介孔硅与大孔硅的结构、电学和气敏特性
孙 鹏 胡 明 李明达 马双云
(天津大学电子信息工程学院,天津300072)
介孔硅与大孔硅的结构、电学和气敏特性
孙 鹏*胡 明 李明达 马双云
(天津大学电子信息工程学院,天津300072)
采用双槽电化学腐蚀法在P型单晶硅表面制备两种多孔硅.根据它们的孔径将它们分为介孔硅和大孔硅.使用扫描电子显微镜(SEM)观察两种多孔硅表面和断面形貌,介孔硅和大孔硅的表面化学键用傅里叶变换红外(FTIR)光谱仪来研究,通过I-V特性测试表征两种多孔硅电学特性,随后在室温下测试其气敏特性.结果表明:介孔硅具有较高的气体灵敏度,大孔硅具有较好的气体响应恢复特性.介孔硅对NO2气体具有较好的选择性,大孔硅对NH3气体具有较好的选择性.
介孔硅;大孔硅;室温;气敏特性
1 Introduction
Several materials with large specific surface areas,such as polymer membranes,1carbon nanotubes,2,3and metal oxide4-6have been studied as prospective candidates for future gas sensors.Porous silicon(PS)is an especially interesting material with large specific surface area,7which is easily produced by electrochemical process.8,9PS formed by this method have diameters varying from tens nanometers10-12to a few of micrometers.13,14It has been classified according to the pore size as micro-PS(≤2 nm),meso-PS(2-50 nm),and macro-PS(>50 nm).15As the specific area of PS may be as high as 700-800 m2·cm-3,16it can be used as gas sensor.Recently,many chemical sensors using PS have been reported to be operated at room temperature,17-19which is not possible for other semiconductor gas sensors.Additionally,it is easy to fabricate with an advantage of controlling the surface morphology through the varia-tion of the formation parameters.Most importantly,PS is compatible with silicon integration technology.20
However,PS contains a large number of interface states and volume traps.This leads to the instability of electro-physical parameters of gas sensor based on porous silicon with poor gas sensing properties due to the significant Fermi-level pinning.21To overcome the defects of PS,a few studies on the modified layer and the catalyst deposition on porous silicon surface were reported.22-24Unfortunately,reversibility and response-recovery time are still the two issues which restrict the PS for practical applications.Actually,the changing of the microstructure of the PS may bring large variation in the gas sensing properties. However,there are only a few articles reporting on the relationship between the microstructure and the gas sensing properties.25,26
In this work,meso-PS and macro-PS were made.We investigated the gas sensing properties of PS at room temperature and focused on both response-recover characteristics and the effect of the microstructure on sensitivity.
2 Experimental
Meso-PS and macro-PS were formed by electrochemical corrosion27of a(100)-oriented mono-crystalline P+-type silicon wafer with 1×10-2-2×10-2Ω·cm resistivity and a(100)-oriented mono-crystalline P-type silicon wafer with 10-15 Ω·cm resistivity in a double-tank cell,respectively.The mass fractions of HF,C2H5OH,and dimethylformamide(DMF)were 40%, 99.7%,and 99.5%,respectively.The preparing conditions of the PS are shown in Table 1.
Pt electrodes of dimensions 3 mm×3 mm were deposited on the top of PS gas sensor using dc magnetron sputtering method.The base pressure was 5×10-4Pa and the sputtering pressure was 2 Pa.The sputtering gas was Ar.The sputtering power was 90 W.The sputtering time was 10 min.Fig.1 is the schematic diagram of the PS gas sensor.


Table 1 Preparing conditions of the meso-PS and macro-PS

Fig.1 Schematic diagram of the PS gas sensor
The average porosity(ε),i.e.,the void fraction in the porous layer,can be obtained by gravimetry via Eq.(1): where m1is the mass of the sample before the etching,m2is the mass of the sample after the formation of the PS layer,and m3is the mass of the sample after the removal of the PS layer in KOH solution(1%,mass fraction).The electronic balance (AR1400,OHAUS,USA)has an accuracy of 0.0001 g.The morphology of PS layers was measured by a field emission scanning electron microscope(FESEM,Nanosem 430,FEI, USA).The surface chemical bonds of the PS were studied by Fourier transform infrared spectroscopy(FTIR,NEXUS470, Thermo,USA).Current-voltage(I-V)characteristics of the PS samples were tested by potentiostat(ZF-9,Zheng Fang Electronics Corporation,China).Gas sensing characteristics of PS samples was measured at room temperature.In this process, they were placed in a chamber of 30 L.Test gas was precisely inserted by using an injector.The gas detection sensitivity(S) of the sensor device has been evaluated using the relation:

where Rr,Ro,and Raare resistances of the sensor device in reducing gas,oxidizing gas,and the ambient atmosphere,respectively.
The gas baseline shift(T)of the sensor device has been evaluated using the relation:

where Rinand Rreare resistances of the sensor device before gas injected and after gas removed,respectively.
3 Results and discussion
3.1 Morphology of meso-PS and macro-PS samples
Fig.2(a,b)show the SEM photographs of two different kinds of PS layers with a symmetrical hole-shaped frame.It can be seen that the average pore sizes are 20 nm and 1.5 μm, respectively,so the two different kinds of PS samples respectively belong to meso-PS and macro-PS.Fig.2(c,d)present the SEM images of the cross-section of PS.The meso-PS shows a tree branch porous silicon structure,while the macro-PS shows an orderly uniform porous silicon structure.The porosity is about 76.8%and 39.3%for meso-PS layer and macro-PS,respectively.As there is lots of silica which has not been etched, the macro-PS has lower porosity.
3.2 FTIR analysis of meso-PS and macro-PS samples
FTIR spectra of the meso-PS and macro-PS in a range of 850-2200 cm-1are shown in Fig.3.Both the spectra of the meso-PS and macro-PS demonstrate the peaks of Si―O―Si stretching mode(1020-1260 cm-1)and the weak peaks of Si―Hxoscillation mode(deformation mode around 850-900 cm-1and stretching mode around 2100 cm-1),which agree well with literature.28It is noted that the density of the surface chemical bonds of meso-PS is larger than that of the macro-PS extending on the entire spectral range.The reason for the phenomenon is that the specific surface area of the meso-PS is larger than that of the macro-PS.As the meso-PS has more surface chemical bonds,more gas can be absorbed on its surface and its sensitivity may be higher than that of the macro-PS.

Fig.2 SEM images of the meso-PS(a,c)and macro-PS(b,d) (a,b)surface morphologies;(c,d)cross-section morphologies
3.3 I-V characteristics of meso-PS and macro-PS samples
Fig.4 shows I-V characteristics of the meso-PS and macro-PS.The voltage was applied between the two Pt electrodes. As the work function of Pt is larger than that of the PS,there is a high-conductance region on the surface of PS.The electrical behavior of the I-V curves exhibits an ohmic contact.The I-V characteristic curves are linear.It also can be seen from Fig.4 that the resistance of the meso-PS is higher than that of the macro-PS.On one hand,by increasing the porosity,the surface area increases.The higher surface area causes higher internal length for flowing charge careers and the surface resistivity becomes higher.On the other hand,as the porosity increases,the effect of the carriers captured by the pore wall is enhanced.So the resistance of the meso-PS is higher than that of the macro-PS.

Fig.3 FTIR spectra of the meso-PS(a)and a range of 850-2200 cm-1
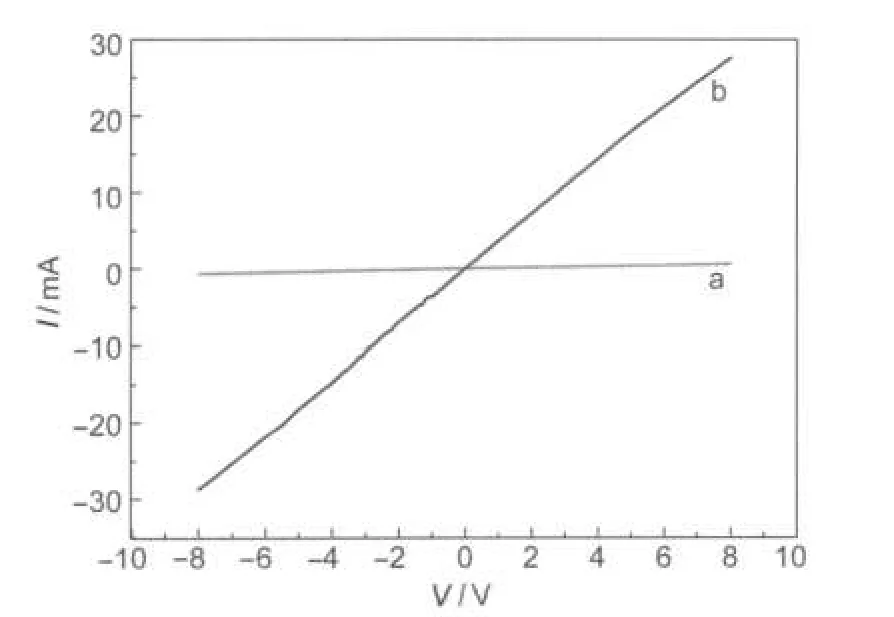
Fig.4 I-V characteristic curves of the meso-PS(a)and macro-PS(b)
3.4 Gas sensing properties of meso-PS and macro-PS samples

Fig.5 Sensitivity(S)curves of the meso-PS(a)and macro-PS(b) to 5×10-5(volume fraction)NH3at room temperature S=Rr/Ra
The gas sensing properties of the meso-PS and macro-PS towards 5×10-5(volume fraction)NH3were tested at room temperature.As shown in Fig.5 the resistance values of PS gas sensors increase to stable values at first when adsorbing NH3.After disengagement from the gas environment,the resistance values decrease gradually to stable values.As NH3is a reducing gas,it makes local and partial reduction of PS surfaces which causes the decrease of defect density.These defects play the role in electron traps into which electrons relax.29,30The decrease of the number of electron traps will induce a large number of free electrons on the surfaces of PS and the number of holes reduces greatly,so that resistance values increase.Therefore,it seems that the electron traps which are diminished by partial reduction of PS in the presence of NH3are responsible for the decrease of free charge carriers in P-type PS.
The gas sensing properties to NH3are shown in Table 2.As shown in Table 2,the sensitivity of meso-PS to NH3is much higher than that of macro-PS.It is also clear from Table 2 that the macro-PS shows faster response-recovery than the meso-PS at room temperature.The porosity of meso-PS is 76.8%, while for macro-PS it is 39.3%.The relative higher porosity can result in higher specific surface area.Thus the meso-PS can absorb more gas,and show higher sensitivity.From the SEM images shown in Fig.2(c,d),it is obvious that the pores of macro-PS are more orderly and uniform than those of meso-PS.It may be the reason for the better response-recovery characteristics of macro-PS.31
As the poor reversibility of the PS,the resistance can not often recover to the initial value.32So there are only a few articles32-34which reported good dynamic response of PS.But in our research,macro-PS shows a good dynamic response characteristic.Fig.6 shows the dynamic responses of macro-PS to NH3gas in various concentrations.As shown in this figure,the measured resistance increases upon exposure to NH3gas.Notably,the resistance could almost recover to its initial value after removal of NH3,indicating a good reversibility of this material.The sensitivity values of the macro-PS upon exposure to 25×10-6,50×10-6,100×10-6,and 200×10-6(volume fraction) NH3are 2.90,4.20,10.24,and 11.65,respectively.
Selectivity is an important factor in gas sensing,and the sensor has to present rather high selectivity for its application.It isknown to all that a PS sensor responds to other gases as well. For this reason,we investigated the sensitivity of the meso-PS and macro-PS sensors to other gases at room temperature.The gas sensitivity of meso-PS and macro-PS to various gases at room temperature are compared and the results are shown in Fig.7.It can be seen that the sensitivity of the meso-PS to NO2is much higher than that to other gases,while the sensitivity of the macro-PS to NH3is much higher than that to other gases. Furthermore,the sensitivity of meso-PS to all of these gases is higher than that of macro-PS,especially to NO2.The different selectivity characteristics of different PS may relate to the dangling bonds on the PS surface.As it is shown in the Fig.3,the peaks of Si―Hxand Si―O―Si bonds on the surface of meso-PS are stronger than those of the macro-PS.The Si―Hxbonds and Si―O―Si bonds play a major role in the adsorption of NO2and NH3,35,36respectively.Therefore,the sensitivity of the meso-PS to testing gases is higher than that of the macro-PS.It is also shown that the density of Si―Hxbonds on the macro-PS surface is very small.That may explain the selectivity of the macro-PS to NH3.

Table 2 Gas sensing properties of meso-PS and macro-PS to NH3

Fig.6 Dynamic responses of macro-PS to various NH3 concentrations at room temperature

Fig.7 Sensitivity of meso-PS(a)and macro-PS(b)to various gases(5×10-5)at room temperature
4 Conclusions
Meso-PS and macro-PS layers were fabricated respectively on single crystal P+-type silicon and P-type silicon surfaces by electrochemical corrosion in a double-tank cell.Then Pt electrodes were deposited on PS layers by dc facing target magnetron sputtering.The normal pore sizes of the meso-PS and macro-PS are about 20 nm and 1.5 μm,respectively.The macro-PS shows an orderly uniform porous silicon structure.The I-V characteristic curves are linear.The meso-PS shows higher sensitivity,and the macro-PS shows better response-recovery characteristics.The two different kinds of PS exhibit different selectivity.
(1) Gao,Y.;Li,X.;Gong,J.;Fan,B.;Su,Z.;Qu,L.J.Phys.Chem. C 2008,112,8215.
(2) Li,W.;Hoa,N.D.;Kim,D.Sensor Actuat.B-Chem.2010,149, 184.
(3) Slobodian,P.;Riha,P.;Lengalova,A.;Svoboda,P.;Saha,P. Carbon 2011,49,2499
(4) Calestani,D.;Zha,M.;Mosca,R.;Zappettini,A.;Carotta,M. C.;Natale,V.D.;Zanotti,L.Sensor Actuat.B-Chem.2010,144, 472.
(5) Qin,Y.X.;Hu,M.;Zhang,J.Sensor Actuat.B-Chem.2010, 150,339.
(6)Yu,L.M.;Fan,X.H.;Qi,L.J.;Ma,L.H.;Yan,W.Appl.Surf. Sci.2011,257,3140.
(7) Ozdemir,S.;Gole,J.L.Curr.Opin.Solid State Mat.Sci.2007, 11,92.
(8) Jalkanen,T.;Tuura,J.;Mäkilä,E;Salonen,J.Sensor Actuat. B-Chem.2010,147,100.
(9)Kim,H.J.;Kim,Y.Y.;Lee,K.W.Curr.Appl.Phys.2010,10, 181.
(10)Yang,H.B.;Hu,M.;Liang,J.R.;Zhang,X.R.;Liu,Z.G.Acta Phys.-Chim.Sin.2008,24,1017.[杨海波,胡 明,梁继然,张旭瑞,刘志刚.物理化学学报,2008,24,1017.]
(11) Vinod,P.N.J.Alloy.Compd.2009,470,393.
(12) Sun,F.Y.;Hu,M.;Sun,P.;Zhang,J.;Liu,B.J.Nanosci. Nanotechnol.2010,10,7739.
(13) Tian,J.;Zheng,D.;Zhang,X.G.;Zhang,B.H.;Xia,B.J.; Yang,H.Acta Phys.-Chim.Sin.2007,23,68.[田 娟,郑 丹,张熙贵,张宝宏,夏保佳,杨 辉.物理化学学报, 2007,23,68.]
(14) Esmer,K.;Kayahan,E.Appl.Surf.Sci.2009,256,1548.
(15) Canham,L.T.Appl.Phys.Lett.1990,57,1046.
(16)Ali,N.K.;Hashim,M.R.;Aziz,A.A.Solid State Electron. 2008,52,1071.
(17) Martinez,H.M.;Rincon,N.E.;Torres,J.;Alfonso,J.E. Microelectron.J.2008,39,1354.
(18) Lewis,S.E.;DeBoer,J.R.;Gole,J.L.;Hesketh,P.J.Sensor Actuat.B-Chem.2005,110,54.
(19) Khoshnevis,S.;Dariani,R.S.;Azim-Araghi,M.E.;Bayindir, Z.;Robbie,K.Thin Solid Films 2006,515,2650.
(20) Barillaro,G.;Strambini,L.M.Sensor Actuat.B-Chem.2008, 134,585.
(21) Mizsei,J.Thin Solid Films 2007,515,8310.
(22) Luongo,K.;Sine,A.;Bhansali,S.Sensor Actuat.B-Chem. 2005,111-112,125.
(23) Galstyan,V.E.;Martirosyan,K.S.;Aroutiounian,V.M.; Arakelyan,V.M.;Arakelyan,A.H.;Soukiassian,P.G.Thin Solid Films 2008,517,239.
(24) Chakane,S.;Gokarna,A.;Bhoraskar,S.V.Sensor Actuat. B-Chem.2003,92,1.
(25) Boarino,L.;Baratto,C.;Geobaldo,F.;Amato,G.;Comini,E.; Rossi,A.M.;Faglia,G.;Lérondel,G.;Sberveglieri,G.Mater. Sci.Eng.B-Adv.2000,69-70,210.
(26) Kanungo,J.;Saha,H.;Basu,S.Sensor Actuat.B-Chem.2010, 147,145.
(27)Fang,Z.Q.;Hu,M.;Zhang,W.;Zhang,X.R.;Yang,H.B.Thin Solid Films 2009,517,2930.
(28) Du,X.W.;Jin,Y.;Zhao,N.Q.;Fu,Y.S.;Kulinich,S.A.Appl. Surf.Sci.2008,254,2479.
(29) Razi,F.;Rahimi,F.;Zad,A.I.Sensor Actuat.B-Chem.2008, 132,40.
(30) Xie,F.Q.;von Blanckenhagen,P.Appl.Phys.Lett.1999,75, 3144.
(31) Salgado,G.G.;Becerril,T.D.;Santiesteban,H.J.;Andrés,E. R.Opt.Mater.2006,29,51.
(32) Pancheri,L.;Oton,C.J.;Gaburro,Z.;Soncini,G.;Pavesi,L. Sensor Actuat.B-Chem.2004,97,45.
(33) Massera,E.;Nasti,I.;Quercia,L.;Rea,I.;Di Francia,G.Sensor Actuat.B-Chem.2004,102,195.
(34) Baratto,C.;Faglia,G.;Comini,E.;Sberveglieri,G.;Taroni,A.; La Ferrara,V.;Quercia,L.;Di Francia,G.Sensor Actuat. B-Chem.2001,77,62.
(35) Yang,G.;He,J.T.;Li,X.J.;Liang,E.J.Journal of Transducer Technology 2004,23,7.[杨 光,何金田,李新建,梁二军.传感器技术,2004,23,7.]
(36)Urbanowicz,A.M.;Shamiryan,D.;Zaka,A.;Verdonck,P.;De Gendt,S.;Baklanov,M.R.J.Electrochem.Soc.2010,157, H565.
October 13,2011;Revised:November 16,2011;Published on Web:November 21,2011.
Microstructure,Electrical and Gas Sensing Properties of Meso-Porous Silicon and Macro-Porous Silicon
SUN Peng*HU Ming LI Ming-Da MA Shuang-Yun
(School of Electronic and Information Engineering,Tianjin University,Tianjin 300072,P.R.China)
Two different kinds of porous silicon(PS)were grown by electrochemical corrosion in a double-tank cell on the surface of single-crystalline P-type silicon.According to their pore sizes,they were denoted as meso-porous silicon(meso-PS)and macro-porous silicon(macro-PS).The surface and cross-section morphologies of the PS were observed by scanning electron microscope(SEM).The surface chemical bonds of the meso-PS and macro-PS were investigated by Fourier transform infrared(FTIR) spectroscopy.The electrical properties of the PS were studied by measuring the I-V characteristics.The gas sensing properties of meso-PS and macro-PS were thoroughly measured at room temperature. Meso-PS has much higher sensitivity,while macro-PS has better response-recovery characteristics. Meso-PS shows better selectivity for NO2,while macro-PS shows better selectivity for NH3.
Meso-porous silicon;Macro-porous silicon;Room temperature;Gas sensing property
10.3866/PKU.WHXB201111212www.whxb.pku.edu.cn
*Corresponding author.Email:sunpengtju@163.com;Tel:+86-13821193235.
The project was supported by the National Natural Science Foundation of China(60771019)and Tianjin Key Research Program ofApplication Foundation andAdvanced Technology,China(11JCZDJC15300).
国家自然科学基金(60771019)和天津市应用基础及前沿技术研究计划(11JCZDJC15300)资助项目
O647

