Preparation and Properties of a New Slurry Cast High Energy AP/Al/CMDB Propellant
FAN Xue-zhong,WANG Han,LI Ji-zhen,ZHANG Wei ,WEI Hong-jian,LIU Xiao-gang
(1.Xi′an Modern Chemistry Research Institute,Xi′an 710065,China;2.Science and Technology on Combusition and Explosion Laboratory,Xi′an Modern Chemistry Research Institute,Xi′an 710065,China)
Introduction
CMDB propellants containing AP and Al(AP/Al/CMDB propellants),which combine the merits such as higher theoretical specific impulse(Isp),excellent burning performance and mechanical properties,as well as lower costs of both double base propellants(DBP)based on NC/NG and composite propellants(CP)based on oxidizer(AP)and binder(Hydroxyl terminated polybutadiene)[1],have been widely used in free loading rocket motors and missiles,etc.[2].In the past decades,the major research work for AP/Al/CMDB propellants was focused on the enhancement of the energy(Isp)of this kind of propellant.The theoreticalIspof a typical AP/Al/CMDB propellant investigated by C.N.Divekar was about 2 499N·s·kg-1[3].Another type of AP/Al/CMDB propellant was reported by National Aeronautics and Space Administration(NASA)with their theoreticalIspin the range of 2 549-2 598N·s·kg-1.In addition,the AP/Al/CMDB propellants with theoreticalIspof 2 548-2 597N·s·kg-1were reported by Hau[4].
It was found experimentally and theoretically that an increase inIspof propellants can extend the flight range of the rockets or missiles and enhance payload capability of the rockets or missiles[5].Therefore,in order to further improve the energy(Isp)of AP/Al/CMDB propellants,a new high energy AP/Al/CMDB propellant was designed and prepared by an increase in contents of the solid fillers(AP and Al)and optimization of their mass ratios in the propellant formulation.Its thermodynamic performances,processibility,burning behavior,chemical stability,mechanical property,sensitivities,thermal decomposition behavior,and the flame structure were further thoroughly studied.
1 Experiment
1.1 Materials and equipments
AP power(The fourth research institute of CASC),Al power(Gaizhou metal power factory),NC(N%=12.0,Sichuan chuanan chemical factory),RDX(Gansu baiyin yinguang chemical material factory).
RS300HAKKE rheometer(Haake Co.,Germany),Model ZDHW-2temperature-constant automatic calorimeter(Xi′an Modern Chemistry Research Institute,China),Model WL-1impact sensitivity apparatus(Xi′an Modern Chemistry Research Institute,China),Model WM-1 friction sensitivity apparatus(Xi′an Modern Chemistry Research Institute,China),Model SM-1static spark sensitivity apparatus(Xi′an Modern Chemistry Research Institute,China),Model YC-1instrument(Xi′an Modern Chemistry Research Institute,China), Model TA 2850 instrument(TA Co.,USA),Model TA DSC 910Sdifferential scanning calorimeter(TA Co.,USA),thermal video system(Xi′an Modern Chemistry Research Institute,China),INSTRON 4505 material test apparatus(Instron Co.,British).
1.2 Preparation of propellants
All the propellant samples were mixed in a 2 liter vertical planetary mixer in vacuum at 25℃for 60minutes.The samples were then cast in vacuum and cured at 70℃for 96h[6].
1.3 Characterization methods
Thermodynamic performances were calculated by using the Neng Xing Software and Database(China)at a standard expansion ratio of 100∶1(pressure,10MPa).
Processibility was determined on the RS300 HAKKE rheometer by using coaxial cylinder detectors at shear rate of 0-10s-1at 25℃.
The constant-volume heats of explosion(Qv)were measured by using a Model ZDHW-2temperature-constant automatic calorimeter with sample mass of 5.0g and reference sample of standard double-base gun propellant in vacuum.
Impact sensitivity was analyzed by employing the fall hammer method(2.0kg drop hammer)in a Model WL-1impact sensitivity apparatus with sample mass of 30mg and reference sample of standard RDX.Impact sensitivity was expressed by critical height of 50% probability of explosion(H50,cm).
Friction sensitivities were obtained by a Model WM-1friction sensitivity apparatus with pendulum angle of 66degree,sample mass of 20mg,sample clamp pressure of 2.45MPa and reference sample of standard tetryl.Friction sensitivity was expressed by probability of explosion(P,%).
Static spark sensitivity testing was performed on a Model SM-1static spark sensitivity apparatus with sample mass of 10mg and reference sample of standard RDX.Static spark sensitivity impact sensitivity was expressed by critical static spark energy of 50%probability of explosion(E50,mJ).
Flash point was measured by a wood alloy bath method with sample mass of 2.50g at a heating rate of 20℃·min-1.
Color-changing times was measured by methyl violet tests with sample mass of 2.50g at 120℃.Color-changing time was expressed by the time from the beginning to color change of methyl violet test paper.
Vacuum stability test was performed in a Model YC-1instrument with sample mass of 5.00g at 90℃for 48h.
Thermogravimetry(TG)analysis was carried out on a Model TA 2 850 TGA instrument with sample mass of 2.0mg at a heating rate of 5℃·min-1,and in dynamic nitrogen atmosphere at a flow rate of 100mL·min-1.
Differential scanning calorimeter(DSC)analysis was carried out on a Model TA DSC 910Sdifferential scanning calorimeter in static nitrogen atmosphere with sample mass of 2.0mg at a heating rate of 5℃·min-1,and in static nitrogen atmosphere with pressure of 0.1MPa.
Mechanical properties were determined by uniaxial tensile test on a INSTRON 4 505 material test apparatus with gauge length of dumbbellshaped samples of 100mm at cross speed of 100mm·min-1.
The burning rates were tested with the Crawford measurement method[7]in the pressure range of 1-22MPa.The sample strands with dimensions of 150mm ×5mm×5mm were coated with polyethylene alcohol and ignited by 0.1mm nickel wire.Burning rates were determined for 100mm measuring distance by disruption of 0.1mm nickel wires drawn through the strands.
Flame structures were obtained by a thermal video system in nitrogen atmosphere at pressures of 1MPa,3MPa and 5MPa,respectively[8].The sample with dimensions of 5mm×5mm×15mm were ignited by 0.1mm nickel wire.
2 Results and discussions
2.1 Formulation and thermodynamic performance
2.1.1 Effects of contents of solid fillers on the energy of AP/Al/CMDB propellants
It was theoretically and experimentally found that an increase in solid fillers such as AP,RDX,Al,etc.,in propellants can increase the energy of the propellants[3-4,9].The effects of the contents of solid fillers including AP and Al(mass ratio 2∶1)on energy of the AP/Al/CMDB propellants were studied by means of thermodynamic calculation and measurement of constant-volume explosion heat.The results were shown in Figure 1and Table 1 ,respectively.

Fig.1 Effects of the contents of solid fillers on the theoretical specific impulse of AP/Al/CMDB propellants
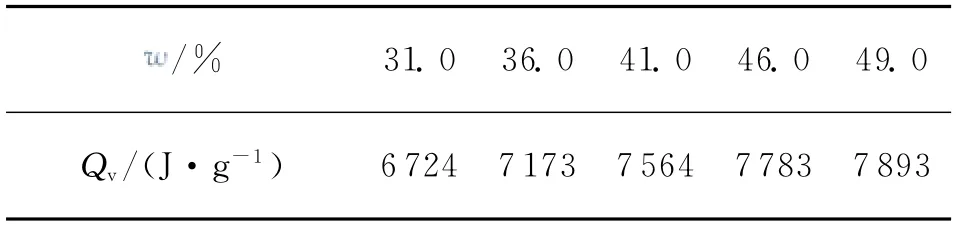
Table 1 Effects of the contents of solid fillers on the explosion heat of AP/Al/CMDB propellants at constant volume
It can be seen from Fig.1 that the theoretical specific impulse of AP/Al/CMDB propellant with 41%of solid fillers reaches to the maximum value of 2 628N·s·kg-1.It was also experimentally found from Table 1 that the determined explosion heat of AP/Al/CMDB propellants at constant volume increases gradually with the increase of the contents of solid fillers in propellants.Therefore,the content of solid fillers in AP/Al/CMDB propellant formulation was selected to be 41%.
2.1.2 Effects of mass ratio of Al to AP on the energy of AP/Al/CMDB propellants
The mass ratio of Al to AP in AP/Al/CMDB propellants can also affect its energy[10].The theoretical specific impulse and determined explosion heat(at constant volume)of the AP/Al/CMDB propellants including 41%of solid fillers with various mass ratios of Al to AP were shown in the Figure 2and Table 2 ,respectively.
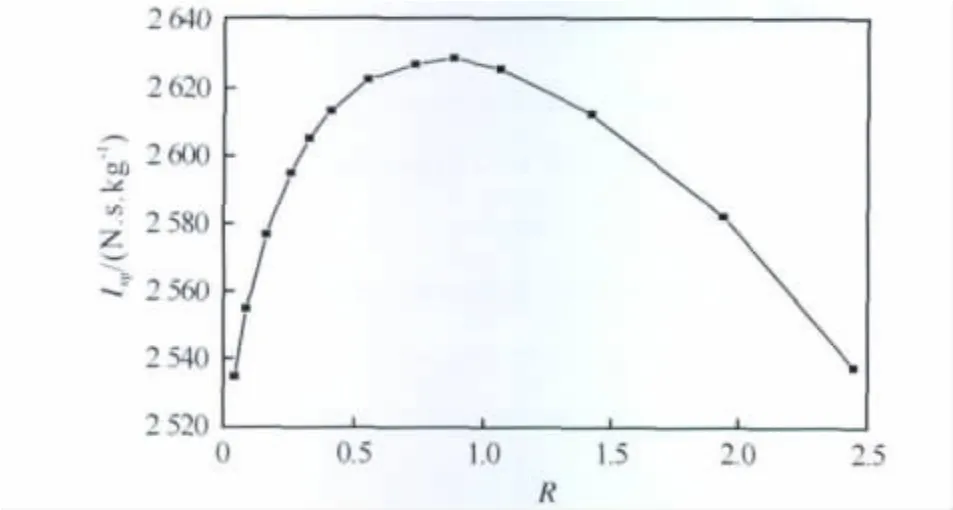
Fig.2 Effect of mass ratios of Al to AP on the theoretical specific impulse of AP/Al/CMDB propellants with 41%of solid fillers

Table 2 Effect of mass ratios of AP to Al on explosion heat of AP/Al CMDB propellant with 41%of solid fillers at constant volume
It can be seen from Figure 2and Table 2 that the theoretical specific impulse and the determined explosion heat of the propellant at constant volume reach to the maximum values of 2 637N·s·kg-1and 7 604J·g-1,respectively,at the mass ratio of 0.95.Therefore,the mass ratio of Al to AP in the AP/Al/CMDB propellant formulation with 41%solid fillers was selected to be 0.95.
2.1.3 Formulation of AP/Al/CMDB propellant and its thermodynamic performances
From the above results and the general requirements of mechanical properties,preparation processibility of AP/Al/CMDB propellant,a high energy AP/Al/CMDB propellant formulation containing 20% NC,30% NG,21% AP,20% Al,4.0%combustion catalysts,and 5.0%others was designed.Its theoretical thermodynamic performances were listed in Table 3.
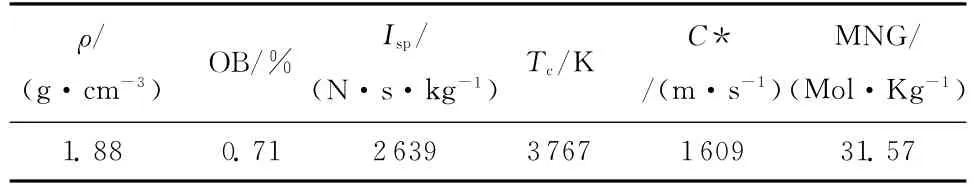
Table 3 The theoretical thermodynamic performances of the designed AP/Al/CMDB propellant
The results in Table 3 showed that the theoretical specific impulse of the designed AP/Al/CMDB propellant reaches to 2 639N·s·kg-1at a standard expansion ratio of 100 ∶1(pressure,10MPa),which is significantly higher than those of the propellants reported previously[3-4].Meanwhile,its theoretical density,oxygen balance and characteristic velocity arrive at 1.88g·cm-3,0.71 and 1 609m·s-1,respectively.
2.2 Preparation processibility
The preparation processibility of the propellant could be characterized by the yield stress and apparent viscosity of the propellants slurry[11].The yield stress and the apparent viscosity of the AP/Al/CMDB propellant slurry were calculated by using HAKKE program from the flow and viscosity curves shown in Figure 3,which was obtained by using the RS300HAKKE rheometer.The processibility of the propellant was listed in Table 4.

Fig.3 Flow and viscosity curves of the AP/Al/CMDB propellant slurry
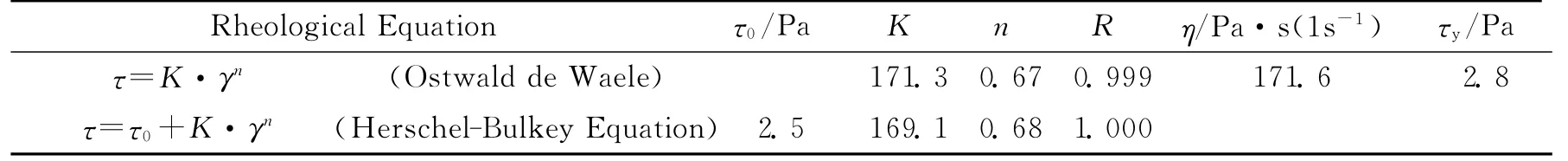
Table 4 The processibility of the AP/Al/CMDB propellant
It can be seen from Figure 3that the slurry of the AP/Al/CMDB propellant show a shearthinning behavior as the shear rate increases and the slurry appear the yield behavior at certain shear rate.It was indicated by above phenomena that the slurry of AP/Al/CMDB propellant is a typical pseudoplastic liquid,which can be made to flow and maintain a given flow rate as undergoing agiven force[12].It was found from curve-fitting with rheological equation that the flow curve is in agreement with both Ostwald de Waele and Herschel-Bulkey Equations with the correlation coefficientsR≥0.999and pseudoplastic indexn=0.67and 0.68,respectively.The apparent viscosity of the slurry at the shear rate of 1s-1is 171.6Pa·s and the yield stress is 2.8Pa.It means that the slurry of AP/Al/CMDB propellant has good flowing and leveling properties[13].
2.3 Mechanical properties
The mechanical properties of the AP/Al/CMDB propellant performed by uniaxial tensile test were listed in the Table 5.

Table 5 Mechanical Properties of the AP/Al/CMDB propellant
It was experimentally found that the propellant has acceptable maximum tensile strength at both 20℃and 50℃for the purpose of practical application by the addition of an appropriate amount of bonding agents and process aids,although the nitrocellulose(binder)content in the AP/Al/CMDB propellant was decreased to 20%in order to improve the energy of propellant by increasing the contents of solid fillers in the propellant.The mechanical properties of the AP/Al/CMDB propellant could meet the needs of free loading rocket motor.
2.4 Combustion characteristics
The combustion characteristics of the AP/Al/CMDB propellant were generally difficult to be modified due to large contents of AP and Al in the propellant.For the purpose of modifying the combustion characteristics of designed propellant,the combustion catalysts composed of lead salt,copper salt and carbon black,which were generally employed to improve the combustion characteristics of the CMDB propellant[9,14-15],were used herein.The combustion characteristics of the AP/Al/CMDB propellant were listed in the Table 6.

Table 6 Combustion characteristics of the AP/Al/CMDB propellant at 25℃
It was indicated from Table 6 that the cata-lysts composed of lead salts,copper salt and carbon black could effectively improve the combustion characteristics of the AP/Al/CMDB propellant.In particular,the pressure exponents of the AP/Al/CMDB propellant containing catalysts were decreased to 0.47(0.70 without catalysts)in the pressure range of 1-10MPa and 0.37(0.83without catalysts)in the pressure range of 10-20MPa.In the pressure range of 10-20MPa,the burning rate is weakly dependent on the pressure compared to the AP/Al/CMDB propellant without catalysts.It was concluded that the proposed AP/Al/CMDB propellants has excellent burning per-formance,which could meet the requirements of solid rocket motors(n<0.5).
2.5 Sensitivity and chemical stability
The chemical stability and sensitivity of propellant to external stimulation should be investigated to guarantee its safe applications,storage,preparation and transportation.For our new AP/Al/CMDB propellant formulation,the testing results of the sensitivities and chemical stabilities were as follows:the vacuum stability,flash point and color-changing time are 5.01mL,203.5℃and 54minutes,respectively.And the impact(H50),friction(P)and static spark sensitivity(E50)of the AP/Al/CMDB propellant are 27cm,61%and 179 mJ,respectively.TheH50is higher than that of the reported AP/Al/CMDB propellant containing 30% NC,35% desensitized NG,21% AP and 14% Al[16].The results above indicated that the new AP/Al/CMDB propellant can be used safely.
2.6 Thermal decomposition behavior
The thermal decomposition characteristics of the AP/Al/CMDB propellant were investigated by using the TG and DSC techniques.Both TG and DSC curves of the AP/Al/CMDB propellant were shown in Figure 4.
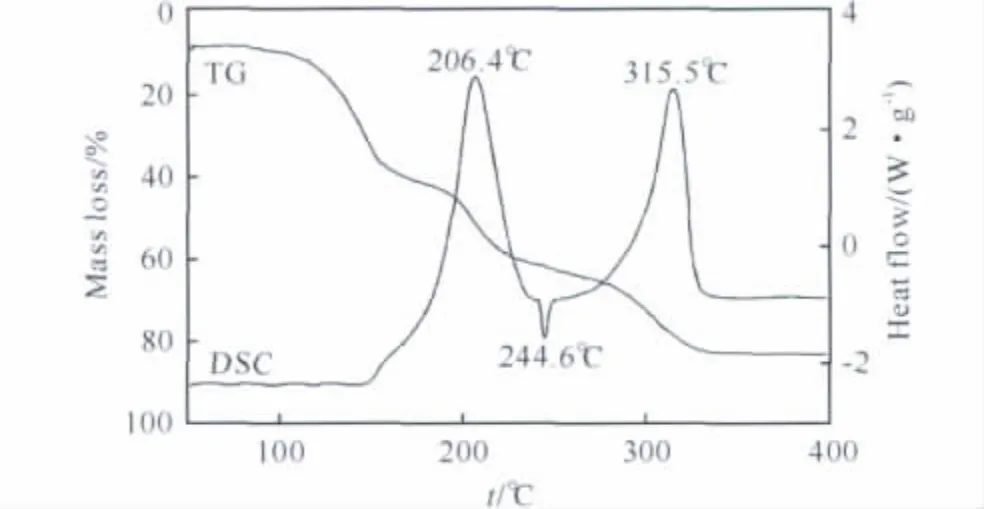
Fig.4 TG and DSC curves of the AP/Al/CMDB propellant at a heating rate of 5℃·min-1
As the AP/Al/CMDB propellant was heated,three mass loss stages in the temperature ranges of 105-160℃,160-235℃and 235-338℃were observed on its TG curve.The corresponding mass losses of the three stages are 31.7%,26.6%and 25.6%,respectively.Correspondingly,the DSC curve of the propellant showed two big exothermic peaks and one small endothermic peak.The first mass loss stage mainly results from the volatilization of NG.Due to smaller endothermic enthalpy of NG volatilization at pressure of 0.1MPa,the endothermic peak of the volatilization could not be evidently observed on the DSC curve.The second weight loss stage is mainly attributed to the decompositions of NC and DINA[17],which results in an exothermic peak at 206.4℃on the DSC curve.In the second weight loss stage,smaller endothermic peak at 244.6℃is related to crystal transition of AP[18].In the last weight loss stage,AP,combustion catalysts,and stabilizers were completely consumed.These consumptions result in intensive exothermic reactions,which correspond to an exothermic peak at 315℃on the DSC curve.At the end of testing,the residue(21.6%)was metal ox-ides and carbon black.
2.7 Flame structure characterization
The flame and its turbulence of the AP/Al/CMDB propellant can be identified clearly by Figure 5.
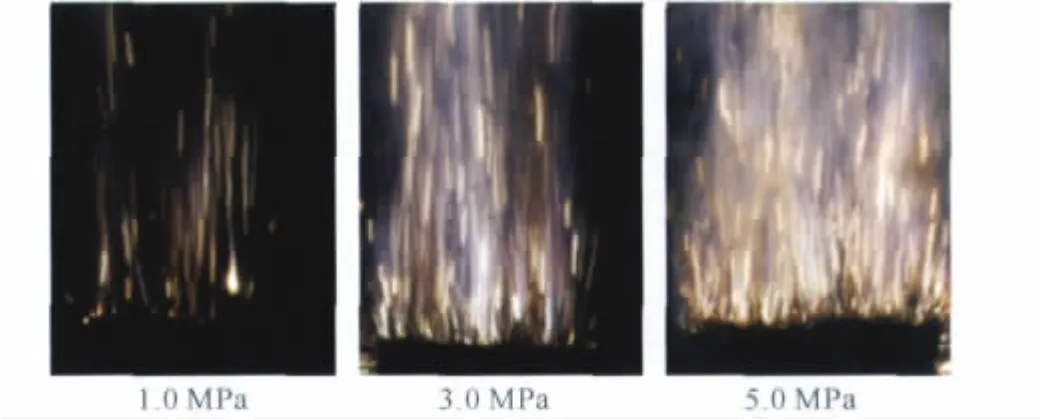
Fig.5 Flame photographs of the AP/Al/CMDB propellant at three different pressures
As shown in Figure 5,both bluish and yellowish flames could be seen.Observations for the burning surface also revealed that the bluish flames are formed above AP particles on the burning surface.These flames result from the combustions of ammonia and perchloric acid,which are decomposition products of AP[19].Surrounding the bluish flames,the yellowish luminous flame stream is formed.The yellowish luminous flame is produced by the decomposed gases of AP particles and the double-base matrix(NC and NG)[20].The bluish flames and yellowish flames overlap each other and form the whole flame contour of the propellant.And the large-sized luminous flames in flame photographs are attributed to combustion flames of Al.Additionally,it can be seen from the flame photographs that the brightness and turbulence of the flames increase as pressure increases.These phenomena are in agreement with the facts that burning rates increase as the pressure increases.Whereas,the dark zone existed in the premixed flame could not be observed in the flames of the propellant.From these phenomena described above,the flame of the AP/Al/CMDB propellant can be classified as diffusion flame[20-21].
4 Conclusions
(1)A novel slurry cast AP/Al/CMDB propellant with high theoretical specific impulse(Isp=2 639N·s·Kg-1at 100∶1 expansion ratio,10MPa)has been successfully designed and prepared by an increase in contents of solid fillers(AP and Al)and optimization of their mass ratio in the propellant formulation.
(2)The new propellant has good processibility and mechanical properties,sufficient chemical stability and sensitivities,which could meet the needs of free loading rocket motor.
(3)The propellant also possess excellent burning performance,especially lower pressure exponents(0.47in the pressure range of 1-10MPa,0.37in the pressure range of 10-20MPa).
(4)The TG curve of the AP/Al/CMDB propellant has three mass loss stages,while its DSC curve shows two decomposition stages.
(5)The flame of the new AP/Al/CMDB propellant is the typical diffusion flame.
[1]Kulkarni A R,Bhat V K,Phdke S P,et al.Simplified burn-rate model for CMDB propellants[J].Defense Science Journal,1990,40(3):255-262.
[2]Bhat V K,Singh H.Cross-linked slurry cast composite double base propellants:mechanical properties[J].Defense Science Journal,1987,37(1):39-44.
[3]Diver kar C N,Asthana S N,et al.Combustion and thermal studies on Al/Ti/Ni/Zr composite modified double-base systems[J].Journal of Propulsion and Power,2003,19(4):614-622.
[4]Hau Z L,Fen Z G,Wang E P,et al.The energy and pressure exponent of composite modified double base propellants[J].Prop,Expl,Pyro,1992,17(2):59-62.
[5]Singh H.Current trend if R &D in the field of high energy materials(HEMs)-an Overview[J].Propulsion(Japanese),2005:120-126.
[6]Stanley N F.Slurry cast propellant method:US,4080411[P].1978.
[7]Menke K,Heintz T,Schweikert W,Keicher T,et al.Formulation and properties of AND/GAP propellant[J].Prop,Expl,Pyro,2009,34(3):218-230.
[8]Yan Q L,Song Z W,Shi X B,et al.Combustion mechanism of double-base propellant containing nitrogen heterocyclic nitroamines(II):The temperature distribution of the flame and its chemical structure[J].Acta Astronautica,2009,64(5-6):602-614.
[9]Muthiah Rm.,Varghese T L,Rao S S,and et al.Realization of an eco-friendly solid propellant based on HTPB-HMX-AP system for launch vehicle applica-tions[J].Propellant,Explosive,Pyrotechnics,1998,23(2):90-93.
[10]Thomas N,Howard G,Cutforth.Solid propellant selection and characterization,SP-8064[R].[S.l.]:The National Aeronautics and Space Administration(NASA),1971:24-29.
[11]Muthiah Rm,Krishnamurthy V N,Gupta B R..Rheology of HTPB propellant:development of generalized correlation and evaluation of pot life[J].Propellants,Explosives,Pyrotechnics,2004,21(4):186-192.
[12]Schramm G.A practical approach to rheology and rheometer[M].Karlsruhe:Gebrueder HAAKE Gmbh,1994:14-19.
[13]Muthiah R M,Hirshnamurthy V K,Gupta B R.Rheology of HTPB propellant.Ⅰ:Effect of solid loading,oxidizer practical size and aluminum content[J].Journal of Applied Polymer Science,1992,44:2043-2052.
[14]Raman K V,Singh H.Ballistic modification of RDXBased CMDB propellants[J].Propellant,Explosive,Pyrotechnics,2004,13(5):149-151.
[15]Han X,Wang T F,Lin Z K,et al.RDX/AP-CMDB Propellants containing fullerenes and carbon black additive[J].Defense Science Journal,2009,59(3):284-293.
[16]Asthana S N,Athawale B K,Singh H.Impact,friction,shock sensitivity and DDT behavior of advanced CMDB Propellant[J].Defense Science Journal,1989,39(1):99-103.
[17]Fan X Z,Li J Z,Zhang L Y W,et al.Characteristics of the smokeless CMDB propellants with 1,3,3-trinitroazetidine t[J].Chinese Journal of Explosives and Propellants,2005,28(4):35-39.
[18]Bircumshaw L L,Newman B H.The thermal decomposition of ammonium perchlorate.I.Introduction,experimental,analysis of gaseous products,and thermal decomposition experiments[C]//Proceedings of the Royal Society of London.Series A,Mathematical and Physical Sciences.[S.l.]:Royal Society Publishing,1954,227:115-119.
[19]Kubota N.Propellant and explosives:thermochemical aspects of combustion[M].New York:John Wiley &Sons Inc,2004:183-188.
[20]Parr T P,Hanson-Parr D M.Solid Propellant diffusion flame structure[C]//Symposium(International)on Combustion.Naples:[s.n.],1996,26:1981-1876.
[21]Kubota N.Propellant and explosives:thermochemical aspects of combustion[M].New York:John Wiley &Sons Inc,2004:157-162.

