Early changes of hepatic hemodynamics measured by functional CT perfusion in a rabbit model of liver tumor
Guo-Lin Ma, Rong-Jie Bai, Hui-Jie Jiang, Xue-Jia Hao, Xu-Peng Dong, Da-Qing Li, Xin-Ding Liu and Lai Wei
Harbin, China
Early changes of hepatic hemodynamics measured by functional CT perfusion in a rabbit model of liver tumor
Guo-Lin Ma, Rong-Jie Bai, Hui-Jie Jiang, Xue-Jia Hao, Xu-Peng Dong, Da-Qing Li, Xin-Ding Liu and Lai Wei
Harbin, China
BACKGROUND:Early detection and treatment of hepatocellular carcinoma is crucial to improving the patients' survival. The hemodynamic changes caused by tumors can be serially measured using CT perfusion. In this study, we used a CT perfusion technique to demonstrate the changes of hepatic hemodynamics in early tumor growth, as a proof-of-concept study for human early hepatocellular carcinoma.
METHODS:VX2 tumors were implanted in the liver of ten New Zealand rabbits. CT perfusion scans were made 1 week (early) and 2 weeks (late) after tumor implantation. Ten normal rabbits served as controls. CT perfusion parameters were obtained at the tumor rim, normal tissue surrounding the tumor, and control liver; the parameters were hepatic blood flow, hepatic blood volume, mean transit time, permeability of capillary vessel surface, hepatic arterial index, hepatic arterial perfusion and hepatic portal perfusion. Microvessel density and vascular endothelial growth factor were correlated.
RESULTS:At the tumor rim, compared to the controls, hepatic blood flow, hepatic blood volume, permeability of capillary vessel surface, hepatic arterial index, and hepatic arterial perfusion increased, while mean transit time and hepatic portal perfusion decreased on both early and late scans (P<0.05). Hepatic arterial index increased (135%,P<0.05), combined with a sharp increase in hepatic arterial perfusion (182%,P<0.05) and a marked decrease in hepatic portal perfusion (-76%,P<0.05) at 2 weeks rather than at 1 week (P<0.05). Microvessel density and vascular endothelial growth factor showed significant linear correlations with hepatic blood flow, permeability of capillary vessel surface and hepatic arterial index, but not with hepatic blood volume or mean transit time.
CONCLUSION:The CT perfusion technique demonstrated early changes of hepatic hemodynamics in this tumor model as proof-of-concept for early hepatocellular carcinoma detection in humans.
(Hepatobiliary Pancreat Dis Int 2012;11:407-411)
liver tumor; computed tomography; hemodynamic; animal model; perfusion imaging
Introduction
In recent years, the incidence and mortality of hepatocellular carcinoma (HCC) have been increasing worldwide. Early detection and treatment of HCC are crucial to improving patient survival.[1]Imaging examinations are commonly applied to the diagnosis of HCC and provide non-invasive means of tumor visualization, such as MRI, traditional CT or both combined, but they also have difficulty in visualizing small HCCs.[2]It is, therefore, important to develop an alternative method to evaluate early HCC.
Angiogenesis is responsible for the vascular changes seen in both primary and metastatic blood flow. Angiogenesis can be assessed by CT perfusion techniques due to its alteration of vascular perfusion, blood volume, and permeability. In this study, we used CT perfusion parameters to determine the physiologic changes of angiogenesis in VX2 liver tumors. Our aim was to assess early alterations in hepatic hemodynamics in the VX2 model using this functional imaging method.
Methods
Animal model
This study was approved by the Institutional AnimalCare and Use Committee of the Second Affiliated Hospital, Harbin Medical University. Ten New Zealand rabbits (weighing 2.5-3.0 kg) served as controls. Ten additional rabbits were implanted with VX2 liver tumors, using cell lines derived from rabbit papilloma virusinduced skin cancer.[3,4]The animals were anesthetized with Sumianxin (0.2 mL/kg, Changchun University of Agriculture and Animal Science) by intramuscular injection. Fresh VX2 tumor tissue masses of 1-2 mm3were implanted locally into a tunnel established with an ophthalmic nipper in the left lobe of the liver.[5]
Data acquisition and perfusion imaging
The rabbits were fasted for 8 hours before scanning and anesthetized by intramuscular injection of Sumianxin. CT perfusion scans were performed at 1 and 2 weeks after tumor implants. The scans performed at the first week after implantation corresponded to "early" tumoral development, whereas the scans performed at the second week were considered as "late" tumor progression. CT imaging was completed on a multi-slice spiral CT (Lightspeed 16-slice spiral CT; GE Healthcare, Milwaukee, WI, USA). To select the scanning range, a plain CT scan of the liver was done before beginning perfusion scanning. The CT scanning parameters were as follows: 80 kVp, 120 mAs, slice thickness 3 mm, matrix 512×512, FOV15 cm, contrast medium IV injection in marginal ear vein 1 mL/s (1.0-1.5 mL/kg body weight).
The CT perfusion parameters were hepatic blood flow (HBF, mL/min/100 mg body weight), hepatic blood volume (HBV, mL/100 mg), mean transit time (MTT, sec), permeability of capillary vessel surface (PS, mL/min/100 mg), hepatic arterial index (HAI), hepatic arterial perfusion (HAP, mL/min/100 mg body weight) and hepatic portal perfusion (HPP, mL/min/100 mg body weight). The perfusion images were analyzed by a radiologist, and focal abnormalities were recorded. Regions of interest (ROIs) were carefully drawn as large as possible based on enhanced CT images. Vessels were avoided and not included in ROIs as far as possible. CT perfusion parameters were obtained three times at each area (control, tumor rim, and normal tissue surrounding the tumor) and the mean value of the three measurements was used in the analysis. For the control liver tissue, ROIs were drawn randomly on the normal liver parenchyma. For the tumor rim, ROIs were placed in those regions seen as "ring" enhancement on enhanced CT. For "normal" liver tissue, ROIs were placed on liver parenchyma at least 1 cm away from the tumor; these did not show any abnormality on pathological examination. Equivalent ROIs were placed on the early and late scanning images.
Pathology
The liver was fixed in 10% buffered neutral formalin and embedded routinely in paraffin for immunohistochemical study, with hematoxylin and eosin (HE), vascular endothelial growth factor (VEGF) and CD34 staining, using the methods described in a previous study.[5,6]Microvessel density (MVD) was measured by anti-CD34 staining.
Statistical analysis
The data were expressed as mean±SD. All analyses were carried out using SPSS version 11.5 (SPSS Inc., Chicago, IL., USA). A two-tailed Student'sttest was used to determine differences in perfusion parameters between the tumor rim and surrounding normal tissues. The correlations between MVD, VEGF and the CT perfusion parameters were analyzed using Pearson's correlation coefficient. Changes in the different perfusion parameters during tumor growth were evaluated by analysis of variance (ANOVA), comparing the effects of time (early and late scans). APvalue <0.05 was considered statistically significant.
Results
Pathological findings
On gross specimen examination, tumor diameter ranged from 10 to 20 mm (mean 16.5). The tumor was clearly visible and showed some central necrosis. A thick layer of granulation tissue surrounded the remainder of the lesion. Tumor cells had large trachychromatic nuclei in a nestlike distribution (Fig. 1A). Tumor tissue was processed for immunohistochemical analysis to demonstrate MVD (Fig. 1B) and VEGF protein levels (Fig. 1C). Positive linear correlations were found between the values of HBF, PS, HAI, and HAP versus MVD and VEGF (P<0.05). On the other hand, HPP showed a negative correlation with MVD and VEGF measurements (P<0.01). There were no correlations between MTT and HBV versus MVD and VEGF. The delimitation between interstitial tissue and the tumor was clear and maximal staining density was observed in the tumor rim, which was seen as "ring" enhancement on the arterial phase of contrast-enhanced CT (Fig. 1D). In addition, an area of intense perfusion was noted at the tumor rim (Fig. 1E).
CT imaging
In all rabbits, regions demonstrating both CT enhancement and tumoral perfusion enlarged with tumor growth. Intense enhancement was seen at the tumor rim on arterial phase contrast-enhanced CT. The contour of the tumor was clearly delineated in the increasedperfusion area on HAI, HBF, HBV, and PS maps, and in the lower perfusion area on the MTT map (Fig. 2).

Fig. 1. A: Tumor cells had a nest-like distribution. Many immature capillaries and abundant fibrous tissue surrounded the tumor (HE staining, original magnification ×100). B: Immunohistochemical staining for positive expression of CD34 (original magnification × 100). The vessels were stained brown and high MVD was apparent. C: Immunohistochemical staining for positive expression of VEGF (original magnification ×40). The cytoplasm of tumor cells was stained brown. Black arrows in A, B and C show the delimitation between interstitial tissue and the tumor tissue. D: On the arterial phase CT image, the central portion of the tumor became hypoattenuated, the enhancement was seen as "ring" enhancement at the tumor rim (black arrow), and no abnormal enhancement surrounded the tumor (white arrow). E: Functional CT perfusion map of HAI; a higher perfusion area was seen at the tumor rim(black arrow), and normal liver tissues surrounded the tumor (white arrow).
CT perfusion parameters
CT perfusion parameters were measured three times for each tumor tissue versus control, i.e.,n=30 for each group. The ANOVA showed significant differences in parameters in the tumor group at early scans compared to late scans (Fig. 3). At the tumor rim, the perfusion parameters HBF, HBV, HAI, HAP, and PS increased (P<0.05), but MTT and HPP decreased, compared to the control group. On the early scans, CT perfusion parameters that were related to hepatic hemodynamics caused by the tumors showed an increase in HAI (73%,P<0.05) with an increased HAP (90%,P<0.05) and a decreased HPP (-37%,P<0.05). Compared to normal regions, the perfusion parameters HBF, HBV, HAI, HAP, and PS in the tumor rim were higher at 2 weeks than at 1 week post-implant (P<0.05). HAI increased (135%,P<0.05) in combination with a sharp increase in HAP (182%,P<0.05) and a marked decrease in HPP(-76%,P<0.05). The values of the surrounding "normal" perfusion parameters did not change significantly with tumor growth (Fig. 4).
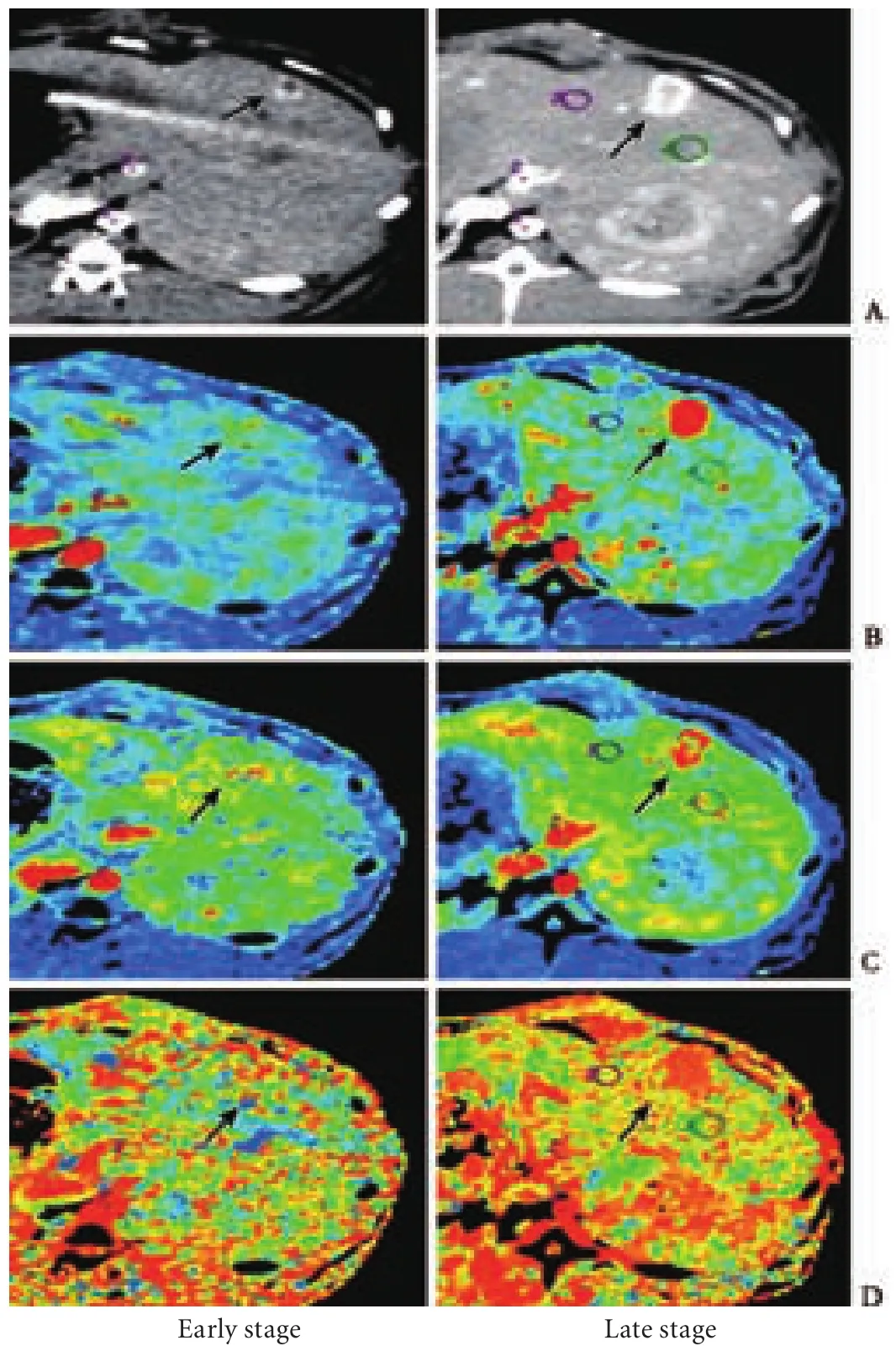
Fig. 2. Contrast-enhanced CT images and CT perfusion maps in the early and late stage of tumor growth. Black arrows indicate the boundary of the tumor. Red represents the highest measurement value, while yellow, green, blue and black represent decreasing measurement values. A: Contrast-enhanced CT images in the arterial phase; B: Functional maps of HBF; C: Functional maps of HBV; D: Functional maps of MTT.

Fig. 3. CT perfusion parameters in control and tumor rim from early and late scans (n=30). *: compared to the control group, in tumor rims on early and late scans after implantation, HBF, HAP, HBV and PS significantly increased (P<0.05), and HPP and MTT values significantly decreased (P<0.05).

Fig. 4. HAI (A), HAP (B) and HPP (C) mean values of control and tumor rim on the early and late scans. In "pathological normal" liver tissue, HAI, HAP and HPP were similar to those in the control group and remained almost unchanged over time. In the tumor rim, HAI and HAP were elevated, and HPP decreased compared to the control group.
Discussion
The VX2 tumor has a high rate of successful implantation, stable histology, and is capable of neovascularization. The pathological features of VX2 tumors are similar to those of human HCC, making this animal model especially suitable for imaging tumor angiogenesis.[7-10]Our pathological results showed many immature capillaries and much fibrous tissue in the tumor rim, where the most intensive angiogenesis occurred.[11,12]This was seen as areas of ring enhancement on contrast-enhanced CT, corresponding to high peripheral perfusion, increased blood volume, and elevated permeability on the CT perfusion maps. Thus, it is important to make tumor rims the ROIs for the assessment of tumor angiogenesis. In the present study, we evaluated the timeline underlying changes of parameters on the CT perfusion images, in order to develop a tumor model as a proof-of-concept study for early HCC detection in human subjects.
In our study, normal liver tissue without tumor implantation served as the control. There were no differences in the perfusion parameters between the control group and the "normal" tissue. The presence of "normal" perfusion in liver tissue implied normal blood flow. This result also suggested that blood flow in some areas was not influenced by the implanted tumor. Thus, CT perfusion quantifies not only the vascular physiology of the tumor itself, but also other blood flow changes of the surrounding parenchyma. Also, both local and distant blood flow caused by tumors can be evaluated by CT perfusion parameters. More importantly, the control group or the "normal" tissue adjacent to the tumor can be considered as a benign state, and the tumor rim seems to reflect a malignant state. Thus, CT perfusion seems to be able to differentiate benign from malignant states of liver tumors based on their perfusion parameters.
As we concluded in the previous study,[5]HAI is still the most important parameter used to detect regions "that are prone to develop into tumor" on early scans. Therefore, early tumors are visually detectable with this parameter. On the early scan, compared to the normal region, there was an inversion between the arterial and portal blood flow in the tumor, as a high HAP (90%) and a low HPP (-37%). On the later scan, there was a sharp increase in HAP (182%) coinciding with a decrease in HPP (-76%). Thus, the CT perfusion technique can be used to evaluate hepatic hemodynamic changes, including arterial and portal blood supply, and thus determine liver tumor vascular physiology.[13-15]
Our study had several limitations. The main limitation was due to the retrospective placing the ROI on the CT perfusion image, based on contrast-enhanced CT images and pathological findings. This indicated that our ROIs represented the perfusion parameters of specific regions. Thus, focal abnormal changes of perfusion images involved with the tumor may not be detected freely without knowing the specific areas of the liver beforehand. Therefore, it is important for us to develop a perfusion technique to obtain perfusion parameters throughout the entire liver, which would allow a more objective assessment of vascular physiology in earlier tumor development. Currently, dual-energy CT technology has just started in clinical settings.[16]Pure 80-kVp data acquired from a dual-energy CT scannerproduce greater differences in attenuation between hepatic lesions and the surrounding liver, potentially improving the early assessment and detection of liver tumors. In the future, this technique may be suitable for assessing more promising parameters of early vascular physiology in liver tumors.
Another limitation of our study was a mismatch between CT perfusion parameters and pathological measurement at the early stage because of differences in the time when the CT scans were performed compared to the time when the pathology measurement was done. Thus, some important pathological results of early tumor may have been lost. Thus, the relationship between the CT perfusion parameters and pathological findings of early tumors requires further investigation. Moreover, our results may not be applicable to studies of early HCC in humans, since HCC frequently occurs in cirrhotic patients, and the VX2 tumor model in our study was not developed using a background of liver cirrhosis. Therefore, further experimental studies are needed to establish an animal model simulating the lesions in human liver carcinogenesis (from liver cirrhosis to early HCC).
In conclusion, this multi-parametric CT perfusion imaging technique is sensitive, specific and non-invasive. It has the potential to detect the early hemodynamic changes in hepatic tumors and thus has a major advantage in the evaluation of angiogenesis in human early HCC.[17-19]
Contributors:MGL and BRJ contributed equally to the article. MGL and JHJ proposed the study. MGL, BRJ and JHJ analyzed data and prepared the manuscript. HXJ and DXP completed histopathological examinations of liver biopsy specimens. LDQ and LXD performed the imaging scans. WL prepared animal tests and revised the manuscript. JHJ is the guarantor.
Funding:This work was supported by a grant from the Educational Committee of Heilongjiang Province (11541166).
Ethical approval:This study was approved by our Institutional Animal Care and Use Committee.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
林肯携由新款林肯MKC、全新林肯航海家Nautilus、全新林肯飞行家Aviator preview、全新林肯领航员Navigator、林肯大陆Continental和林肯MKZ六款车型组成的全系阵容,焕新亮相2018广州车展。全系车型均采用了重新设计的家族式林肯星辉式前格栅,呈现出林肯“静谧之旅”理念下优雅精炼的风貌。
1 Yang L, Parkin DM, Ferlay J, Li L, Chen Y. Estimates of cancer incidence in China for 2000 and projections for 2005. Cancer Epidemiol Biomarkers Prev 2005;14:243-250.
2 Bruix J, Sherman M; Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology 2005; 42:1208-1236.
3 Nasseri M, Wettstein FO. Cottontail rabbit papillomavirusspecific transcripts in transplantable tumors with integrated DNA. Virology 1984;138:362-367.
4 Nasseri M, Wettstein FO. Differences exist between viral transcripts in cottontail rabbit papillomavirus-induced benign and malignant tumors as well as non-virus-producing and virus-producing tumors. J Virol 1984;51:706-712.
5 Jiang HJ, Zhang ZR, Shen BZ, Wan Y, Guo H, Shu SJ. Functional CT for assessment of early vascular physiology in liver tumors. Hepatobiliary Pancreat Dis Int 2008;7:497-502.
7 Lijowski M, Caruthers S, Hu G, Zhang H, Scott MJ, Williams T, et al. High sensitivity: high-resolution SPECT-CT/MR molecular imaging of angiogenesis in the Vx2 model. Invest Radiol 2009;44:15-22.
8 Deng G, Zhao DL, Li GC, Yu H, Teng GJ. Combination therapy of transcatheter arterial chemoembolization and arterial administration of antiangiogenesis on VX2 liver tumor. Cardiovasc Intervent Radiol 2011;34:824-832.
9 Maehara N. Experimental microcomputed tomography study of the 3D microangioarchitecture of tumors. Eur Radiol 2003;13:1559-1565.
10 Stewart EE, Chen X, Hadway J, Lee TY. Correlation between hepatic tumor blood flow and glucose utilization in a rabbit liver tumor model. Radiology 2006;239:740-750.
11 Kan Z, Phongkitkarun S, Kobayashi S, Tang Y, Ellis LM, Lee TY, et al. Functional CT for quantifying tumor perfusion in antiangiogenic therapy in a rat model. Radiology 2005;237: 151-158.
12 Ogawa M, Yamamoto H, Nagano H, Miyake Y, Sugita Y, Hata T, et al. Hepatic expression of ANG2 RNA in metastatic colorectal cancer. Hepatology 2004;39:528-539.
13 Choi SH, Chung JW, Kim HC, Baek JH, Park CM, Jun S, et al. The role of perfusion CT as a follow-up modality after transcatheter arterial chemoembolization: an experimental study in a rabbit model. Invest Radiol 2010;45:427-436.
14 Fournier LS, Cuenod CA, de Bazelaire C, Siauve N, Rosty C, Tran PL, et al. Early modifications of hepatic perfusion measured by functional CT in a rat model of hepatocellular carcinoma using a blood pool contrast agent. Eur Radiol 2004;14:2125-2133.
15 Cuenod C, Leconte I, Siauve N, Resten A, Dromain C, Poulet B, et al. Early changes in liver perfusion caused by occult metastases in rats: detection with quantitative CT. Radiology 2001;218:556-561.
16 Okada M, Kim T, Murakami T. Hepatocellular nodules in liver cirrhosis: state of the art CT evaluation (perfusion CT/ volume helical shuttle scan/dual-energy CT, etc.). Abdom Imaging 2011;36:273-281.
17 Ippolito D, Sironi S, Pozzi M, Antolini L, Ratti L, Alberzoni C, et al. Hepatocellular carcinoma in cirrhotic liver disease: functional computed tomography with perfusion imaging in the assessment of tumor vascularization. Acad Radiol 2008;15:919-927.
18 Sahani DV, Holalkere NS, Mueller PR, Zhu AX. Advanced hepatocellular carcinoma: CT perfusion of liver and tumor tissue--initial experience. Radiology 2007;243:736-743.
19 Tsushima Y, Funabasama S, Aoki J, Sanada S, Endo K. Quantitative perfusion map of malignant liver tumors, created from dynamic computed tomography data. Acad Radiol 2004;11:215-223.
December 14, 2011
Accepted after revision February 17, 2012
Author Affiliations: Department of Radiology, China-Japan Friendship Hospital, Beijing 100029, China (Ma GL); Department of Radiology, Beijing Jishuitan Hospital, Beijing 100035, China (Bai RJ); Department of Radiology, Second Affiliated Hospital, Harbin Medical University, Harbin 150086, China (Jiang HJ, Hao XJ, Dong XP, Li DQ, Liu XD and Wei L)
Hui-Jie Jiang, PhD, Department of Radiology, Second Affiliated Hospital, Harbin Medical University, Harbin 150086, China (Tel: 86-451-86605576; Email: jhjemail@163.com)
© 2012, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(12)60199-4
 Hepatobiliary & Pancreatic Diseases International2012年4期
Hepatobiliary & Pancreatic Diseases International2012年4期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Gastric- and intestinal-type marker expression in invasive ductal adenocarcinoma of the pancreas
- Expression of SOCS3 throughout liver regeneration is not regulated by DNA methylation
- A common variant in the precursor miR-146a sequence does not predispose to cholangiocarcinoma in a large European cohort
- Muscarinic acetylcholine receptor M3 in proliferation and perineural invasion of cholangiocarcinoma cells
- Hepatocyte differentiation of mesenchymal stem cells
- Early control of short hepatic portal veins in isolated or combined hepatic caudate lobectomy
