Muscarinic acetylcholine receptor M3 in proliferation and perineural invasion of cholangiocarcinoma cells
Yu-Jie Feng, Bing-Yuan Zhang, Ru-Yong Yao and Yun Lu
Qingdao, China
Muscarinic acetylcholine receptor M3 in proliferation and perineural invasion of cholangiocarcinoma cells
Yu-Jie Feng, Bing-Yuan Zhang, Ru-Yong Yao and Yun Lu
Qingdao, China
BACKGROUND:Cholangiocarcinoma, a type of malignant tumor, originates from epithelial cells of the bile duct. Perineural invasion is common path for cholangiocarcinoma metastasis, and it is highly correlated with postoperative recurrence and poor prognosis. It has been reported that muscarinic acetylcholine receptor M3 (mAChR M3) is widely expressed in digestive tract cancer, and may play an important role in the proliferation, differentiation, transformation and carcinogenesis of tumors. This study was to explore the effect of mAChR M3 on the growth of cholangiocarcinoma cellsin vitroand provide a new approach to the pathogenesis and treatment of cholangiocarcinoma.
METHODS:Streptavidin-biotin complex immunohistochemistry was carried out to assess the expression of mAChR M3 in surgical specimens of cholangiocarcinomas (40 cases) and normal bile duct tissues (9), as well as to investigate nerve infiltration. The cholangiocarcinoma cells were treated with different concentrations of selective M-receptor agonist pilocarpine and M-receptor blocker atropine sulfate to induce changes in cell proliferation. The experimental data were analyzed by the Chi-square test.
RESULTS:The strongly-positive expression rate of mAChR M3 was much higher in poorly-differentiated (69%, 9/13) than in well- and moderately-differentiated cholangiocarcinomas (30%, 8/27) (χ2=5.631,P<0.05). The strongly-positive mAChR M3 expression rate in hilar cholangiocarcinoma (50%, 14/28) was higher than that in cholangiocarcinomas from the middle and lower common bile duct (25%, 3/12) (χ2=2.148,P<0.05).Cholangiocarcinomas with distant metastasis had a stronglypositive expression rate (75%, 9/12), which was much higher than those without distant metastasis (29%, 8/28) (χ2=7.410,P<0.01). The absorbance value in the pilocarpine+atropine group was significantly higher than the corresponding value in the pilocarpine group.
CONCLUSIONS:The expression of mAChR M3 is influenced by the extent of differentiation, distant metastasis and the site of cholangiocarcinoma. It also plays a key role in the proliferation and metastasis of cholangiocarcinoma.
(Hepatobiliary Pancreat Dis Int 2012;11:418-423)
cholangiocarcinoma; mAChR M3; immunohistochemistry; perineural invasion; proliferation
Introduction
Cholangiocarcinoma, a malignant tumor, originates from epithelial cells of the bile duct. The development of cholangiocarcinoma is currently considered to be a multivariate and multi-step pathological process, involving oncogene activation, tumor suppressor gene inactivation, tumor metastasis, abnormalities in apoptosis and cell cycle, and genetic and epigenetic changes.[1]Studies[2-4]reported that the muscarinic acetylcholine receptor M3 (mAChR M3) is widely expressed in digestive tract cancer, and may play an important role in the proliferation, differentiation, transformation and carcinogenesis of tumors. So far, data on the expression of mAChR M3 and its function in the proliferation of cholangiocarcinoma cells are scarce. This study was undertaken to explore the effect of mAChR M3 on the growth of cholangiocarcinoma cells by evaluating its expression levels and cellular proliferation. The results may provide new ideas for pathogenesis studies and the treatment of cholangiocarcinoma.
Methods
Sources of specimens
Surgical specimens were taken from 40 cholangiocarcinoma patients who had been treated from January 2008 to June 2010 in our hospital. These patients with complete clinical data were included in the experimental group, and none of them received preoperative chemotherapy or radiotherapy. The specimens were fixed in 10% formalin, embedded in paraffin, serially sectioned at 3 µm, stained with HE, and confirmed pathologically. Specimens of the normal bile duct from nine liver transplantation donors were chosen as the control group. The cholangiocarcinoma cell line QBC939 was obtained from the Shanghai Institute Cell Library.
Main reagents, supplies and preparation of drugs
The mAChR M3 human polyclonal antibody, SP-9000 immunohistochemistry universal kit and concentrated DAB kit were purchased from Zhongshan Biotechnology Co. Ltd. (Beijing, China). RIMP1640 medium and fetal bovine serum (FBS) were from Hyclone (USA). Cell Counting Kit-8 (CCK-8) was from Beyotime (China), while pilocarpine powder and atropine sulfate were from Sigma (St. Louis, MO).
Pilocarpine was first dissolved to 30 mmol/L, filtersterilized, and diluted with pure 1640 medium before use. Pure 1640 medium was also used for dilution of atropine sulfate.
Cell culture and passage
Cholangiocarcinoma cells were cultured in 1640 medium supplemented with 10% FBS at 37 ℃ in a 5% CO2incubator. The medium was changed regularly until adherent cells grew into a dense monolayer, when the cells were digested with 0.25% trypsin and inoculated into culture bottles. Logarithmic phase cells were selected and digested with 0.25% trypsin. The growth of cells was observed under an inverted microscope every day and photographed regularly.
Expression and localization of mAChR M3
Streptavidin-biotin complex (SABC) staining was performed to detect the expression and localization of mAChR M3. Each biopsy was pretreated and the procedures were carried out according to the manufacturer's instructions, with PBS instead of primary antibody as negative control. Suspended cells at 1×105/mL were inoculated into 6-well culture plates (2 mL/well) and cultured for 48 hours. Then, the coverslips with cells were removed from the plates and fixed for 10 minutes in 100% acetone at 4 ℃ to detect the location of mAChR M3. Positive staining showed the basement membrane as brownish-yellow. A negative result indicates that the percentage of cholangiocarcinoma cells showing positive staining was <25%, while a positive result indicates that the percentage was between 25% and 55%. If the percentage was >55%, the staining was described as "strongly positive".
Detection of cholangiocarcinoma cell proliferation and effects of pilocarpine and atropine
Logarithmic phase cells were inoculated into 96-well cell culture plates at 5000 cells/well (100 µL/well) and incubated at 37 ℃ in a 5% CO2incubator for 24 hours. The adherent cells were removed by washing twice with PBS, and then pilocarpine (final concentrations 1, 0.5 and 0.1 mmol/L), atropine sulfate (final concentrations 0.1 and 0.01 mmol/L), or pilocarpine+atropine sulfate were added. The experiment was repeated simultaneously in five wells for each group, with a negative control group with medium instead of drug. The adherent cells were treated for 24, 48 or 72 hours. Then we added 10 µL of CCK-8 solution to each well and incubated for 3 hours. Finally the absorbance was measured at 450 nm.
Growth inhibition rate=(1-drug group A/control group A)×100%.
Statistical analysis
The data were expressed as mean±SD and were analyzed by SPSS 17.0. Rate comparison was made using the Chi-square test. Multiple group comparisons were made using ANOVA, and the LSD method was used to compare the two tests. APvalue less than 0.05 was considered statistically significant.
Results
Expression of mAChR M3
The expression of mAChR M3 was visible in the cell membrane and basement membrane of the normal bile duct, and the rate of positive expression was 33% (3/9). Discontinuous weakly-positive immune reactants were detected in the basement membrane in some sections, with little expression in the cytoplasm (Fig. 1A). In all cholangiocarcinoma specimens, the rates of positive and strongly-positive expression of mAChR M3 were 90% (36/40) and 42.5% (17/40), respectively. Continuous or coarse granular staining was observed in the cell membrane and basement membrane in cholangiocarcinoma, with strongly-positive staining in the cytoplasm of some sections (Fig. 1B). Comparedwith the normal bile duct, the intensity of mAChR M3 expression increased in the cell membrane and basement membrane of cholangiocarcinoma. Neural infiltration was widely seen in cholangiocarcinoma, with an incidence of 87.5% (35/40) (Fig. 2). The immunohistochemical reaction of cholangiocarcinoma cells was positive, and the positive reaction was granular or distributed in flakes, mainly located on the cell membrane and in the cytoplasm (Fig. 3).

Fig. 1. A: Normal expression of mAChR M3 in bile duct (SABC, original magnification ×200). Heterogeneous expression of mAChR M3, with discontinuous weakly-positive immune reactant on the basement membrane and some weak expression in the cytoplasm. B: mAChR M3 expression in cholangiocarcinoma (SABC, original magnification ×200). Continuous or coarse granular staining on the cell membrane and basement membrane of cholangiocarcinoma, with strongly-positive staining in the cytoplasm in some sections.

Fig. 2. Nerve infiltration in cholangiocarcinoma. Invasive tumor growth among nerve fibers.
Relationship between mAChR M3 expression and clinical pathology
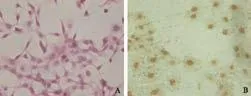
Fig. 3. A: HE staining in cholangiocarcinoma cells (SABC, original magnification ×400). B: mAChR M3 expression in cholangiocarcinoma cells (SABC, original magnification ×400). The immuno-histochemical reaction of cholangiocarcinoma cells was positive, with granular reaction products or distributed in flakes mainly in the cell membrane and cytoplasm.
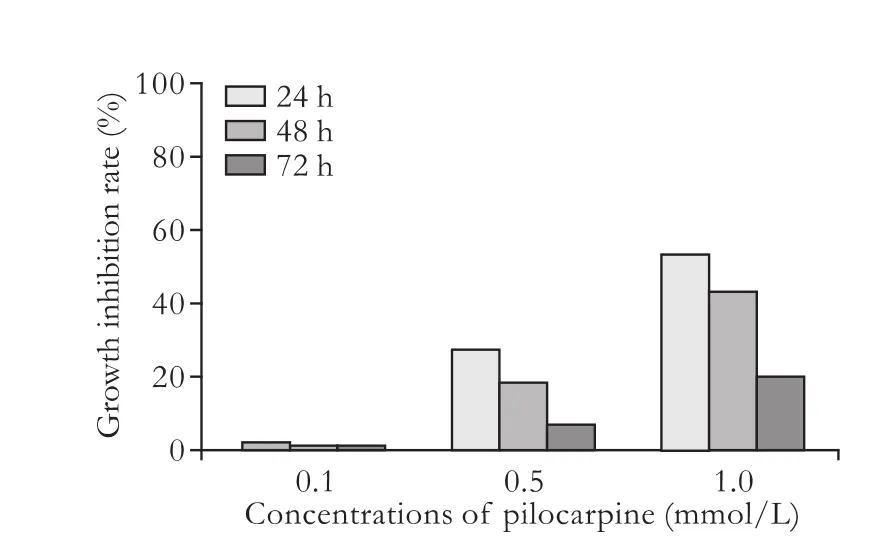
Fig. 4. Proliferation of cholangiocarcinoma cells with different concentrations of pilocarpine.
The experimental group comprised specimens from 27 men and 13 women. Their ages ranged from 44 to 75 (mean 61.65) years. Thirty-one were older than 55 years and 9 were younger than 55 years. Twenty-seven patients had tubular adenocarcinoma, 9 had papillary adenocarcinoma, and 4 had mucinous adenocarcinoma. The numbers of patients with well-, moderately- or poorly-differentiated cholangiocarcinoma were 7, 20 and 13, respectively. The tumor was found in the hilus in 28 patients, in the middle common bile duct in 9, and in the lower common bile duct in 3. Surgical exploration and biopsy specimens confirmed no peritumoral and distant lymph node metastasis in 28 patients and metastasis in 12. The sites of metastasis included the hepatoduodenal ligament, pancreas, lymph nodes, liver, and omentum. The strongly-positive expression rate of mAChR M3 was significantly higher in specimens of poorly-differentiated cholangiocarcinoma (69%, 9/13) than in those of welland moderately-differentiated cholangiocarcinoma (30%, 8/27) (χ2=5.631,P<0.05). The strongly-positive expression rate in hilar cholangiocarcinoma specimens (50%, 14/28) was higher than in those from the middle and lower common bile duct (25%, 3/12) (χ2=2.148,P<0.05). Cholangiocarcinoma with distant metastasis had a strongly-positive expression rate of 75% (9/12), which was significantly higher than that in cholangiocarcinoma without distant metastasis (29%, 8/28) (χ2=7.410,P<0.01).
Effects of pilocarpine and atropine on proliferation of cholangiocarcinoma cells
Pilocarpine inhibited the proliferation of cholangiocarcinoma cells in a concentration-dependent manner. The groups treated with 1 and 0.5 mmol/L pilocarpinewere inhibited compared with the negative control group (P<0.01), and there was no significant difference between the group treated with 0.1 mmol/L pilocarpine and the negative control group. The inhibitory effect was time-dependent and weakened gradually with time. The inhibitory effect decreased at 24, 48, and 72 hours in the groups treated with 1 and 0.5 mmol/L pilocarpine (P<0.05), and there was no difference in the inhibitory effect between different time points in the group treated with 0.1 mmol/L pilocarpine (Fig. 4). The absorbance value from the pilocarpine+atropine group was significantly higher than the corresponding value from the pilocarpine group, showing that atropine sulfate blocked the inhibition of proliferation by pilocarpine. There was no difference in absorbance values between the atropine sulfate and negative control groups, showing that atropine itself had no effect on the proliferation of cholangiocarcinoma cells (Table).
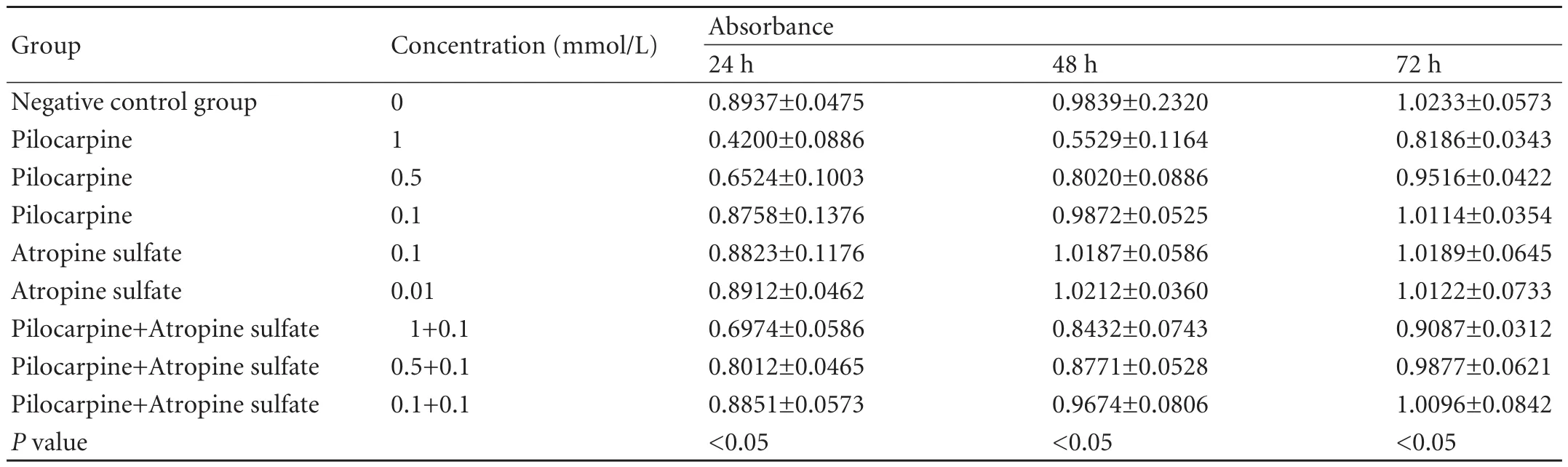
Table. Inhibition of cholangiocarcinoma cell proliferation by pilocarpine and atropine
Discussion
Many studies[5-7]have focused on the interactions between neurotransmitters and tumors, especially tumors with perineural infiltration characteristics, such as cholangiocarcinoma. Studies[8,9]have shown the abnormal expression of many neurotransmitter receptors in tumors, hence neurotransmitter and receptor agonists can influence the proliferation, differentiation and metastasis of tumor cells, and receptor antagonists can block these effects.
The vine-like neural plexus in the human liver ligament can be divided into pre- and post-liver components. The pre-liver component comprises the left celiac ganglion and branches of the left vagus nerve (the cystic duct, gallbladder, and biliary and pancreaticobiliary-common bile duct branches) forming a sheath around the hepatic artery, and entering the liver along this artery. The post-liver component comprises the right celiac ganglion and the right branch of the vagus nerve, which are distributed mainly along the extrahepatic bile duct and portal vein, with its branch communicating to the branch of the pre-liver component. Sensory fibers of the right phrenic nerve are distributed within the coronary ligament, falciform ligament and liver capsule,[10]and some of the fibers combine with the liver ante- and metaplex, along with the fibers of the hepatic plexus distributed into the exterior and interior biliary system. These nerve plexuses include sympathetic and parasympathetic nerves, which predominate throughout the liver. These nerve fibers are all distributed in the hepatic artery, vena portae hepatica, liver interior and extrahepatic bile duct. The sympathetic innervation originates from the celiac ganglia, while the parasympathetic innervation comes from the vagus.[11]Therefore, the bile duct system is an autonomic organ dominated by a wide range of nerves. This has a very important implication: the parasympathetic nervous system in the bile duct plays an important role in malignant transformation.
Acetylcholine (ACh) is the main neurotransmitter of the parasympathetic system. ACh acts on the cholinergic receptors (AChRs) of effector cells to regulate their functions. Previous work revealed that AChRs effectively regulate cellular transformation and differentiation.[12]There are two types of AChRs: nicotinic (nAChRs) and muscarinic (mAChRs). nAChRs are ligand-gated ion channel proteins, while mAChRs belong to the G protein-coupled receptor family.[13]They have different mechanisms, structures and biological effects. ACh and its receptors have been found in epithelial cells (respiratory, digestive, and urinary tracts, and epidermis), mesothelial cells (pleura and pericardium), and endothelial, muscle and immune cells (monocytes, granulocytes, alveolar macrophages and mast cells).[14-16]We found that mAChR M3 wasexpressed in cholangiocarcinoma cells, mainly in the cell membrane and cytoplasm. The expression was related to the differentiation, metastasis, and location of the tumor. The lower the degree of differentiation, the higher the strongly-positive expression rate. The strongly-positive expression rate was significantly higher in cholangiocarcinoma specimens from patients with distant metastasis than in those without metastasis, suggesting that mAChR M3 plays important roles in the differentiation and metastasis of cholangiocarcinoma.
A study[4]reported that ACh may play an important role in the proliferation, differentiation, transformation and tumorigenesis of normal somatic cells. Many experiments have demonstrated ACh expression in a variety of tumors,[12,17]including lung cancer which is the most common malignant tumor, and other malignant tumors in various organs. In addition to carcinogenesis, diseases caused by cumulative factors with ACh involvement are far beyond the scope of nerve conduction for this excitatory neurotransmitter. It was reported that mAChR M3 is expressed in the cholangiocarcinoma cell line Mz-ChA-1, and that IP3 signaling is activated and intracellular Ca2+levels increase in cholangiocarcinoma cells cultured with the mAChR agonist choline carbonate.[18]At present, many studies[19]indicate that after stimulation by different molecules, IP3 and Ca2+levels play an important role in inhibiting the growth of bile duct cancer. Our study found that pilocarpine inhibited the proliferation of cholangiocarcinoma cells in a concentration-dependent manner, and the inhibitory effect gradually decreased with time. We conclude that the over-expression of mAChR M3 in cholangiocarcinoma and the secretion of ACh may be a cellular response and a defense mechanism against tumors. These results lead to a new understanding of ACh in the human body or broader biological systems acting two roles: ACh in non-neural cells as a local signaling molecule is involved in the regulation of cell morphology and function; and ACh in neurons as a neurotransmitter mediates rapid exchange between nerve cells and their effectors.
From this point of view, we speculate that the growth of cholangiocarcinoma may be regulated by the parasympathetic nervous system, which provides new approaches to the drug treatment of cholangiocarcinoma.
Contributors:FYJ, ZBY and LY designed the research. FYJ carried out the cytology and immunohistochemistry studies; YRY discussed the results and analyzed data. FYJ and LY wrote the paper. All authors read and approved the final manuscript. LY is the guarantor.
Funding:None.
Ethical approval:Not needed.
Competing interest:No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
1 Khan SA, Taylor-Robinson SD, Toledano MB, Beck A, Elliott P, Thomas HC. Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol 2002;37: 806-813.
2 Park YS, Cho NJ. Enhanced proliferation of SNU-407 human colon cancer cells by muscarinic acetylcholine receptors. BMB Rep 2008;41:803-807.
3 Frucht H, Jensen RT, Dexter D, Yang WL, Xiao Y. Human colon cancer cell proliferation mediated by the M3 muscarinic cholinergic receptor. Clin Cancer Res 1999;5: 2532-2539.
4 Wegener C, Hamasaka Y, Nässel DR. Acetylcholine increases intracellular Ca2+via nicotinic receptors in cultured PDF-containing clock neurons of Drosophila. J Neurophysiol 2004;91:912-923.
5 Schuller HM, Porter B, Riechert A. Beta-adrenergic modulation of NNK-induced lung carcinogenesis in hamsters. J Cancer Res Clin Oncol 2000;126:624-630.
6 Drell TL 4th, Joseph J, Lang K, Niggemann B, Zaenker KS, Entschladen F. Effects of neurotransmitters on the chemokinesis and chemotaxis of MDA-MB-468 human breast carcinoma cells. Breast Cancer Res Treat 2003;80:63-70.
7 Masur K, Niggemann B, Zanker KS, Entschladen F. Norepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by beta-blockers. Cancer Res 2001;61:2866-2869.
8 Radu A, Pichon C, Camparo P, Antoine M, Allory Y, Couvelard A, et al. Expression of follicle-stimulating hormone receptor in tumor blood vessels. N Engl J Med 2010;363:1621-1630.
9 Leu FP, Nandi M, Niu C. The effect of transforming growth factor beta on human neuroendocrine tumor BON cell proliferation and differentiation is mediated through somatostatin signaling. Mol Cancer Res 2008;6:1029-1042.
10 Natsis K, Paraskevas G, Papaziogas B, Agiabasis A. "Pes anserinus" of the right phrenic nerve innervating the serous membrane of the liver: a case report (anatomical study). Morphologie 2004;88:203-205.
11 Tsuneki K, Ichihara K. Electron microscope study of vertebrate liver innervation. Arch Histol Jpn 1981;44:1-13.
12 Trombino S, Cesario A, Margaritora S, Granone P, Motta G, Falugi C, et al. Alpha7-nicotinic acetylcholine receptors affect growth regulation of human mesothelioma cells: role of mitogen-activated protein kinase pathway. Cancer Res 2004; 64:135-145.
13 Miyazawa A, Fujiyoshi Y, Unwin N. Structure and gating mechanism of the acetylcholine receptor pore. Nature 2003; 423:949-955.
14 Sastry BV, Sadavongvivad C. Cholinergic systems in nonnervous tissues. Pharmacol Rev 1978;30:65-132.
15 Wessler I, Kirkpatrick CJ, Racké K. Non-neuronal acetylcholine, a locally acting molecule, widely distributed in biological systems: expression and function in humans. Pharmacol Ther 1998;77:59-79.
16 Klapproth H, Reinheimer T, Metzen J, Münch M, Bittinger F, Kirkpatrick CJ, et al. Non-neuronal acetylcholine, a signallingmolecule synthezised by surface cells of rat and man. Naunyn Schmiedebergs Arch Pharmacol 1997;355:515-523.
17 Song P, Sekhon HS, Proskocil B, Blusztajn JK, Mark GP, Spindel ER. Synthesis of acetylcholine by lung cancer. Life Sci 2003;72:2159-2168.
18 Elsing C, Hübner C, Fitscher BA, Kassner A, Stremmel W. Muscarinic acetylcholine receptor stimulation of biliary epithelial cells and its effect on bile secretion in the isolated perfused liver. Hepatology 1997;25:804-813.
19 McGill JM, Gettys TW, Basavappa S,Fitz G. GTP-binding proteins regulate high conductance anion channels in rat bile duct epithelial cells. J Membrane Biol 1993;133:253-261.
Accepted after revision February 25, 2012
Universities are full of knowledge; the freshmen bring a little in and the seniors take none away, so knowledge accumulates.
—Abbott Lawrence Lowell
April 9, 2011
Author Affiliations: Second Department of General Surgery (Feng YJ, Zhang BY and Lu Y) and Central Laboratory of Molecular Biology (Yao RY), Affiliated Medical College Hospital, Qingdao University, Qingdao 266003, China
Yun Lu, MD, Second Department of General Surgery, Affiliated Medical College Hospital, Qingdao University, 16 Jiangsu Road, Qingdao 266003, China (Tel: 86-532-82911362; Email: cloudylucn@126.com)
© 2012, Hepatobiliary Pancreat Dis Int. All rights reserved.
10.1016/S1499-3872(12)60201-X
 Hepatobiliary & Pancreatic Diseases International2012年4期
Hepatobiliary & Pancreatic Diseases International2012年4期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- Gastric- and intestinal-type marker expression in invasive ductal adenocarcinoma of the pancreas
- Expression of SOCS3 throughout liver regeneration is not regulated by DNA methylation
- Early changes of hepatic hemodynamics measured by functional CT perfusion in a rabbit model of liver tumor
- A common variant in the precursor miR-146a sequence does not predispose to cholangiocarcinoma in a large European cohort
- Hepatocyte differentiation of mesenchymal stem cells
- Early control of short hepatic portal veins in isolated or combined hepatic caudate lobectomy
