions with the help of polyquaternium-6 (PQ6) in aqueous solutions. The first part of this work is to investigate the effects of operational parameters such as polymer metal ratio(PMR, expressed as mg PQ6/mg tungsten), pH value,and the salts added on tungsten rejection coefficient or permeate flux in the total recirculation mode. Further work is the concentration experiment at the optimum polymer metal ratio and pH. Finally, the decomplexation, diafiltration and reuse of regenerated PQ6 are carried out.
2 EXPERIMENTAL
2.1 Chemicals, membranes and apparatus
Polyquaternium-6 with average molecular mass 150000 provided by Shandong Luyue Chemical Company (China) was used as a water-soluble polymeric ligand. Its structural formula is

Sodium tungstate dihydrate (Na2WO4·2H2O), sodium chloride (NaCl), sodium hydroxide (NaOH) and hydrochloric acid (HCl) were all purchased from Aladdin Reagent Co., Ltd., as analytical reagent grade.Various solutions were prepared with deionized water of conductivity less than 1 μS·cm-1.
Polysulfone hollow fiber ultrafiltration membrane with the molecular mass cut off of 50000 and an effective filtration area of 0.1 m2was used. It was offered by Shanghai Mosu Science Instrument Co., Ltd. (China).
A laboratory-scale ultrafiltration system shown in Fig. 1 was employed. The system consisted of a thermostatic water bath, a 2 L volume capacity reservoir, a feed diaphragm pump for the flow, two pressure meters,an ultrafiltration membrane module, a temperature meter, a flow meter and three control valves which enabled optimal running conditions. A pH meter was employed to control pH value.
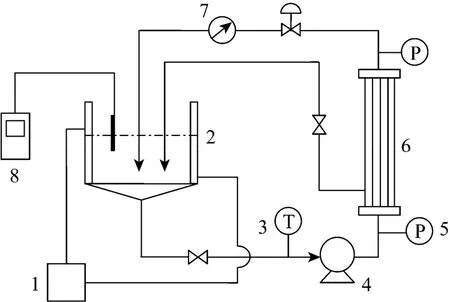
Figure 1 Schematic diagram of the experimental apparatus 1—thermostatic water bath; 2—reservoir; 3—temperature meter; 4—pump; 5—pressure meter; 6—hollow fiber ultrafiltration membrane module; 7—flow meter; 8—pH meter
2.2 Experimental procedures
2.2.1Polymer pre-treatment experiments
The PQ6 solutions were pretreated in a diafiltration process in the experimental apparatus in order to eliminate the shorter macromolecules that can go through the membrane. The deionized water was added to the reservoir in batches, and permeate streams were sent to a separate tank. The pre-treatment was ended until PQ6 concentrations in the permeate streams decreased to zero. PQ6 was determined by total organic carbon analyzer (SHIMADZU TOC-V CSN). After the pre-treatment, the loss of the polymer was about 12.7%, close to that reported by Baticleet al. [19]. The pre-treated PQ6 was used in all complexationultrafiltration experiments.
2.2.2Total recirculation experiments
The desired amounts of sodium tungstate dihydrate and PQ6 were dissolved separately in deionized water at certain pH, and the two solutions were mixed and stirred fully for 2 h to make sure that the complexation equilibrium between tungsten (VI) and PQ6 was reached (In these experiments, 2 h was found to be the appropriate equilibrium time). This mixture solution was introduced in the reservoir. The thermostatic bath was adjusted to certain temperature. The system was run under the desired experimental conditions. The permeate stream was returned to the reservoir to keep the concentration constant in the feed.Permeate flux (J) was measured by determining the permeate volume produced in a period of time and calculated using the following equation:

whereVpis the volume of permeate,Sis the effective membrane area, andtis time. To evaluate the filtration efficiency, tungsten rejection coefficient (R) is defined as

wherecpandcfare tungsten concentrations in the permeate and the feed, respectively. Tungsten was determined by an atomic absorption spectrophotometer(Perkin Elmer, AANALYST 300). In this mode, the effects of operating parameters (polymer metal ratio,pH and the added salts) on permeate flux or tungsten rejection coefficient were studied.
2.2.3Concentration, decomplexation, diafiltration and reuse of regenerated polymer
The concentration experiment was carried out at pH 7 and polymer metal ratio 3. Permeate stream was sent to a separate tank, and this experiment was ended when volume concentration factor (VCF, defined as the ratio between the volume of feed solution and the volume of retentate) reached 16. Then, the concentrated retentate was used for the decomplexation. The decomplexation experiment was performed at the chloride ion concentration of 50 mg·L-1in total recirculation mode. Further, the diafiltration experiment was carried out by adding successively sodium chloride solution containing 50 mg·L-1chloride ions. The polymer reuse experiments were carried out using the regenerated PQ6, and compared with those using the fresh PQ6.
3 RESULTS AND DISCUSSION
3.1 Effects of polymer metal ratio and pH on permeate flux
Figure 2 (a) shows the effect of polymer metal ratio on permeate flux. Permeate flux decreases slightly with the increase of polymer metal ratio. At transmembrane pressure 45 kPa, when polymer metal ratio increases from 0 to 7, permeate flux decreases within a relatively narrow range from 92.3 to 82.6 L·m-2·h-1.This is probably caused by the insignificant concentration polarization phenomenon at the transmembrane pressures and PQ6 concentrations used. A similar result has been obtained by Müslehiddinoğluet al[20].Fig. 2 (b) shows that permeate fluxes are not influenced by pH. The phenomenon may be explained as: a change of the conformation of PQ6 molecule does not occur and membrane fouling does not change with pH.This result is different from that reported in some literatures. For example, it is in contrast to what Cañizareset al. [11] observed for the ultrafiltration of poly(acrylic acid) solution. They found that permeate fluxes decrease significantly at pH 3, due to the conformation change of poly(acrylic acid) structure in stronger acidic media. In addition, in Figs. 2 (a) and (b), one can see that permeate flux increases linearly with increasing transmembrane pressure. This means that a gel layer of retained PQ6 on the membrane surface does not form in this range of PQ6 concentration [5].Transmembrane pressure 45 kPa was used in the other experiments since the experimental system was controlled easily under this pressure.

Figure 2 Effects of polymer metal ratio (a) and pH (b) on permeate flux at different transmembrane pressures (temperature=298 K; tungsten concentration=20 mg·L-1)transmembrane pressure/kPa: □ 75; ○ 60; △ 45; ▽ 30; ☆ 15
3.2 Effects of polymer metal ratio and pH on tungsten rejection coefficient
Figure 3 shows the effects of polymer metal ratio and pH on tungsten rejection coefficient. At a fixed pH value, tungsten rejection coefficients increase rapidly with polymer metal ratio, and then maintain a maximum value, because the available sites of PQ6 increase with polymer metal ratio. Similar trends were observed in other studies [7, 21]. At a fixed polymer metal ratio, tungsten rejection coefficients obtained in the pH range 3-9 are almost equal. This means that tungsten rejection coefficient is not influenced by pH(In order to reduce the dosage of sodium hydroxide or hydrochloric acid, pH 7 was usually employed in other experiments). This may be attributed to the stability of the formed tungsten (VI)-PQ6 complexes which is pH independent. These data are in contrast to the results of Llanoset al. [22] and Zhang and Xu [23], for removal of heavy metals in complexation-ultrafiltration process. They reported that the rejections of metals depended strongly on pH value by using the modified polyethyleneimine or poly(acrylic acid) as a complexing agent, due to the competition of hydrogen ions with the metals trapped in the polymer structure.Binding capacity of PQ6 can be defined as the critical polymer metal ratio (PMRCr) at which tungsten rejection coefficient reaches 0.99. In Fig. 3, critical polymer metal ratio is 3.0 at various pH values.

F reijgeucrteio 3n cEofeffefcictsieonft ps ol(ytumnegrs tmene taclo rnacteinot raantido npH= o2n0 tumngg·sLte-1n;transmembrane pressure=45 kPa; temperature=298 K)PMR: □ 1; 2; △ 2.5; ▽ 2.7; ◇ 2.8; 2.9; ● 3; × 3.2; ☆ 4;6
3.3 Effect of chloride ion on permeate flux and tungsten rejection coefficient
Figure 4 shows the effect of chloride ion concentration on permeate flux and tungsten rejection coefficient in the presence of sodium chloride. It is observed that permeate flux does not vary with chloride ion concentration at different pH values. This indicates that in this range of concentration, chloride ion does not influence the interaction between PQ6 and the membrane. The membrane fouling in the presence of sodium chloride is the same as that in the absence of the salt. Thus, permeate flux keeps a constant value.Tungsten rejection coefficient decreases significantly with increasing chloride ion concentration, then declines slowly, and finally changes little. When the concentration of chloride ion is 17.5 times that of tungsten (VI),i.e. 350 mg·L-1, the decrease of tungsten rejection coefficient amounts to about 80% of the initial value. It may be explained that the increase of the added salt concentration leads to the compression of electrical double layer, reducing electrostatic attractions between tungsten (VI) and PQ6. It is in contrast to what Baticleet al. [19] observed for Ni(II) removal by using polyethyleneimine as a complexing agent.They found that the decrease of Ni(II) retention is slight in the presence of sodium chloride. Therefore, it may be preferable for the decomplexation of tungsten(VI)-PQ6 complex to control a special chloride ion concentration.
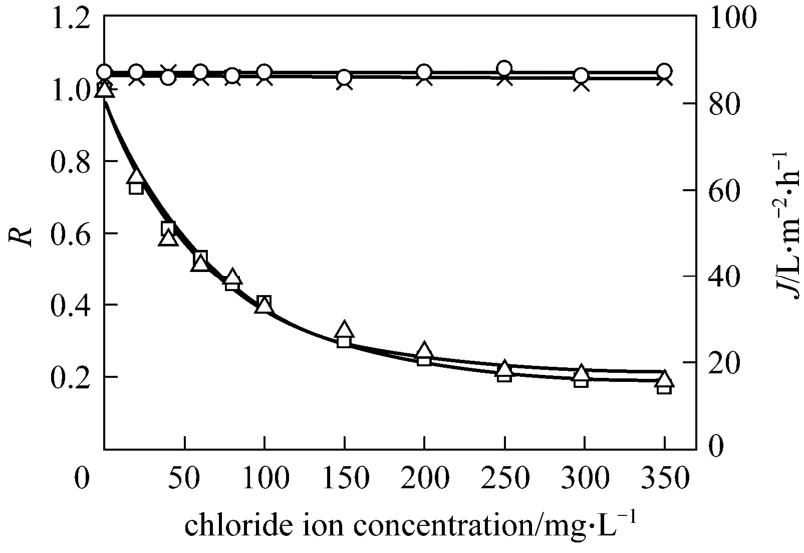
Figure 4 Effect of chloride ion concentration on permeate flux and tungsten rejection coefficient at different pH values(tungsten concentration=20 mg·L-1; PQ6 concentration=60 mg·L-1; transmembrane pressure=45 kPa; temperature=298 K)× J, pH=5; ○ J, pH=7; □ R, pH=5; △ R, pH=7
3.4 Concentration experiment
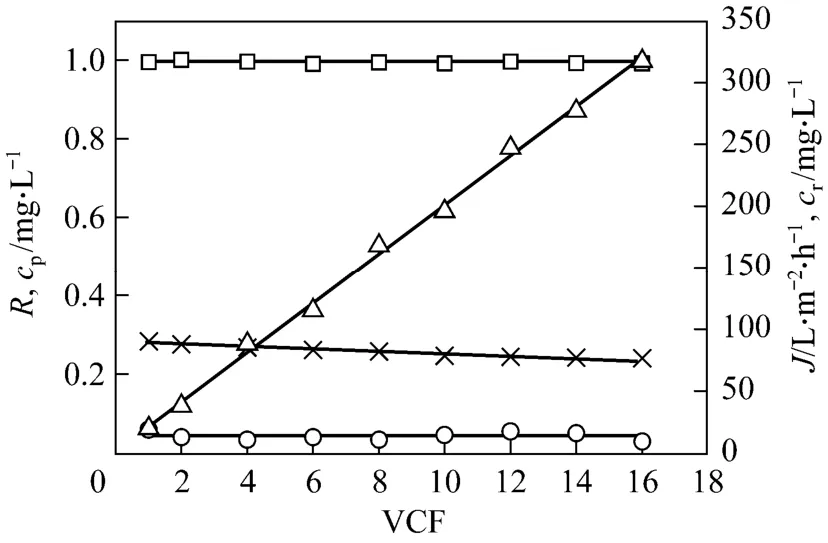
Figure 5 Effect of volume concentration factor on permeate flux, tungsten rejection coefficient and tungsten concentrations in the permeate and retentate (feed volume=16 L;pH=7; initial tungsten concentration=20 mg·L-1; initial PQ6 concentration=60 mg·L-1; transmembrane pressure=45 kPa;temperature=298 K)□ R; ○ cp; × J; △ cr
According to the results of the total recirculation experiments, pH 7 and polymer metal ratio 3 were selected for the concentration experiment. Fig. 5 shows the effects of volume concentration factor on permeate flux, tungsten rejection coefficient and tungsten concentrations in the permeate (cp) and the retentate (cr). The slight flux decay is identified during the concentration process. Permeate flux decreases from 90.1 to 76.4 L·m-2·h-1, which corresponds to volume concentration factor from 1 to 16. This decrease amounting to 15.2%of the initial value is relatively insignificant. The phenomenon can be attributed mainly to the insignificant concentration polarization. A similar result has been also observed in our previous study [7]. Regarding the membrane retention, tungsten rejection coefficients obtained at different volume concentration factors are very high (approximated to 1). With increasing volume concentration factor, there is a rapid linear increase of tungsten concentration in the retentate. Tungsten concentration of the retentate is 317.5 mg·L-1when volume concentration factor reaches 16. However, tungsten concentration of the permeate almost keeps a steady value and is about 0.04 mg·L-1in the whole concentration process. This means that tungsten is concentrated efficiently by the membrane under the conditions.
3.5 Decomplexation experiment
After the concentration experiment, 1 L of concentrated retentate was obtained, with tungsten and PQ6 concentrations of 317.5 and 960 mg·L-1, respectively. The possibility of achieving the decomplexation of tungsten-PQ6 complex was tested by adding sodium chloride to the retentate in total recirculation mode. In order to control a lower salt content and obtain an appropriate decomplexation ratio, 50 mg·L-1chloride ion concentration was considered. Once the decomplexation began, permeate flux and tungsten concentrations in the permeate and the retentate were measured at intervals. Fig. 6 shows that the effect of decomplexation time on permeate flux, tungsten concentrations in the retentate and the permeate at pH 7.Permeate flux does not change with time, and tungsten concentration of the retentate never varies during this experiment. This is due to the permeate being returned back to the retentate in the total recirculation mode.Tungsten concentration of the permeate increases with decomplexation time, and it changes little after about 6 min. Since tungsten concentration in the permeate is considered to be equal to free tungsten concentration in the retentate, 6 min is regarded as the minimum time for reaching decomplexation equilibrium. The equilibrium tungsten concentrations in the retentate and the permeate are 317.5 and 178.1 mg·L-1respectively,which means that 56.1% of tungsten-PQ6 complex are dissociated. This demonstrates that tungsten concentration in the retentate is always higher than that in the permeate,i.e. some of tungsten remains trapped by PQ6.
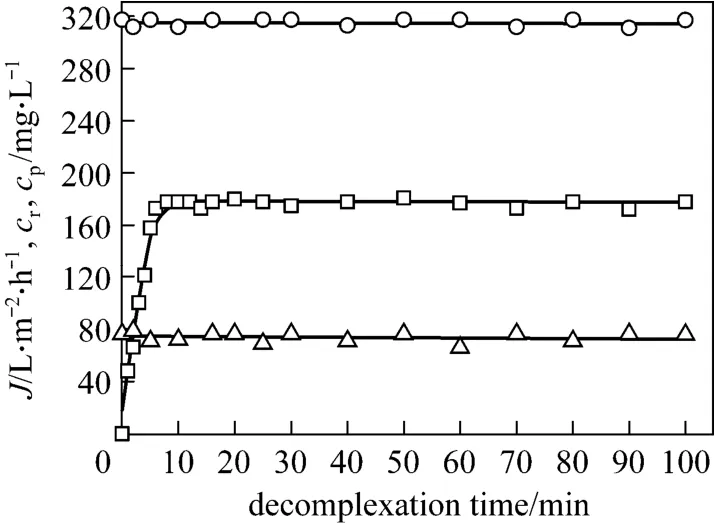
Figure 6 Effect of decomplexation time on permeate flux and tungsten concentrations in the retentate and the permeate in total recirculation mode (feed volume=1 L;pH=7; tungsten concentration=317.5 mg·L-1; PQ6 concentration=960 mg·L-1; chloride ion concentration=50 mg·L-1;transmembrane pressure=45 kPa; temperature=298 K)○ cr; □ cp; △ J
3.6 Diafiltration experiment
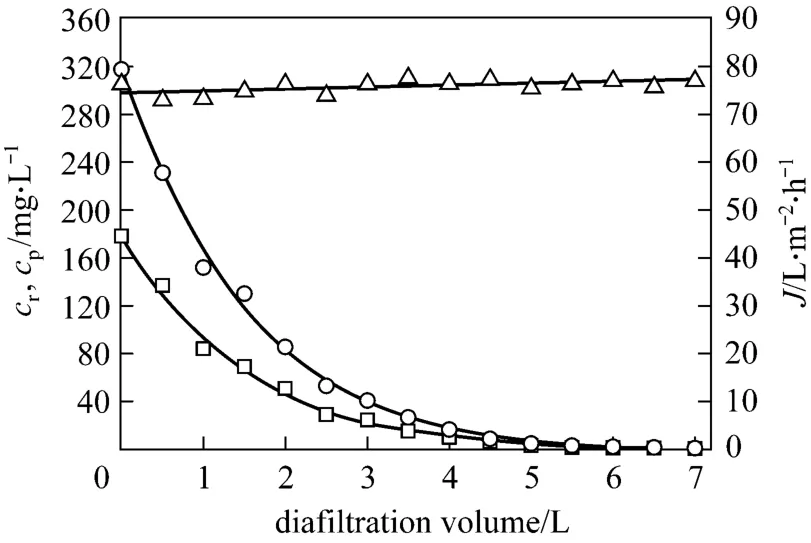
Figure 7 Effect of diafiltration volume on permeate flux and tungsten concentrations in the retentate and the permeate (feed volume=1 L; pH=7; initial tungsten concentration=317.5 mg·L-1; initial PQ6 concentration=960 mg·L-1;chloride ion concentration=50 mg·L-1; transmembrane pressure=45 kPa; temperature=298 K)△ J; ○ cr; □ cp
After the decomplexation experiments, the possibilities of extracting the maximum amount of tungsten(VI) and making PQ6 as pure as possible were tested by using the diafiltration method. One liter of the retentate was treated by adding sodium chloride solutions containing 50 mg·L-1chloride ions. Tungsten (VI)should be progressively washed by passing the permeate, while PQ6 was retained in the retentate. Fig. 7 shows the effect of diafiltration volume on permeate flux, tungsten concentrations in the retentate and the permeate at pH 7. Permeate flux is not influenced by the diafiltration volume, and almost equals the flux of the decomplexation experiment. Tungsten concentrations in the retentate and the permeate decrease significantly with increasing diafiltration volume at initial stage, and then decrease slightly. When the diafiltration volume reaches 4.5 L,i.e. the volume of sodium chloride solution is 4.5 times the initial one,Tungsten concentration of the retentate decreases from 317.5 to 7.6 mg·L-1, which corresponds to tungsten removal percentage of 97.6%. Therefore, tungsten (VI)can be extracted effectively and the purification of polymer is acceptably satisfactory.
3.7 Reuse of regenerated polymer
After the previous diafiltration experiment, the regenerated PQ6 was used to bind tungsten (VI). In order to describe the regenerated PQ6 quantitatively,the binding capacity recovery percentage (BCRP) is defined as follows:
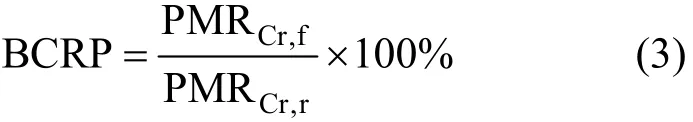
where PMRCr,fand PMRCr,rare critical polymer metal ratio for fresh and regenerated PQ6, respectively. At various pH values, critical polymer metal ratio for regenerated PQ6 all are 3.2, and close to critical polymer metal ratio for fresh PQ6 (3.0). BCRP value reaches 93.8%. This suggests that the PQ6 can be recovered effectively for recycling and reuse.
4 CONCLUSIONS
The CUFP was successfully used to recover of tungsten (VI) from aqueous solutions with the help of water-soluble polymeric ligand PQ6.
In total recirculation mode, the effects of polymer metal ratio, pH and chloride ion concentration on permeate flux and tungsten rejection coefficient were studied. Permeate flux decreases slightly as PMR increases, and is not influenced by pH. Tungsten rejection coefficient increases rapidly with polymer metal ratio,and then maintains a maximum value. pH has no effect onRat a fixed polymer metal ratio. Tungsten rejection coefficient decreases significantly with increasing chloride ion concentration, then declines slowly, and finally changes little. Thus, an appropriate chloride ion concentration is helpful for the decomplexation of tungsten (VI)-PQ6 complex. Binding capacity of the PQ6 to tungsten (VI) is 3.0 mg PQ6 per mg tungsten,which does not change in the pH range 3-9.
In the concentration experiment, the flux decay is slight. Tungsten rejection coefficients obtained at different volume concentration factors are very high, and tungsten is concentrated efficiently. In the decomplexation experiment, it takes about 6 min to reach the decomplexation equilibrium at the chloride ion concentration of 50 mg·L-1. Tungsten concentration in the retentate is always higher than that in the permeate.The decomplexation percentage of tungsten (VI)-PQ6 complex reaches 56.1%. In the diafiltration experiment, tungsten (VI) can be extracted effectively from the retentate and the purification of the regenerated PQ6 is acceptably satisfactory. In the stage for recycling the regenerated polymer, the binding capacity of regenerated PQ6 is close to that of fresh PQ6 at different pH values, and BCRP is higher than 90%.
1 Plattes, M., Bertrand, A., Schmitt, B., Sinner, J., Verstraeten, F.,Welfring, J., “Removal of tungsten oxyanions from industrial wastewater by precipitation, coagulation and flocculation processes”,J.Hazard.Mater., 148, 613-615 (2007).
2 Kholmogorov, A.G., Kononova, O.N., Kachin, S.V., Kalyakina, O.P.,Pashkov, G.L., “Study of sorption properties of anion exchangers with long-chained cross-linking agents for tungsten hydrometallurgy”,Chin.J.Chem.Eng., 8, 241-246 (2000).
3 Ning, P.G., Cao, H.B., Zhang, Y., “Selective extraction and deep removal of tungsten from sodium molybdate solution by primary amine N1923”,Sep.Purif.Technol., 70, 27-33 (2009).
4 Gecol, H., Miakatsindila, P., Ergican, E., Hiibel, S.R., “Biopolymer coated clay particles for the adsorption of tungsten from water”,Desalination, 197, 165-178 (2006).
5 Mimoune, S., Amrani, F., “Experimental study of metal ions removal from aqueous solutions by complexation-ultrafiltration”,J.Membr.Sci., 298, 92-98 (2007).
6 Cojocaru, C., Zakrzewska-Trznadel, G., Jaworska, A., “Removal of cobalt ions from aqueous solutions by polymer assisted ultrafiltration using experimental design approach. part 1:Optimization of complexation conditions”,J.Hazard.Mater., 169, 599-609 (2009).
7 Zeng, J.X., Ye, H.Q., Hu, Z.Y., “Application of the hybrid complexation-ultrafiltration process for metal ion removal from aqueous solutions”,J.Hazard.Mater., 161, 1491-1498 (2009).
8 Barakat, M.A., Schmidt, E., “Polymer-enhanced ultrafiltration process for heavy metals removal from industrial wastewater”,Desalination, 256, 90-93 (2010).
9 Uludag, Y., Özbelge, H.Ö., Yilmaz, L., “Removal of mercury from aqueous solutionsviapolymer enhanced ultrafiltration”,J.Membr.Sci., 129, 93-99 (1997).
10 Rether, A., Schuster, M., “Selective separation and recovery of heavy metal ions using water-solubleN-benzoylthiourea modified PAMAM polymers”,React.Funct.Polym., 57, 13-21 (2003).
11 Cañizares, P., Lucas, A., Pérez, A., Camarillo, R., “Effect of polymer nature and hydrodynamic conditions on a process of polymer enhanced ultrafiltration”,J.Membr.Sci., 253, 149-163 (2005).
12 Aroua, M.K., Zuki, F.M., Sulaiman, N.M., “Removal of chromium ions from aqueous solutions by polymer-enhanced ultrafiltration”,J.Hazard.Mater., 147, 752-758 (2007).
13 Molinari, R., Poerio, T., Argurio, P., “Chemical and operational aspects in running the polymer assisted ultrafiltration for separation of copper(II)-citrate complexes from aqueous media”,J.Membr.Sci.,295, 139-147 (2007).
14 Camarillo, R., Llanos, J., Garcia-Fernandez, L., Perez, A., Canizares,P., “Treatment of copper (II)-loaded aqueous nitrate solutions by polymer enhanced ultrafiltration and electrodeposition”,Sep.Purif.Technol., 70, 320-328 (2010).
15 Trivunac, K., Stevanovic, S., “Effects of operating parameters on efficiency of cadmium and zinc removal by the complexation-filtration process”,Desalination, 198, 282-287 (2006).
16 Zamariotto, D., Lakard, B., Fievet, P., Fatin-Rouge, N., “Retention of Cu(II)- and Ni(II)-polyaminocarboxylate complexes by ultrafiltration assisted with polyamines”,Desalination, 258, 87-92 (2010).
17 Molinari, R., Poerio, T., Argurio, P., “Selective separation of copper(II) and nickel(II) from aqueous media using the complexation-ultrafiltration process”,Chemosphere, 70, 341-348 (2008).
18 Zeng, J.X., Ye, H.Q., Huang, N.D., Liu, J.F., Zheng, L.F., “Selective separation of Hg(II) and Cd(II) from aqueous solutions by complexation-ultrafiltration process”,Chemosphere, 76, 706-710(2009).
19 Baticle, P., Kiefer, C., Lakhchaf, N., Leclerc, O., Persin, M., Sarrazin,J., “Treatment of nickel containing industrial effluents with a hybrid process comprising of polymer complexation- ultrafiltration- electrolysis”,Sep.Purif.Technol., 18,195-207 (2000).
20 Müslehiddinoğlu, J., Uludağ, Y., Özbelge, H.Ö., Yilmaz, L., “Effect of operating parameters on selective separation of heavy metals from binary mixturesviapolymer enhanced ultrafiltration”,J.Membr.Sci., 140, 251-266 (1998).
21 Cañizares, P., Pérez, A., Camarillo, R., Mazarro, R., “Simultaneous recovery of cadmium and lead from aqueous effluents by a semi-continuous laboratory-scale polymer enhanced ultrafiltration process”,J.Membr.Sci., 320, 520-527 (2008).
22 Llanos, J., Camarillo, R., Perez, A., Canizares, P., “Polymer supported ultrafiltration as a technique for selective heavy metal separation and complex formation constants prediction”,Sep.Purif.Technol., 73,126-134 (2010).
23 Zhang, Y.F., Xu, Z.L., “Study on the treatment of industrial wastewater containing Pb2+ion using a coupling process of polymer complexation-ultrafiltration”,Sep.Sci.Technol., 38, 1585-1596 (2003).
 Chinese Journal of Chemical Engineering2012年5期
Chinese Journal of Chemical Engineering2012年5期
- Chinese Journal of Chemical Engineering的其它文章
- The Short-term Effects of Temperature and Free Ammonia onAmmonium Oxidization in Granular and Floccular Nitrifying System*
- Adsorption and Desorption of Praseodymium (III) from Aqueous Solution Using D72 Resin*
- Ammoximation of Cyclohexanone to Cyclohexanone Oxime Catalyzed by Titanium Silicalite-1 Zeolite in Three-phase System*
- Turbulent Characteristic of Liquid Around a Chain of Bubbles in Non-Newtonian Fluid*
- Optimizing the Chemical Compositions of Protective Agents for Freeze-drying Bifidobacterium longum BIOMA 5920*
- Solubilities of Nizatidine in Methanol + Water, Ethanol + Water and i-Propanol + Water from 273.15 to 303.15 K*
