含10mol%氧化硅的钙磷酸盐玻璃陶瓷的合成及其体外生物活性
李文旭罗 燕赵智博于德珍王福平姜承慧
(1哈尔滨工业大学化学系,哈尔滨 150001)
(2哈尔滨工业大学材料科学与工程学院,哈尔滨 150001)
(3哈尔滨工业大学化工学院,哈尔滨 150001)
含10mol%氧化硅的钙磷酸盐玻璃陶瓷的合成及其体外生物活性
李文旭*,1罗 燕1赵智博1于德珍2王福平3姜承慧3
(1哈尔滨工业大学化学系,哈尔滨 150001)
(2哈尔滨工业大学材料科学与工程学院,哈尔滨 150001)
(3哈尔滨工业大学化工学院,哈尔滨 150001)
采用高分子网络凝胶法合成出SiO2-CaO-P2O5生物玻璃陶瓷,该材料具有较低含硅量和高钙磷比(nCa/nP=1.57)的特点,更接近人体硬组织的成分。将材料在SBF溶液浸泡研究材料的体外生物活性,通过TG/DTA,XRD,FTIR和SEM等方法对粉体和浸泡后的样品表面进行表征,ICP-AES对SBF溶液中钙、磷、硅离子的浓度进行检测。结果表明,氧化硅的添加有利于玻璃陶瓷表面磷灰石晶相的形成;随着浸泡时间的延长,沉积在样品表面的碳酸羟基磷灰石层逐渐由球型突起变为叶片状,溶液中钙、磷离子浓度降低,而硅离子浓度增加,说明材料具有良好的生物活性,适宜作为牙齿和骨骼的替代或修复材料。
生物玻璃陶瓷;高分子网络凝胶法;SiO2-CaO-P2O5;体外生物活性
0 Introduction
Bioactive glass-ceramicsarespecialsystems generally composed of SiO2,CaO and P2O5.In the early 1970s,Hench et al.[1]developed the first bioactive glasses,suggesting a new conceptof bioactive materials.Kokubo et al.[2]studied the bioactive behavior of the prepared bioactive glasses based on CaO-P2O5and CaO-SiO2.The results showed that the CaO-SiO2-based glasses formed an apatite layer on the surface in a simulated body fluid,which contributes to the bioactivity instead of the CaO-P2O5-based glasses,indicating that silica provides special useful sites for the apatite nucleation.
Inspired by the previous studies, recent researches have put more efforts on the bioactive glasses.Ma et al.[3]prepared 58S bioglass with the composition of 58 mol%SiO2,38 mol%CaO,and 4 mol% P2O5via the sol-gel technique at different sintering temperatures.After 7 days soaking in the SBF,the surface of the bioglass sintered at 700℃was fully covered by tiny spherical apatite particles,while that obtained at 1 200℃sintering was just partially covered with the apatite layer,which indicated that the crystallization decreased the bioactivity.Hong et al.[4]prepared BGC(bioactive glass ceramic)nanoparticles (nSi∶nCa∶nP≈66∶27∶7)via the combination of the sol-gel and coprecipitation processes.After soaking in the SBF (the simulated body fluid)for 14 days,the surfaces of BGC particles became coarser,which could be due to the formation of hydroxyapatite in the SBF solution,indicating their excellent bioactivity.Blamurugan et al.[5]developed the 58S bioactive glass(58wt%SiO2-33wt%CaO-9wt%P2O5) via the sol-gel technique.The bioactive properties of the material were determined through the immersion in the SBF solution and characterized by XRD.After soaking in the SBF for 7 days,the maximum diffraction due to the hydroxyapatite was detectable, which indicated the samples were biocompatible.In addition,since some trace elements such as Sr,Zn,Ag or Mg in the human body provide anabolic effects in bone metabolism[6-9],introduction of necessary trace elements into scaffold materials have been used to enhance their bioactivity.
Even though some bioglasses have shown good biological activities,the high content of silica made them different from the hard tissue of human body.In 2010,Leonardi et al.[10]reported the preparation of a novel phosphate-based glass ceramics with a small amount of silica:45%P2O5,3%SiO2,26%CaO,7%MgO,15%Na2O,and 4%K2O,by the melting method.After soaking in the SBF for 3 months,the globular agglomerates of a new phase were clearly distinguished,which confirmed thatthe material prepared was bioactive.Although the lower content of silica of the materials makes it closer to human hard tissues,the biological activity decreases compared with other bioactive materials.
At present,a few methods are utilized to prepare the bioactive glasses.The melting method,as a conventional approach for the glasses preparation,is simple and suitable for massive production[11-12].However,glasses synthesis by this classical method has its own shortcomings,such as composition inhomogeneities and escape of some volatile components such as P2O5asa resultofhightemperature operation[13].Wet chemical methods such as sol-gel and modified sol-gel(Pechini),due to their atomic scale of operation,are more commonly used to avoid from these shortcomings and to obtain glasses with high purity and homogeneity[14].Therefore many efforts have been devoted to the study of the sol-gel derived bioactive glasses containing SiO2,CaO and P2O5as the main components[15-18].
The polyacrylamide gel method used in the experiment is a further improvement of the modified sol-gel (Pechini).In contrasttotheprogressive transformation from viscous to resin in the classic solgel,this method is a time-saving method because the artificial gel formation at low temperature is rapid[19-20].It is also a cheap,reproducible and easily scaled up to obtain a number of fine powders in that the raw materials for polyacrylamide gel are inexpensive and the formation of gel is generally easy[21-22].
This study chooses a ternary system of SiO2-CaO-P2O5with the molar ratio of Si∶Ca∶P of 10 ∶55 ∶35 to prepare the glass-ceramics by a polyacrylamide-gel method.The results show that the glass-ceramics synthesized with a low content of silica and a high calcium-phosphorus ratio present positive biological activity,which could be potentially applied as bone or teeth repair materials or fillers.
1 Experimental
1.1 Synthesis
Firstly,calcium nitrate tetrahydrate (Ca(NO3)2·4H2O),ammonium dibasic phosphate((NH4)2HPO4)and Ethylsilicate (TEOS)was dissolved,respectively,in distilled water,and the Ca(NO3)2·4H2O solution was dropped into TEOS.Then proper citric acid and solution of (NH4)2HPO4were added into the vessel.After the clear sol was obtained,acrylamide (as the monomer)and N,N-methylenebisacrylamide(as lattice reagent)were added to the mixture,respectively.Then the initiator azobisisobutyronitrile was added at 80℃,and the white gels were formed.Finally the glass-ceramics powders were obtained after microwave drying for 10 min and sintering at 900℃for 6 h.
Then the prepared glass-ceramic powders were pressed in cylindrical forms at 10 MPa for 1 min to make the ceramic substrate with 13 mm diameter.Finally the substrate was calcined at 1 200℃for 2 h.
1.2 Characterization
The Thermo gravimetric and differential thermal analyses(TG/DTA,SDT 2960,USA)were carried out with a 10℃·min-1heating rate to 1 200℃ under an air atmosphere.The crystallization of the samples was characterized by X-ray diffraction (Philips Analytical X-ray B.V)usingCu Kα radiation (λ=0.154 06 nm)produced at 40 kV and 55 mA with a step size of 0.02°and a scan peed of 5°·min-1.
In vitro tests were performed by immersing the samples in SBF at 37℃in sterile incubator.During immersion,a constant solid superficial area/liquid volume(0.1 cm-1)ratio[10]was maintained.After being immersed for 7 days and 14 days,samples were rinsed with deionized water and acetone and then dried in air at room temperature.The concentration of calcium,phosphorus and silica in the SBF was monitored by inductively coupled plasma atomic emission spectrometry techniques (ICP-AES;IRISⅡ XSP;USA).The crystallization and structure changes of the samples surface were determined by XRD as described above.The microstructure of the samples immersed in the SBF solution was observed by a scanning electron microscope (SEM,HITACHI S-4800)after being gold sprayed.The IR spectra were measured with a Fourier transform infrared(FTIR,NEXUS-670,THERMONICOLET,USA)atroom temperature.
2 Results and discussion
2.1 Powder and pieces characterization
Fig.1 shows the TG/DTA curves of the prepared dried gel.The 10%weight loss between 30℃and 300℃could be associated with the evaporation of physically absorbed water.The weight loss of 55%correlated to two big exothermic peaks at 336℃and 447℃,respectively,in the DTA curve could be due to the loss of organics (i.e.alkoxy group and carbon chain).The TG curve is stable after 500℃,indicating that almost all water absorbed by the gel powder and organics introduced during the experimental processes have been removed completely.In addition,an endothermic peak around 640℃shown on DTA curve could be due to the glass transition,and a small exothermal peak around 850℃observed might be attributed to the crystallization of the powder.Hence we chose 900℃as the sintering temperature to make the powder partially crystallized.

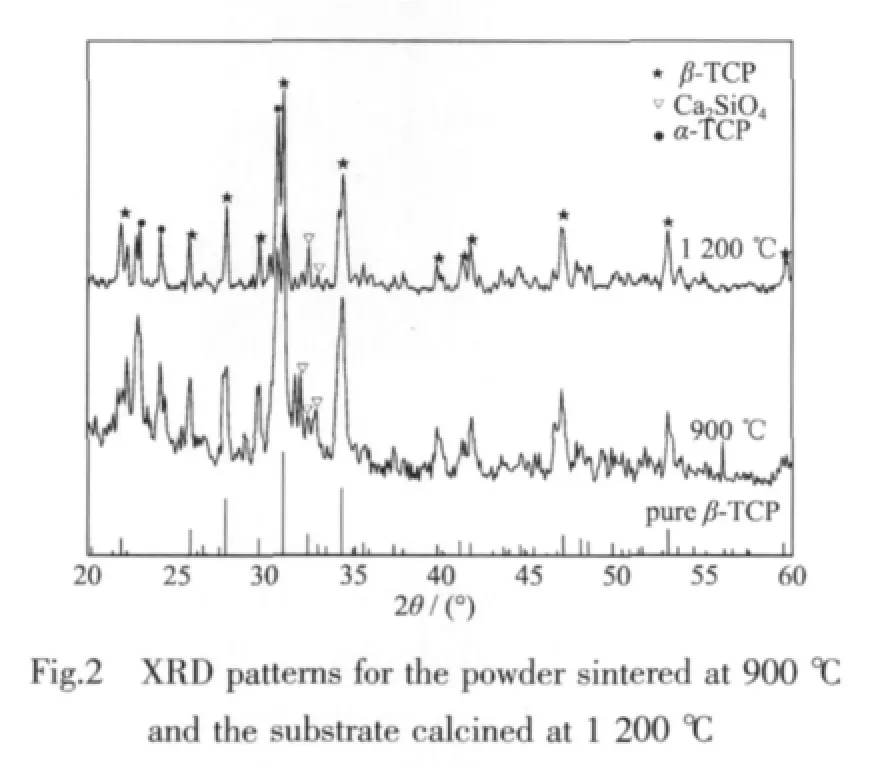
There are only broad peaks presented in XRD pattern of the prepared powder sintered at 900℃,implying its low crystallinity(Fig.2(a)).The intensive diffraction peaks at 2θ=27.76°,31.04°,34.38°associated with β-TCP (PDF#70-2065)and weak diffraction peaks at 2θ=24.16°,22.90°,30.74°corresponding to α-TCP(PDF#70-0364)are detected,suggesting that the β-TCP is the principal phase.In addition,the diffraction peaks at 2θ=32.10°,32.44°,33.00°corresponding to Ca2SiO4(PDF#29-0369)are also observed as a result of the addition of silica.
The maximum XRD diffraction peak of the glassceramic substrate becomes sharper and more independent after calcined at 1 200 ℃ (Fig.2(b)),indicating the further crystallization of the glassceramics.There are no obvious changes of the phase composition,suggesting the β-TCP is stillthe principal phase;similar diffraction peaks associated with Ca2SiO4(PDF#29-0369)and α-TCP (PDF#70-0364)are also seen in the XRD pattern.
2.2 In vitro bioactivity test
To determine the bioactivity of the prepared materials,an in vitro test was carefully designed by using the SBF solution.The XRD patterns of the glass-ceramics before and after immersion in the SBF are compared in Fig.3.Before immersion,the XRD peaks are sharp and independent.After immersed for 7 days,few non-crystalbroad diffraction peaks between 30°and 32°corresponding to the main peaks(211), (112)and (300)and few minorpeaks corresponding to the(222)and(213)of hydroxyapatite(PDF#09-0432)appear,suggesting the formation of an apatite phase.In addition, the reflections corresponding to the β-TCP decrease,and the reflections corresponding to the Ca2SiO4are not detectable probably due to the dissolution of Ca2SiO4or the protection of Ca2SiO4by the apatite layer formed after the soaking.

FTIR spectra ofthe resulting glass-ceramic material after immersed in the SBF for different times are presented in
Fig.4.The broad and strong absorption band at around 1 020 cm-1could be ascribed to the stretch vibration of P-O.The small band appeared at 750~800 cm-1is the typical absorption band of the symmetric stretch vibration of Si-O-Si.

After immersed in the SBF,the stretch vibration of P-O around 1 020 cm-1becomes significantly intensified,while the weak vibration around 1 650 cm-1due to the H-O and CO32-and adsorption bandsat around 1 460,1 420 and 868 cm-1appear.The FTIR spectra do not only suggest the formation of an apatite-like layer but also carbonate hydroxyapatite(CHA)as its composition is.With the extension of the immersion time,the absorption bands of H-O and CO32-intensify and become sharper,while the stretch vibration of Si-O-Si at 750~800 cm-1decreases,further confirming the increased generation ofthe CHA particles.
The surface micrographs of the resulting glassceramic material before and after immersion in the SBF are presented in Fig.5.Before immersion,the surface of the sample is smooth (Fig.5(a,b));after immersed for 7 days,precipitation starts to take place from the initial separate granules to a dense layer with time (Fig.5(c)).High-magnication SEM image(Fig.5(d))further reveals that each spherical bump is consisted of a large number of tiny flake-like crystals of Ca-P similar to the findings reported earlier[23].After immersed for 14 days,the precipitates grow to blades scattered on the surface of the samples evenly,and the superficial area of the blades is about 0.25 μm2with a thickness of 50 nm(Fig.5(e,f)).The change in surface morphology is attributed to the formation of an apatite layer as confirmed by the results of XRD.

The concentrations of Ca,P and Si ions in the SBF solution was measured by inductively coupled plasma atomic emission spectrometry techniques(ICPAES)and are shown in Fig.6.From the Fig.6,one can see that the concentration of Si ion increases(4.106→4.969 →5.385 →5.59 attributed to the dissolution of the Ca2SiO4,and that of P iron decreases (2.604→2.252→2.139→1.927)likely due to the precipitation of an apatite layer.The concentration of Ca iron is also decreased (86.67→85.03→82.69→75.98),butthe rate is different from that of P.This is because that the concentration of Ca ion is controlled by two opposite processes:the dissolution of Ca2SiO4makes it increased whereas the precipitation of an apatite layer makes it decreased.
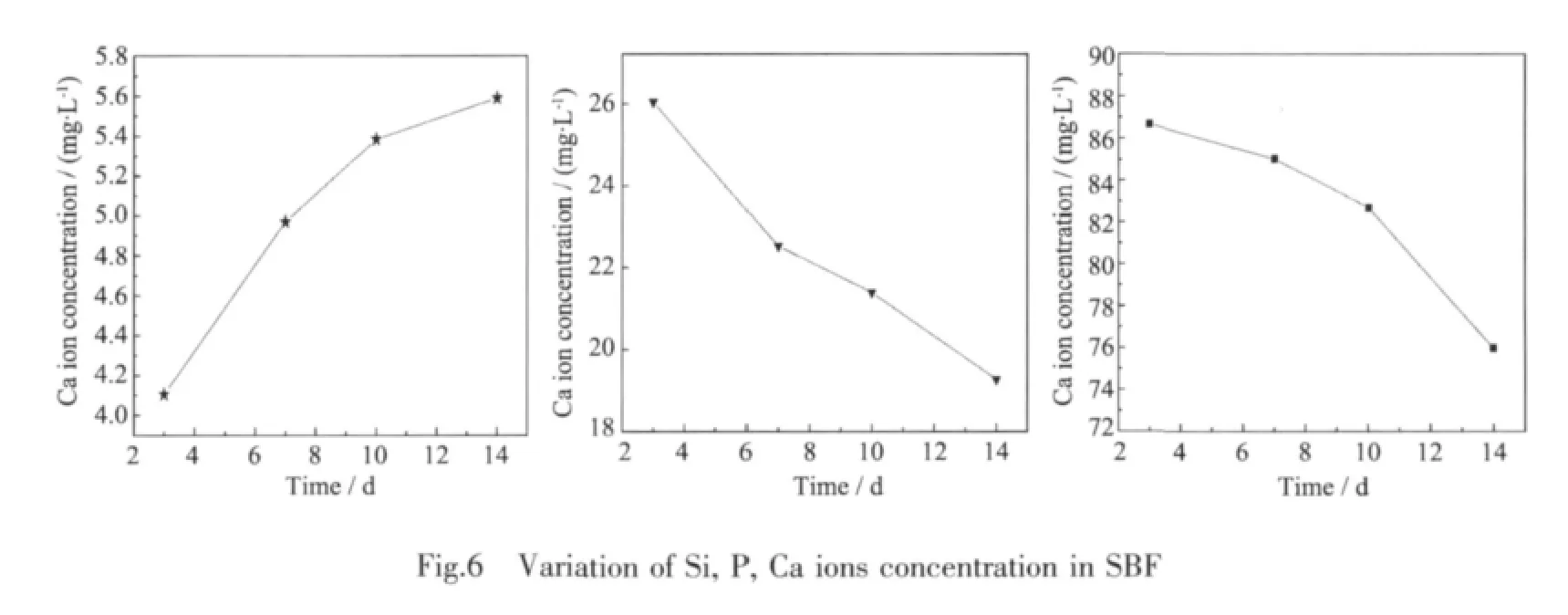
All the results of the characterization mentioned above(XRD,FTIR,SEM and ICP-AES)prove that the surface of the sample is covered with a layer of carbonate hydroxyapatite (CHA),which indicates that the prepared glass-ceramic material has good bioactivity.
2.3 Process discussion of the apatite precipitation
The change trend of the concentrations of Ca,P and Si ions in the SBF solution with time is in agreement with that reported by Hench and Ckark[24].They described that when the bioactive materials were exposed into the SBF solution,the bonding between them to bone would occur through a series of surface reactions.In general,silica provides a good nucleation site for the formation of the apatite as follows.The Si component on the surface of the sample firstly reacts with water,and then the silica network of≡Si-O-Si≡is depredated to lead to the formation of Si-OH groups at the glass-solution interface.The mechanism of the apatite formation induced by silanol involves electrostatic interactions[25-26].Since the pH value of the SBF (7.40)is much greater than the isoelectric point of the silica(2.0),the≡Si-OH groups formed at the interface could readily react as below:≡Si-OH+OH-=≡Si-O-+H2O.As a result,a negative charge is formed at the interface,which enhances electrostatic interaction with the positively charged Ca ions in the fluid.The surface then acquires a positive charge by accumulation of calcium ions.The positive charged surface continually interacts electrostatically with the negatively charged P ions in the SBF,consequently leading to the formation of an amorphous calcium phosphate[25,27].As long as the apatite nuclei are formed[25],they grow spontaneously by consuming the Ca and P ions from the surrounding body fluid.Finally, the amorphous calcium phosphate incorporates OH-and CO32-ions from the solution to form a CHA layer.Hence the existent of silica provides the favorable site for the nucleation of the apatite.
TheCHA isabone-like apatite,which is conductive to the adhesion of the cells for further proliferation as well as new bone formation and reconstruction[28].Therefore,the formation of the CHA on the surfaces of the biomaterials is an important indicator to determine their biological activity.
3 Conclusions
In this study,silica was evenly added into the material by the polyacrylamide gel method.The glassceramic powder with a principal phase of β-TCP was obtained by sintering at 900℃,and the glassceramics were then prepared by calcining at 1 200℃.The resulting glass-ceramic materialwith a low content of silica and a high calcium-phosphorus ratio of 1.57 is closer to the hard tissues of organism.
The results of the in vitro biological activity tests show that with the extension of immersion time,the concentration of Ca and P ions in the SBF solution decreases while the concentration of Si increases.In addition,after immersing for 7 days,the surface of the sample is covered with a dense apatite layer;after immersing for 14 days,the blade-like precipitate with the superficial area of 0.25 μm2and the thickness of 50 nm are firmly deposited,which provides the material prepared with significant physiological response and biological activity.
[1]Hench L L,Splinter R J,Allen W C,et al.J.Biomed.Mater.Res.,1972,2:117-121
[2]Ohtsuki C,Kokubo T,Yamamuro T.J.Non-Cryst.Solids.,1992,143:84-92
[3]Ma J,Chen C Z,Wang D G,et al.Ceram.Int.,2010,36:1911-1916
[4]Hong Z K,Liu A X,Li C.J.Non-Cryst.Solids.,2009,355:368-372
[5]Balamurugan A,Sockalingum G J M,et al.Mater.Lett.,2006,60:3752-3757
[6]Saeed Hesaraki,Mozhdeh Gholami,Sadaf Vazehrad,et al.Mater.Sci.Eng.C,2010,30:383-390
[7]Balamurugan A,Balossier G,Kannan S,et al.Acta.Biomater.,2007,3:255-262
[8]Salinas A J,Román J,Vallet-RegíM,et al.Biomaterials.,2000,21:251-257
[9]Balamurugan A,Balossier G,Laurent-Maquin D,et al.Dent.Mater.,2008,24:343-1351
[10]Leonardi E,Ciapetti G,Baldini N,et al.Acta Biomater.,2010,6:598-606
[11]Franks K,Abrahams I,Georgious G,et al.Biomaterials,2001,22:497-501
[12]Santos J D,Silva P L,Knowles J C,et al.J.Mater.Sci.:Mater.Med.,1996,7:187-189
[13]Brink M,Turunen T,Haponen R P,et al.J.Biomed.Mater.Res.Part A,1997,114:114-121
[14]Zhong J P,Greenspan D C,Biomed J.J.Biomed.Mater.Res.Part B,2000,53:694-701
[15]Coleman N J,Hench L L.Ceram.Int.,2000,26:179-186
[16]Peitl O,Zanotto E D,Hench L L.J.Non-Cryst.Solids.,2001,292:115-126
[17]Sepulveda P,Jones J R,Hench L L.J.Biomed.Mater.Res.,2002,61(2):301-311
[18]ZHANG Xiao-Kai(张晓凯),LIU Wei(刘玮),CHEN Xiao-Feng(陈晓峰).Acta Phys.-Chim.Sinica(Wuli Huaxue Xuebao),2004,17(4):495-498
[19]Song Y,Sun Q,Zhao L R,et al.Mater.Chem.Phys.,2009,113:645-649
[20]Tarancon A,Dezanneau G,Arbiol J,et al.J.Power Sources,2003,118:256-264
[21]Tahmasebpour M,Babaluo A A,Aghjeh M K R.J.Eur.Ceram.Soc.,2008,28:773-778
[22]Tahmasebpour M,Babaluo A A,Shaei S,et al.Powder Technol.,2009,191:91-97
[23]Fu X,Zhang H,Niu S,et al.J.Solid State Chem.,2005,178:603-607
[24]Zhang Y L,Mizuno M,Yanagisawa M,et al.J.Mater.Res.,2003,18:433-441
[25]HenchLL,CkarkDE.J.Non-Cryst.Solids.,1978,28:83-85
[26]Takadama H,Kim H M,Kokubo T,et al.Chem.Mater.,2001,13:1108-1113
[27]Takadama H,Kim H M,Miyaji F,et al.J.Ceram.Soc.Jpn.,2000,108(2):118-121
[28]Vallés Lluch A,Gallego Ferrer G,Monleón Pradas M.Polymer,2009,50:2874-2884
[29]Ducheyne P,Qiu Q.Biomaterials,2004,20:2287-2303
Synthesis and in vitro Bioactivity of Calcium-Phosphate Bioglass-Ceramic with 10mol%Silica
LI Wen-Xu*,1LUO Yan1ZHAO Zhi-Bo1YU De-Zhen2WANG Fu-Ping3JIANG Cheng-Hui3
(1Department of Chemistry,Harbin Institute of Technology,Harbin,150001,China)
(2School of Materials Science&Engineering,Harbin Institute of Technology,Harbin,150001,China)
(3School of Chemical Engineering&Technology,Harbin Institute of Technology,Harbin,150001,China)
The bioactive glass-ceramics based on a SiO2-CaO-P2O5system were synthesized using the polyacrylamide-gel method.The prepared glass-ceramic material is attractive for its low content of silica and high calciumphosphorus molar ratio of 1.57,which is closer to the composition of hard tissues in human body.The bioactivity of the material was assessed by simply immersing it in the simulated body fluid(SBF)for different time durations.The prepared powders and the samples surface after the immersion were characterized by using TG/DTA,XRD,FTIR and SEM techniques.The concentration of Ca,P and Si ions in the SBF were measured by ICP-AES.The results show that the addition of silica contributes to the formation of the apatite on the surface of the glass-ceramics.With the extension of immersion time,the carbonated hydroxyl apatite(CHA)layer deposited is changed from spherical bumps to blades,and the concentration of calcium and phosphorus in the SBF increases while that of silica decreases.The above results indicate that the prepared glass-ceramic material has good bioactivity and may be used as bone and teeth repair material or filler.
bioglass-ceramic;polyacrylamide-gel method;SiO2-CaO-P2O5;in vitro bioactivity
TB321;O611.4
A
1001-4861(2012)10-2264-07
2012-03-23。收修改稿日期:2012-04-10。
哈尔滨工业大学科研创新基金(No.HIT.NSRIF.2010066);黑龙江省自然科学基金(No.E201006)和黑龙江省科技攻关课题(No.GC10A107)资助项目。
*通讯联系人。E-mail:liwx@hit.edu.cn

